Cardiovascular Disease Burden Attributable to High Sodium Intake in China: A Longitudinal Study from 1990 to 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Data
2.2. Definitions of Dietary Risk Factors and Associated Outcomes
2.3. Estimation of High Sodium Intake-Attributed CVD Burden
2.4. Statistical Analyses
3. Results
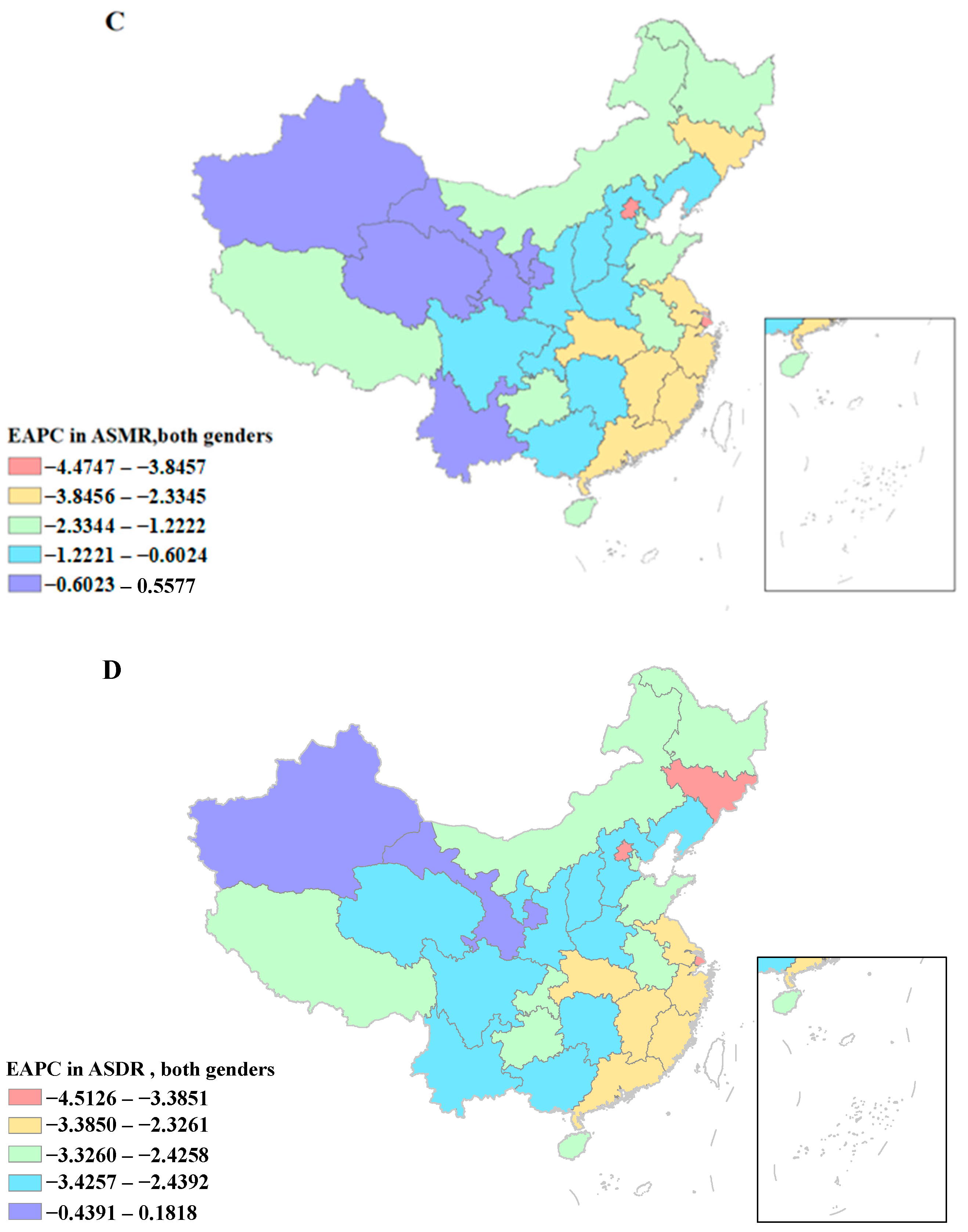
3.1. Deaths and ASMR of CVD Attributable to HSI
3.2. DALYs and ASDR of CVD Attributable to HSI
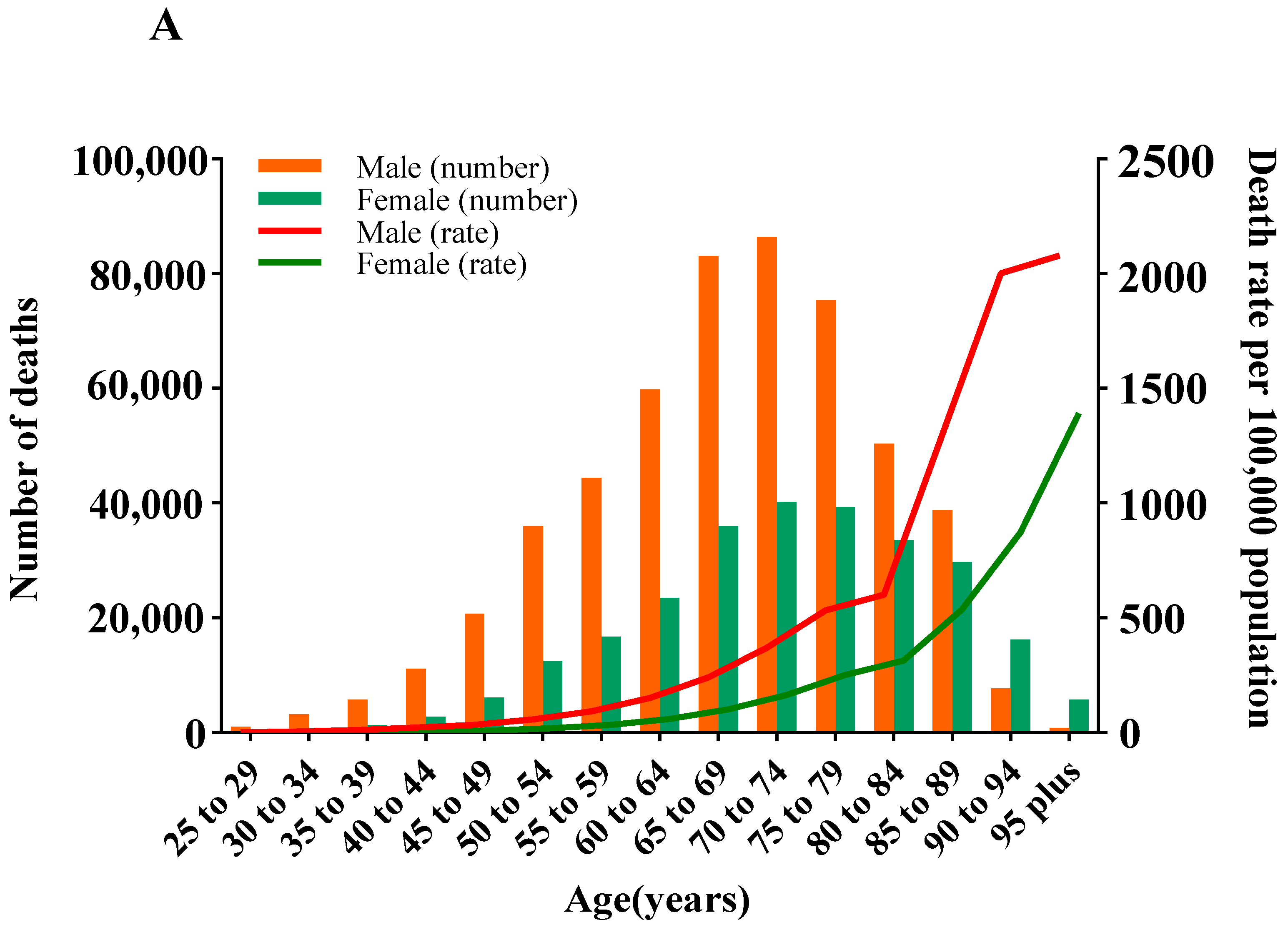
3.3. Deaths and ASMR of CVD Attributable to HSI by Age and Gender
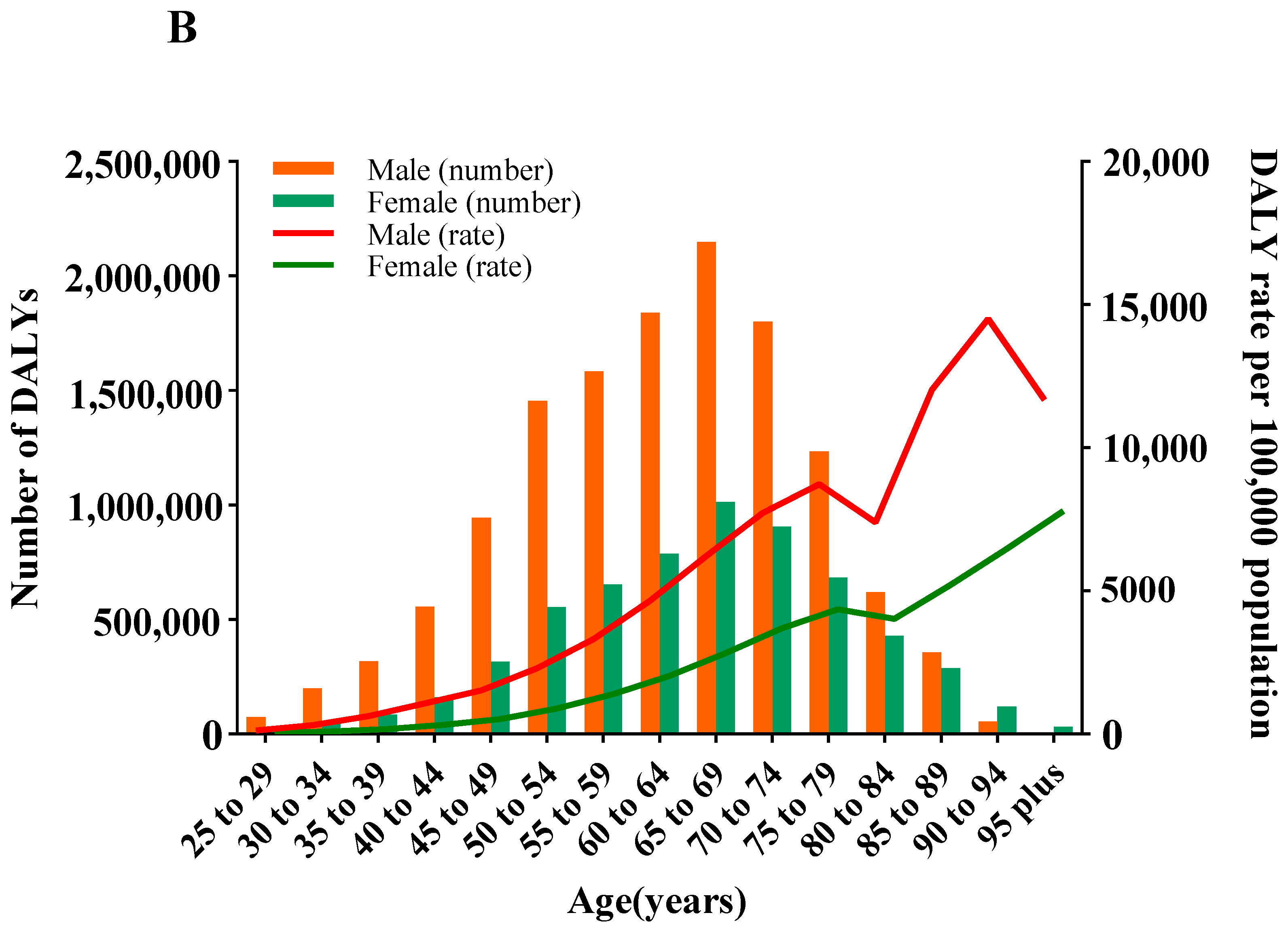
3.4. DALYs and ASDR of CVD Attributable to HSI by Age and Gender
