Seasonal Variation in Vitamin D Status Does Not Interfere with Improvements in Aerobic and Muscular Endurance in Conscripts during Basic Military Training
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Blood Sampling and Analyses
2.3. Assessment of Physical Performance
2.4. Statistical Analysis
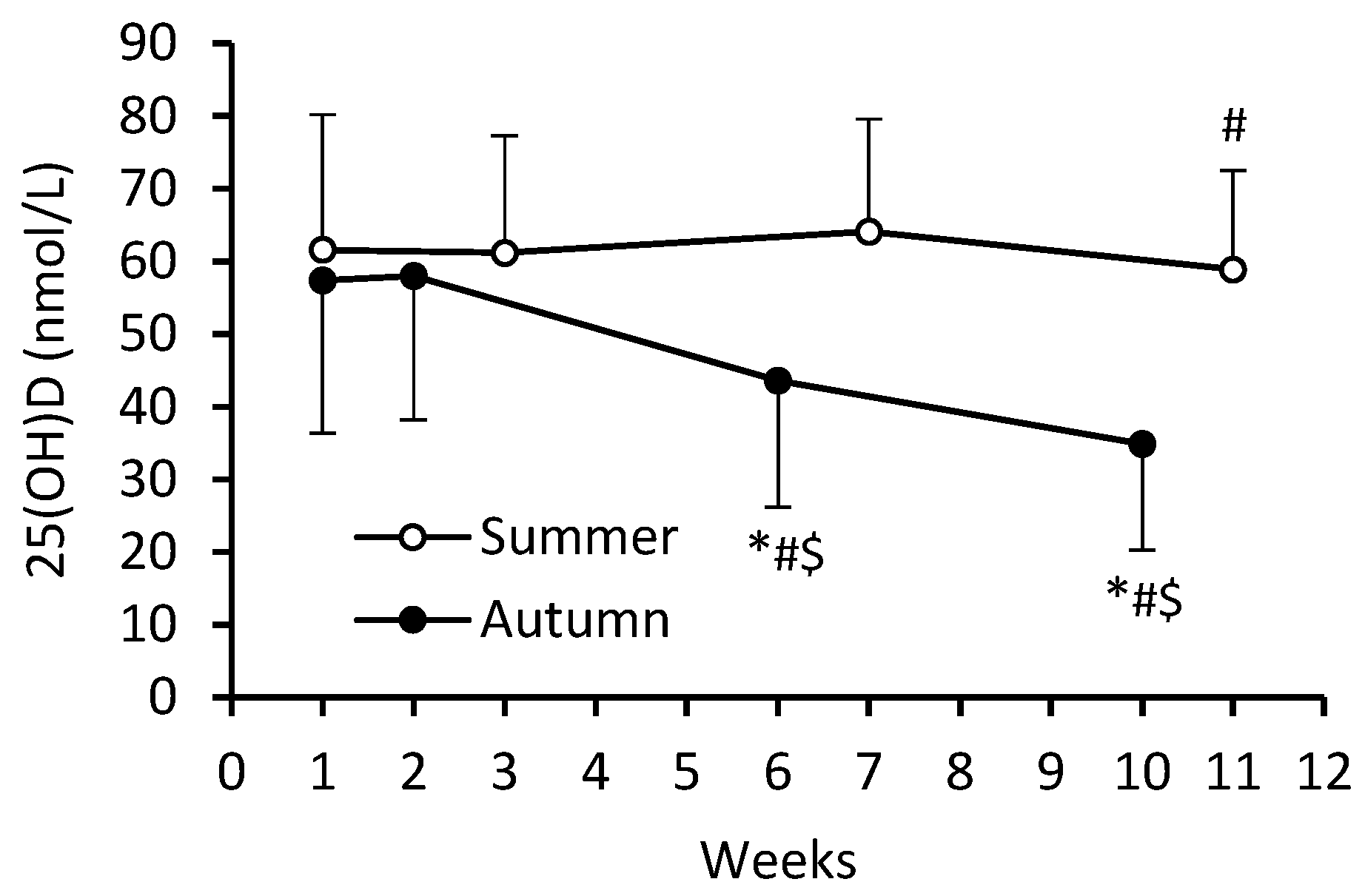
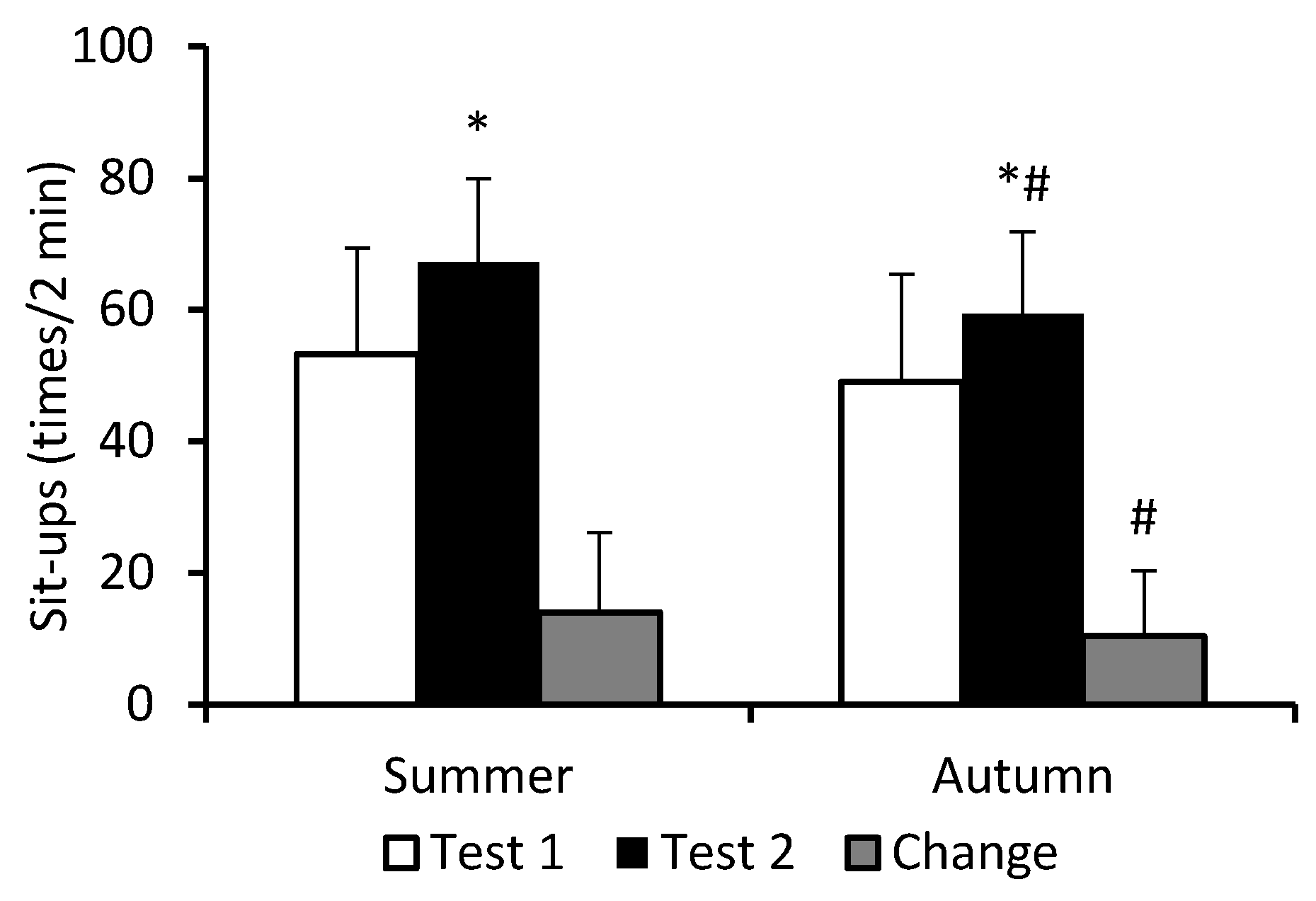
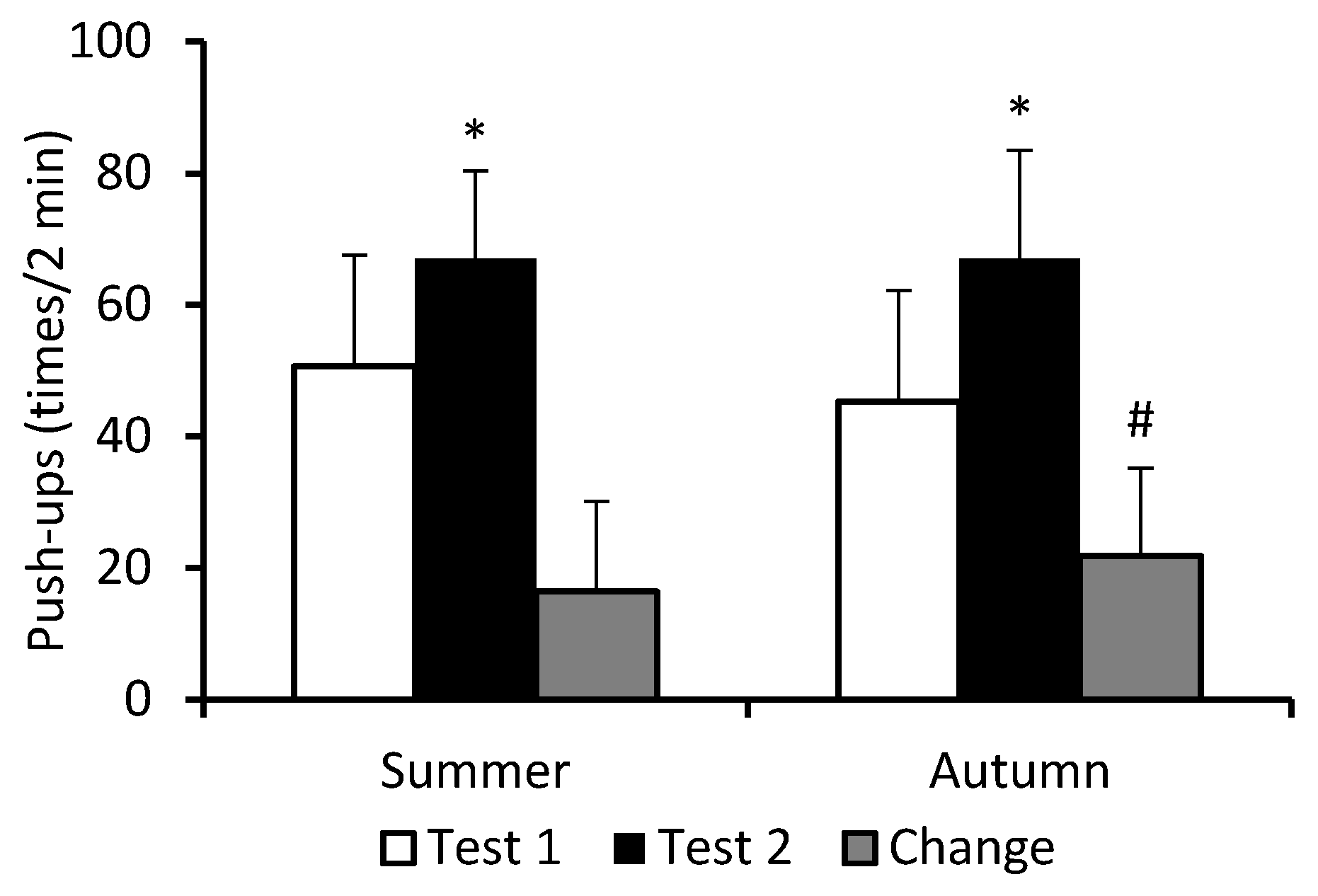
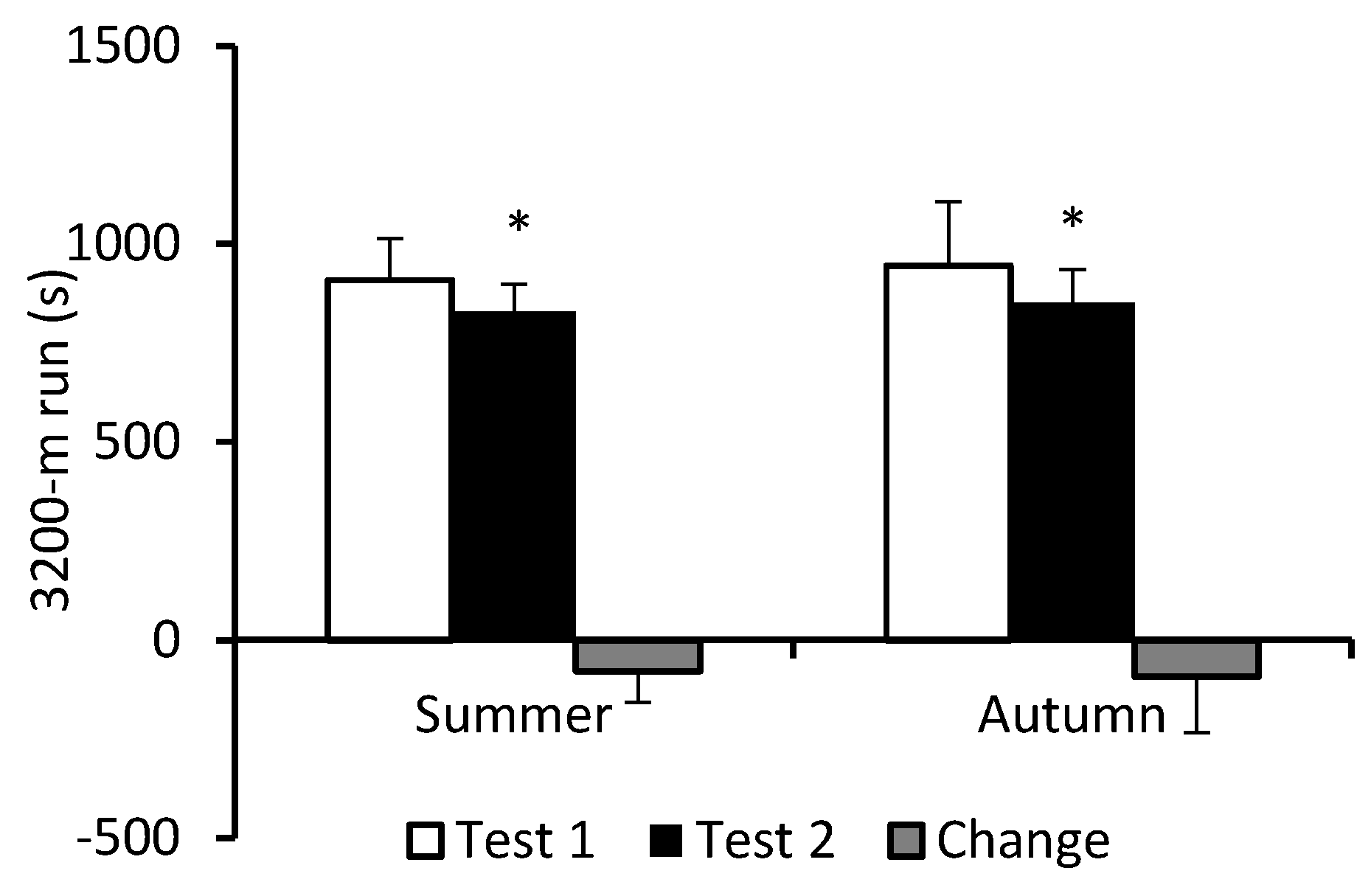
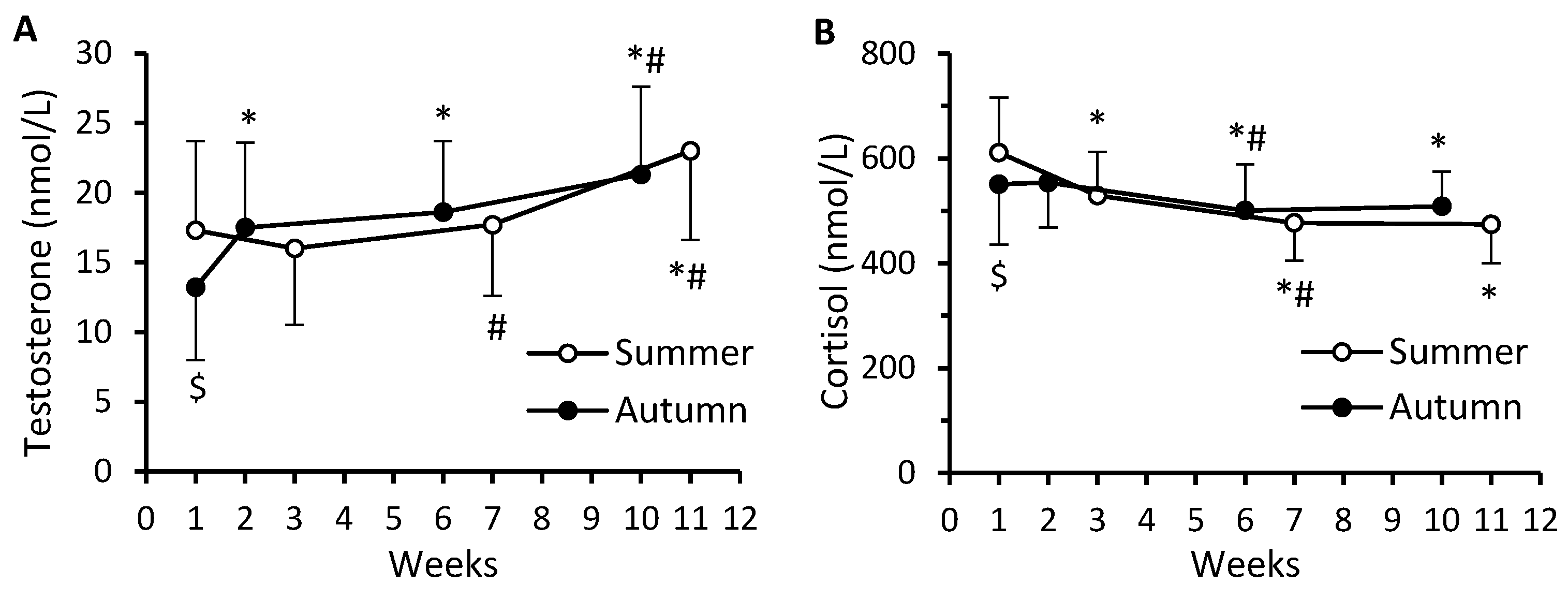
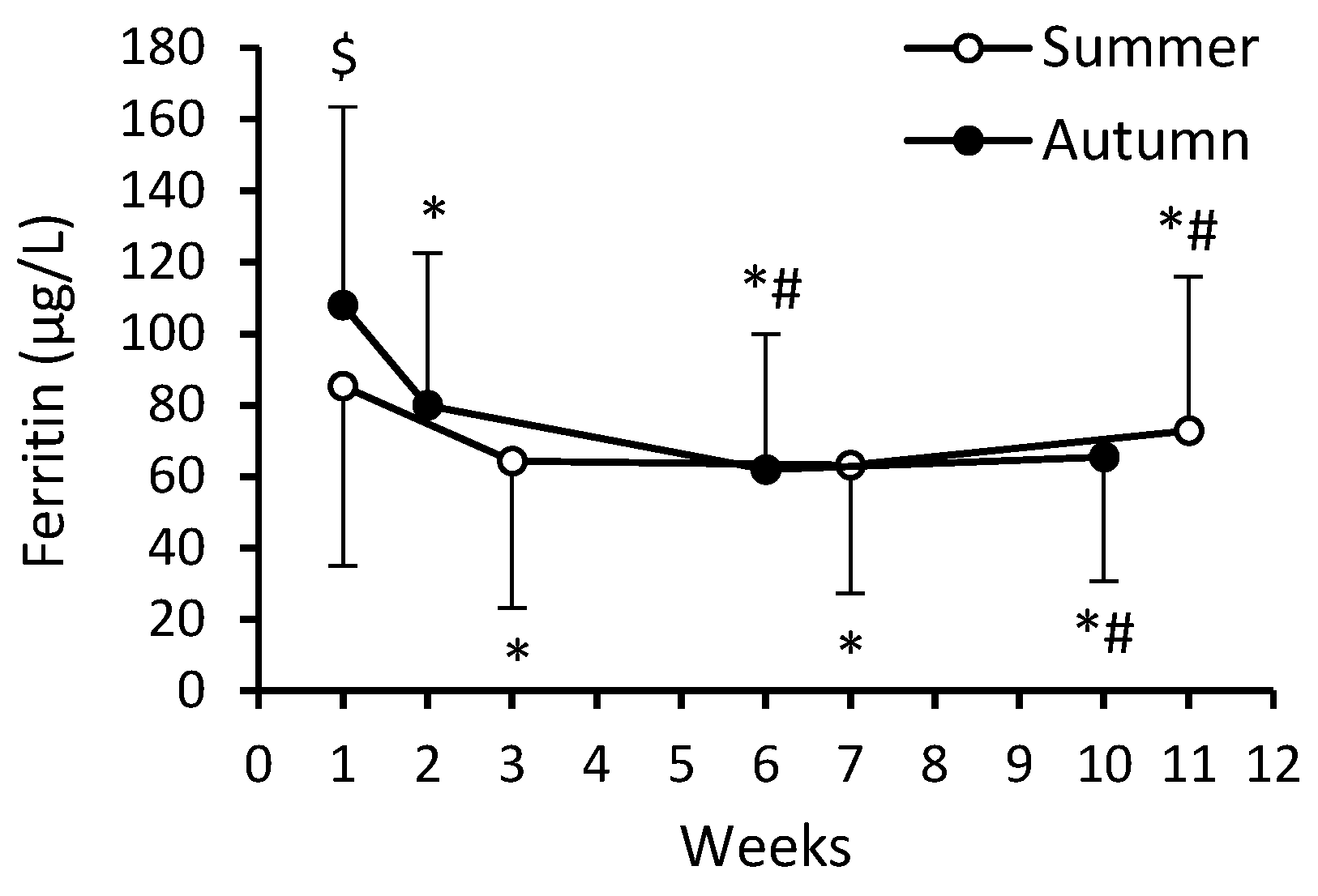
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Owens, D.J.; Fraser, W.D.; Close, G.L. Vitamin D and the athlete: Emerging insights. Eur. J. Sport Sci. 2015, 15, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and cardiovascular disease: An updated narrative review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef] [PubMed]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the athlete: Current perspectives and new challenges. Sports Med. 2018, 48 (Suppl. 1), S3–S16. [Google Scholar] [CrossRef]