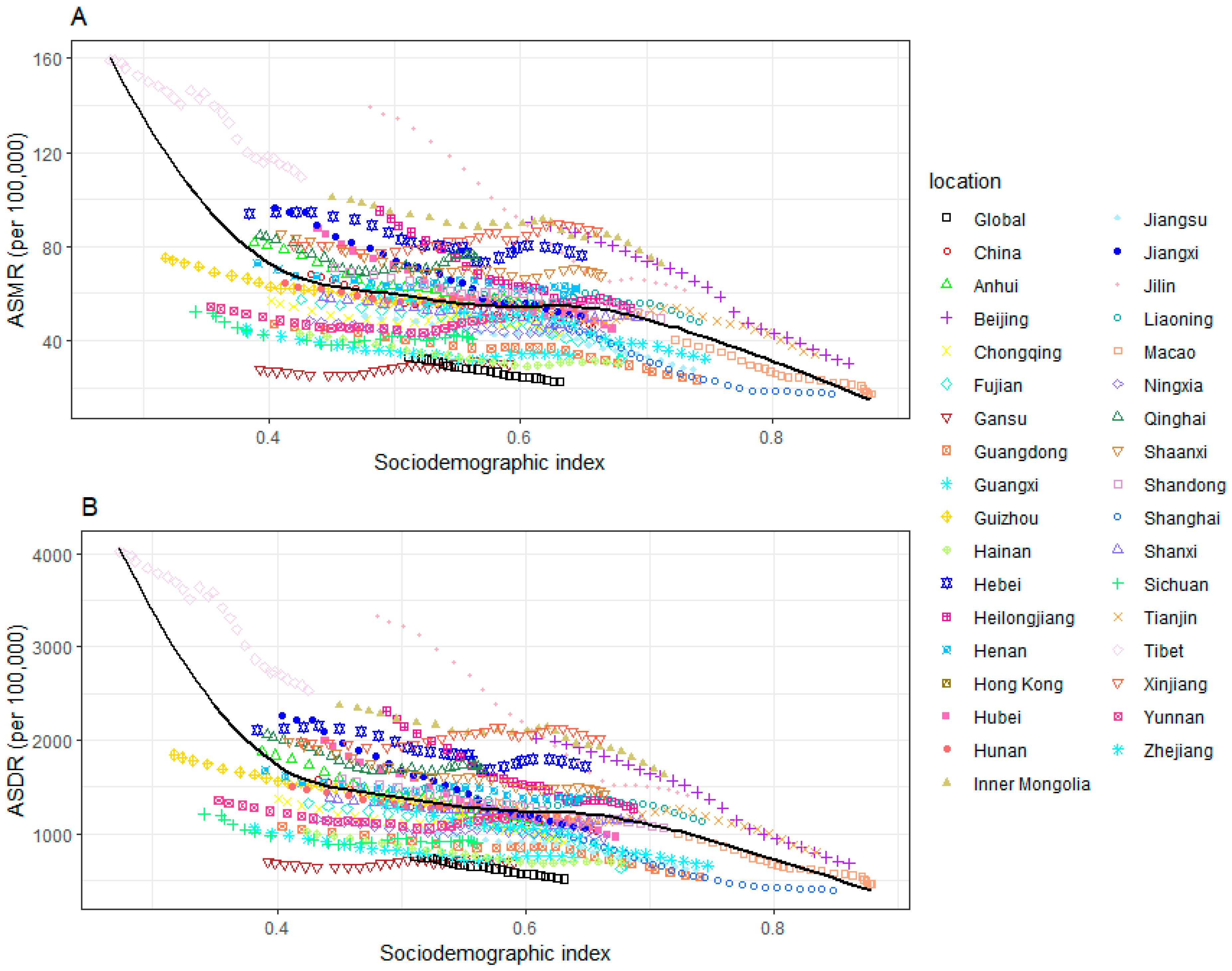
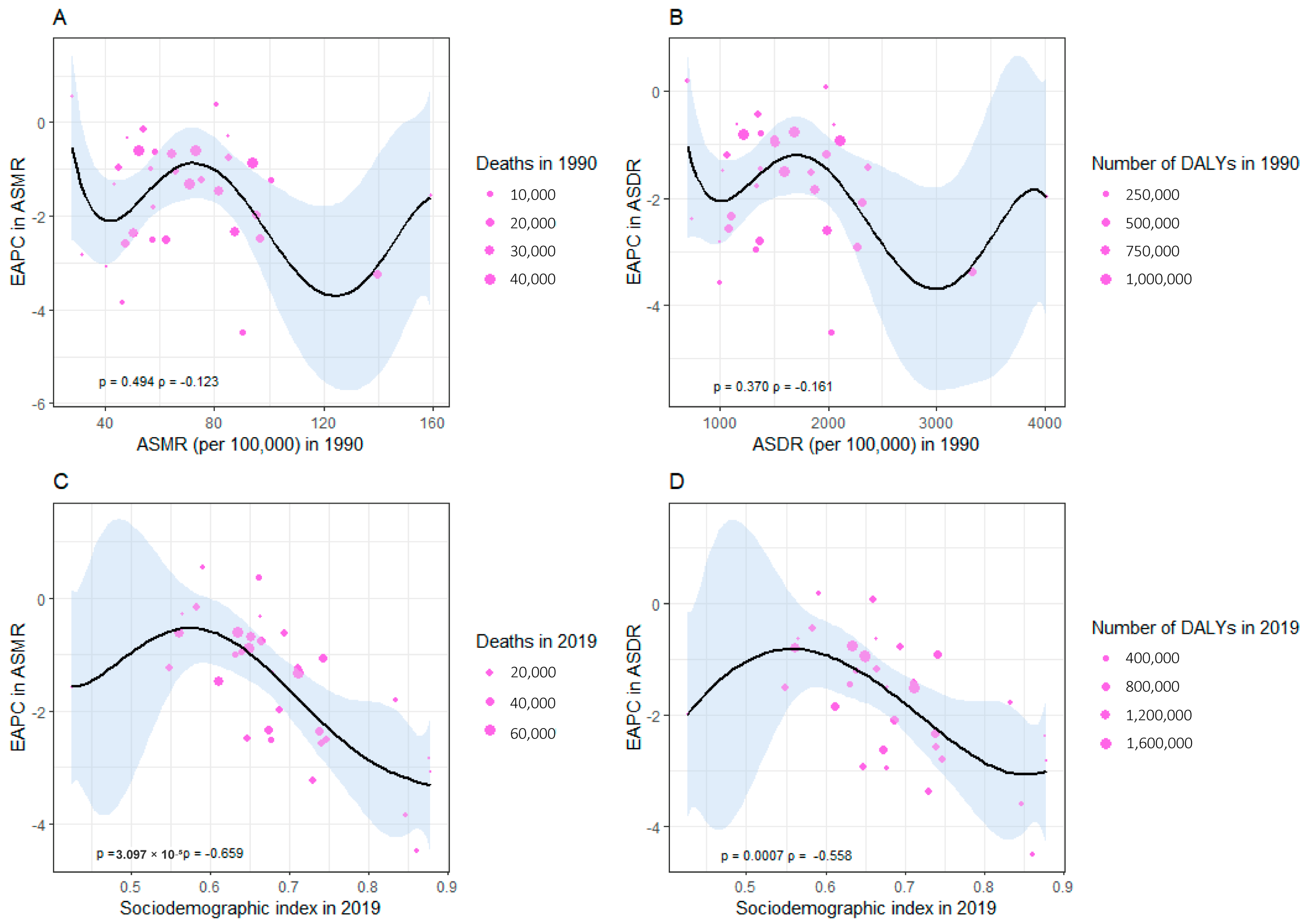
3.5. The Association between ASMR and ASDR of CVD Attributable to HSI and SDI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Cini, K.; Dong, B.; Ma, Y.; Ma, J.; Burgner, D.P.; Patton, G.C. Time Trends in Cardiovascular Disease Mortality across the BRICS: An Age-Period-Cohort Analysis of Key Nations with Emerging Economies Using the Global Burden of Disease Study 2017. Circulation 2020, 141, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Patel, A.; Anderson, C.S.; Dong, J.; Ma, C. Epidemiology of Cardiovascular Disease in China and Opportunities for Improvement. J. Am. Coll. Cardiol. 