- Willis, K.S.; Peterson, N.J.; Larsom-Meyer, D.E. Should we be concerned about the vitamin D status of athletes? Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.E.; Salmon, O.F.; Smith, C.M.; Duarte-Gardea, M.O.; Cramer, J.T. Influences of vitamin D and iron status on skeletal muscle health: A narrative review. Nutrients 2022, 14, 2717. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Medical progress: Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Blau, H.M.; Feldman, D. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology 1986, 119, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DeLuca, H.F. Is the vitamin D receptor found in muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M. Vitamin D and skeletal muscle: Emerging roles in development, anabolism and repair. Calcif. Tissue Int. 2020, 106, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B. Vitamin D and human skeletal muscle. Scand. J. Med. Sci. Sports 2010, 20, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- de la Puente Yagüe, M.; Collado Yurrita, L.; Ciudad Cabañas, M.J.; Cuadrado Cenzual, M.A. Role of vitamin D in athletes and their performance: Current concepts and new trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Cannell, J.J.; Hollis, B.W.; Sorenson, M.B.; Taft, T.N.; Anderson, J.J. Athletic performance and vitamin D. Med. Sci. Sports Exerc. 2009, 41, 1102–1110. [Google Scholar] [CrossRef]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, skeletal muscle function and athletic performance in athletes—A narrative review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Adamkiewicz, D.; Adamkiewicz, M.; Śniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of vitamin D on physical efficiency and exercise performance—A review. Nutrients 2019, 11, 2826. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Tan, Q.; Shao, J.; Yi, M. Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: A systematic review and meta-analysis of randomized controlled trials. J. Int. Soc. Sports Nutr. 2019, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Sist, M.; Zou, L.; Galloway, S.D.R.; Rodriguez-Sanchez, N. Effects of vitamin D supplementation on maximal strength and power in athletes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1163313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Quan, M.; Cao, Z.-B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of vitamin D supplementation by sunlight or oral D3 on exercise performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Heileson, J.L.; McGowen, J.M.; Moris, J.M.; Chapman-Lopez, T.J.; Torres, R.; Funderburk, L.K.; Jeffrey, S.; Forsse, J.S. Body composition, eicosapentaenoic acid, and vitamin D are associated with Army Combat Fitness Test Performance. J. Int. Soc. Sports Nutr. 2022, 19, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Laaksi, A.; Laaksi, I.; Pihlajamäki, H.; Vaara, J.P.; Luukkaala, T.; Kyröläinen, H. Associations of serum 25(OH)D levels with physical performance and anabolic hormones in young men. Front. Physiol. 2023, 14, 1049503. [Google Scholar] [CrossRef] [PubMed]
- Ööpik, V.; Timpmann, S.; Rips, L.; Olveti, I.; Kõiv, K.; Mooses, M.; Mölder, M.H.; Varblane, M.A.; Lille, H.-R.; Gapeyeva, H. Anabolic adaptations occur in conscripts during basic military training despite high prevalence of vitamin D deficiency and decrease in iron status. Mil. Med. 2017, 182, e1810. [Google Scholar] [CrossRef]
- Andersen, N.E.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Rood, J.C.; Young, A.J.; Lieberman, H.R.; McClung, J.P. Vitamin D status in female military personnel during combat training. J. Int. Soc. Sports Nutr. 2010, 7, 38. [Google Scholar] [CrossRef]
- Funderburk, L.K.; Daigle, K.; Arsenault, J.E. Vitamin D status among overweight and obese soldiers. Mil. Med. 2015, 180, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.J.; Morton, K.; Richards, T.; Whyte, G.P.; Pedlar, C.R. Is iron treatment beneficial in, iron-deficient but non-anemic (IDNA) endurance athletes? A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J. The army physical fitness test (APFT): A review of the literature. Mil. Med. 1989, 154, 326–329. [Google Scholar] [CrossRef]
- Kull, M.; Kallikorm, R.; Tamm, A.; Lember, M. Seasonal variance of 25(OH)D in the general population of Estonia, a Northern European country. BMC Public Health 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and exercise performance in professional soccer players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef] [PubMed]