2019, 73, 3135–3147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tong, H.; Jiang, L.; Zhang, Y.; Hu, J. Trends and disparities in China’s cardiovascular disease burden from 1990 to 2019. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-S. Report on cardiovascular health and diseases in China 2021: An updated summary. J. Geriatr. Cardiol. 2023, 20, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Gu, D.; Zhao, D.; Coxson, P.; Wang, Y.C.; Chen, C.-S.; Liu, J.; Cheng, J.; Bibbins-Domingo, K.; Shen, Y.-M.; et al. Future Cardiovascular Disease in China: Markov Model and Risk Factor Scenario Projections from the Coronary Heart Disease Policy Model—China. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wilson, P.W.F.; Kannel, W.B. Beyond Established and Novel Risk Factors: Lifestyle Risk Factors for Cardiovascular Disease. Circulation 2008, 117, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Yusuf, S.; Mente, A.; Gao, P.; Mann, J.F.; Teo, K.; McQueen, M.; Sleight, P.; Sharma, A.M.; Dans, A.; et al. Urinary Sodium and Potassium Excretion and Risk of Cardiovascular Events. Jama 2011, 306, 2229–2238. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary Sodium and Potassium Excretion, Mortality, and Cardiovascular Events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower Levels of Sodium Intake and Reduced Cardiovascular Risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Moran, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.; Ahola, A.; Wadén, J.; Tolonen, N.; Saraheimo, M.; Gordin, D.; et al. The Association Between Dietary Sodium Intake, ESRD, and All-Cause Mortality in Patients with Type 1 Diabetes. Diabetes Care 2011, 34, 861–866. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Report on Sodium Intake Reduction. Available online: https://www.who.int/publications-detail-redirect/9789240069985 (accessed on 1 August 2023).
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; De Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef]
- Fang, K.; He, Y.; Fang, Y.; Lian, Y. Dietary Sodium Intake and Food Sources among Chinese Adults: Data from the CNNHS 2010–2012. Nutrients 2020, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Abbas, K.M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abolhassani, H.; Abreu, L.G.; Abrigo, M.R.M.; et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Zhao, Y.; Zhang, L.; Yang, J.; Zhou, M.; Wang, Z. The burden of ischemic heart disease and type 2 diabetes mellitus attributable to diet high in sugar-sweetened beverages in China: An analysis for the Global Burden of Disease Study 2017. J. Diabetes 2021, 13, 482–493. [Google Scholar] [CrossRef]
- Chen, X.; Du, J.; Wu, X.; Cao, W.; Sun, S. Global burden attributable to high sodium intake from 1990 to 2019. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3314–3321. [Google Scholar] [CrossRef]
- Liu, Z.; Man, Q.; Li, Y.; Yang, X.; Ding, G.; Zhang, J.; Zhao, W. Estimation of 24-hour urinary sodium and potassium excretion among Chinese adults: A cross-sectional study from the China National Nutrition Survey. Am. J. Clin. Nutr. 2024, 119, 164–173. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Y.; Wang, M.; Liu, J.; Bu, X.; Mu, J.; Lu, J. Global cardiovascular diseases burden attributable to high sodium intake from 1990 to 2019. J. Clin. Hypertens. 2023, 25, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef]
- Trieu, K.; Ospanova, F.; Tazhibayev, S.; Jewell, J.; Breda, J.; Santos, J.A.; Webster, J. Sodium and potassium intakes in the Kazakhstan population estimated using 24-h urinary excretion: Evidence for national action. Eur. J. Nutr. 2021, 60, 1537–1546. [Google Scholar] [CrossRef]
- Afroza, U.; Abrar, A.K.; Nowar, A.; Akhtar, J.; Mamun, M.A.A.; Sobhan, S.M.M.; Cobb, L.; Ide, N.; Choudhury, S.R. Salt Intake Estimation from Urine Samples in South Asian Population: Scoping Review. Nutrients 2023, 15, 4358. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.; Todd, J.E.; Mancino, L.; Lin, B.-H. The Impact of Food Away from Home on Adult Diet Quality. ERR-90, U.S. Department of Agriculture, Econ. Res. Serv., February 2010. Adv. Nutr. 2011, 2, 442–443. [Google Scholar] [CrossRef]
- Ju, L.; Yu, D.; Guo, Q.; Fang, H.; Xu, X.; Li, S.; Cheng, X.; Piao, W.; Cai, S.; Zhao, L. Eating out behavior and its impact on obesity among Chinese residents aged 18–59 in 2015. Wei Sheng Yan Jiu 2021, 50, 395–400. [Google Scholar] [CrossRef]
- Du, W.; Wang, H.; Zhang, J.; Zhang, X.; Wei, N.; Li, Y.; Tan, M.; Zhang, P.; He, F.J. Sodium content of restaurant dishes in China: A cross-sectional survey. Nutr. J. 2022, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.G.; Speed, J.S.; Jin, C.; Pollock, D.M. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol.-Ren. Physiol. 2016, 311, F991–F998. [Google Scholar] [CrossRef]
- Kannel, W.B.; Levy, D. Menopause, Hormones, and Cardiovascular Vulnerability in Women. Arch. Intern. Med. 2004, 164, 479. [Google Scholar] [CrossRef]
- World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/ (accessed on 15 August 2023).
- Chen, S.; Shan, L.C.; Tao, W.; Lu, T.; Regan, Á.; Han, H.; Guo, L.; Deng, T.; Wall, P. A survey of Chinese consumers’ knowledge, beliefs and behavioural intentions regarding salt intake and salt reduction. Public Health Nutr. 2020, 23, 1450–1459. [Google Scholar] [CrossRef]
- Mingji, C.; Onakpoya, I.J.; Perera, R.; Ward, A.M.; Heneghan, C.J. Relationship between altitude and the prevalence of hypertension in Tibet: A systematic review. Heart 2015, 101, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Q.; Cai, H.; He, R.; Nima, Q.; Li, Y.; Suolang, D.; Cidan, Z.; Wangqing, P.; Zhao, X.; et al. Association of Tibetan Habitual Food and Metabolic Syndrome Among Tibetan People in China: A Cross-Sectional Study. Front. Nutr. 2022, 9, 888317. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Obarzanek, E.; Cutler, J.A.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The trials of hypertension prevention follow-up study. Arch. Intern. Med. 2009, 169, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.W.Y.; Fong, F.Y.H.; Cheng, F.W.T.; Yan, B.P.Y. Life’s simple 7 and cardiovascular disease risk knowledge in Hong Kong. BMC Cardiovasc. Disord. 2019, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Loke, A.Y.; Chan, K.N. Dietary habits of patients with coronary atherosclerosis: Case–control study. J. Adv. Nurs. 2005, 52, 159–169. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Chen, W.-W.; Gao, R.-L.; Liu, L.-S.; Zhu, M.-L.; Wang, Y.-J.; Wu, Z.-S.; Li, H.-J.; Gu, D.-F.; Yang, Y.-J.; et al. China cardiovascular diseases report 2018: An updated summary. J. Geriatr. Cardiol. JGC 2020, 17, 1. [Google Scholar]
- Wang, M.; Huang, Y.; Song, Y.; Chen, J.; Liu, X. Study on Environmental and Lifestyle Factors for the North–South Differential of Cardiovascular Disease in China. Front. Public Health 2021, 9, 615152. [Google Scholar] [CrossRef]
- Braga, A.L.F.; Zanobetti, A.; Schwartz, J. The time course of weather-related deaths. Epidemiology 2001, 12, 662–667. [Google Scholar] [CrossRef]
- Zhang, S.; Routledge, M.N. The contribution of PM2.5 to cardiovascular disease in China. Environ. Sci. Pollut. Res. 2020, 27, 37502–37513. [Google Scholar] [CrossRef]
- Du, S.; Wang, H.; Zhang, B.; Popkin, B.M. Dietary potassium intake remains low and sodium intake remains high, and most sodium is derived from home food preparation for Chinese adults, 1991–2015 trends. J. Nutr. 2020, 150, 1230–1239. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | 1990 | 2019 | 1990–2019 | |||
|---|---|---|---|---|---|---|
| Deaths Cases, No. (95% UI) | ASMR per 100,000 No. (95% UI) | Deaths Cases, No. (95% UI) | ASMR per 100,000 No. (95% UI) | PAFs % (95% UI) | EAPC (%) in ASMR No. (95% CI) | |
| Global | 1,215,850.67 (397,663.19 to 2,505,379.89) | 33.16 (10.39 to 69.96) | 1,715,381.13 (454,452.62 to 3,713,240.37) | 21.51 (5.58 to 47.20) | 0.09 (0.02 to 0.19) | −1.53 (−1.57 to −1.50) |
| China | 512,346.4 (233,687.7 to 853,907.61) | 68.52 (27.97 to 119.98) | 788,587.8 (307,537.43 to 1,385,711.49) | 42.46 (15.40 to 76.86) | 0.15 (0.06 to 0.28) | −1.45 (−1.55 to −1.35) |
| Gender (China) | ||||||
| Male | 311,061.16 (151,828.83 to 490,285.48) | 89.96 (39.84 to 150.10) | 524,270.43 (225,211.66 to 876,595.25) | 62.57 (24.39 to 109.97) | 0.17 (0.07 to 0.29) | −0.96 (−1.08 to −0.83) |
| Female | 201,285.24 (73,041.74 to 371,881.92) | 52.16 (17.36 to 100.50) | 264,317.36 (67,441.92 to 539,511.81) | 26.86 (6.52 to 55.72) | 0.12 (0.03 to 0.24) | −2.18 (−2.29 to −2.07) |
| Socio-demographic Index (SDI) | ||||||
| High SDI | 3772.18 (370.52 to 4293.03) | 52.22 (22.38 to 88.22) | 4575.81 (1609.17 to 6936.32) | 19.16 (6.52 to 36.28) | 19.16 (0.05 to 0.28) | −3.56 (−3.71 to −3.02) |
| High-middle SDI | 20,309.93 (9732.94 to 28,932.90) | 76.44 (30.92 to 132.48) | 31,137.83 (12,152.52 to 49,705.38) | 42.89 (14.48 to 84.42) | 0.15 (0.05 to 0.27) | −1.88 (−2.23 to −1.63) |
| Middle SDI | 16,908.47 (7177.02 to 28,677.93) | 67.49 (25.61 to 121.99) | 26,349.53 (9721.99 to 54,485.84) | 48.46 (17.31 to 91.24) | 5.98 (0.05 to 0.27) | −0.93 (−1.22 to −0.66) |
| Low-middle SDI | 6740.50 (2819.47 to 7834.64) | 87.39 (38.45 to 154.12) | 10,841.83 (3600.87 to 20,705.91) | 61.33 (23.33 to 111.58) | 0.16 (0.06 to 0.28) | −0.74 (−0.88 to −0.60) |
| Characteristics | 1990 | 2019 | 1990–2019 | |||
|---|---|---|---|---|---|---|
| DALYs, No. (95% UI) | ASDR per 100,000 No. (95% UI) | DALYs, No. (95% UI) | ASDR per 100,000 No. (95% UI) | PAFs % (95% UI) | EAPC (%) in ASDR No. (95% CI) | |
| Global | 30,468,479.33 (10,829,442.92 to 59,419,298) | 756.66 (262.03 to 1495.60) | 40,540,674.51 (12,262,912.95 to 83,022,361.27) | 490.68 (146.27 to 1010.73) | 0.10 (0.03 to 0.21) | −1.54 (−1.57 to −1.50) |
| China | 13,852,385.09 (6,916,321.04 to 22,011,605.74) | 1582.78 (751.65 to 2585.91) | 19,308,628.39 (8,894,786.58 to 31,852,222.15) | 954.80 (425.86 to 1600.97) | 0.19 (0.09 to 0.32) | −1.61 (−1.68 to −1.53) |
| Gender | ||||||
| Male | 8,608,041.4 (4,574,858.95 to 13,276,567.48) | 2008.91 (1004.58 to 3173.03) | 13,190,360.85 (6,551,116.67 to 21,235,740.84) | 1358.90 (646.75 cto 2217.05) | 0.22 (0.11 to 0.34) | −1.13 (−1.22 to −1.03) |
| Female | 5,244,343.69 (2,247,167.81 to 9,012,808.73) | 1196.65 (482.56 to 2093.97) | 6,118,267.54 (1,979,661.12 to 11,147,594.56) | 587.74 (185.00 to 1082.24) | 0.15 (0.05 to 0.28) | −2.44 (−2.53 to −2.35) |
| Socio-demographic Index (SDI) | ||||||
| High SDI | 96,492.83 (88,944.24 to 198,188.75) | 1185.88 (415.09 to 1959.03) | 109,499.06 (62,723.97 to 218,135.01) | 456.96 (205.66 to 826.28) | 0.19 (0.09 to 0.31) | −3.33 (−3.58 to −3.07) |
| High-middle SDI | 542,598.62 (286,974.19 to 869,579.72) | 1820.67 (876.75 to 3011.52) | 770,508.40 (350,208.69 to 1,320,252.39) | 971.97 (378.71 to 1660.43) | 0.19 (0.08 to 0.32) | −2.02 (−2.28 to −1.84) |
| Middle SDI | 461,382.41 (220,529.43 to 851,330.99) | 1591.61 (778.84 to 2691.05) | 638,164.44 (290,164.88 to 989,275.51) | 1074.08 (437.44 to 1876.64) | 0.19 (0.09 to 0.32) | −1.20 (−1.41 to −0.95) |
| Low-middle SDI | 192,364.27 (118,846.97 to 325,071.57) | 2185.11 (1025.61 to 3633.15) | 274,191.05 (106,753.79 to 491,301.09) | 1412.95 (638.08 to 2380.01) | 0.20 (0.09 to 0.32) | −1.10 (−1.23 to −0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Shen, W.; Wang, A.; Fang, H.; Wang, Q.; Li, H.; Liu, S.; Shen, Y.; Liu, A. Cardiovascular Disease Burden Attributable to High Sodium Intake in China: A Longitudinal Study from 1990 to 2019. Nutrients 2024, 16, 1307. https://doi.org/10.3390/nu16091307
Jiang L, Shen W, Wang A, Fang H, Wang Q, Li H, Liu S, Shen Y, Liu A. Cardiovascular Disease Burden Attributable to High Sodium Intake in China: A Longitudinal Study from 1990 to 2019. Nutrients. 2024; 16(9):1307. https://doi.org/10.3390/nu16091307
Chicago/Turabian StyleJiang, Liying, Wanying Shen, Anqi Wang, Haiqin Fang, Qihe Wang, Huzhong Li, Sana Liu, Yi Shen, and Aidong Liu. 2024. "Cardiovascular Disease Burden Attributable to High Sodium Intake in China: A Longitudinal Study from 1990 to 2019" Nutrients 16, no. 9: 1307. https://doi.org/10.3390/nu16091307
APA StyleJiang, L., Shen, W., Wang, A., Fang, H., Wang, Q., Li, H., Liu, S., Shen, Y., & Liu, A. (2024). Cardiovascular Disease Burden Attributable to High Sodium Intake in China: A Longitudinal Study from 1990 to 2019. Nutrients, 16(9), 1307. https://doi.org/10.3390/nu16091307