- Barringer, N.D.; Kotwal, R.S.; Lewis, M.D.; Funderburk, L.K.; Elliott, T.R.; Crouse, S.F.; Smith, S.B.; Greenwood, M.; Kreider, R.B. Fatty acid blood levels, vitamin D status, physical performance, activity, and resiliency: A novel potential screening tool for depressed mood in active duty soldiers. Mil. Med. 2016, 181, 1114–1120. [Google Scholar] [CrossRef]
- Aspray, T.J.; Bowring, C.; Fraser, W.; Gittoes, N.; Javaid, M.K.; Macdonald, H.; Patel, S.; Selby, P.; Tanna, N.; Francis, R.M. National Osteoporosis Society vitamin D guideline summary. Age Ageing 2014, 43, 592–595. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Viru, A.; Viru, M. Biochemical Monitoring of Sport Training; Human Kinetics: Champaign, IL, USA, 2001. [Google Scholar]
- Chicharro, J.L.; López-Mojares, L.M.; Lucía, A.; Pérez, M.; Alvarez, J.; Labanda, P.; Calvo, F.; Vaquero, A.F. Overtraining parameters in special military units. Aviat. Space Environ. Med. 1998, 69, 562–568. [Google Scholar]
- Salonen, M.; Huovinen, J.; Kyröläinen, H.; Piirainen, J.M.; Vaara, J.P. Neuromuscular performance and hormonal profile during military training and subsequent recovery period. Mil. Med. 2019, 184, e113–e119. [Google Scholar] [CrossRef]
- Tait, J.L.; Drain, J.R.; Corrigan, S.L.; Drake, J.M.; Main, L.C. Impact of military training stress on hormone response and recovery. PLoS ONE 2022, 17, e0265121. [Google Scholar] [CrossRef] [PubMed]
- Tanskanen, M.M.; Kyröläinen, H.; Uusitalo, A.L.; Huovinen, J.; Nissilä, J.; Kinnunen, H.; Atalay, M.; Häkkinen, K. Serum sex hormone-binding globulin and cortisol concentrations are associated with overreaching during strenuous military training. J. Strength Cond. Res. 2011, 25, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Damas-Fuentes, M.; Boughanem, H.; Molina-Vega, M.; Tinahones, F.J.; Fernández-García, J.C.; Macías-González, M. 25-hydroxyvitamin and testosterone levels association through body mass index: A cross-sectional study of young men with obesity. Front. Endocrinol. 2022, 13, 960222. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Platz, E.A.; Willett, W.C.; Giovannucci, E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin. Endocrinol. 2012, 77, 106–112. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, S.; Martorella, A.; Coccia, F.; Castellini, C.; Minaldi, E.; Totaro, M.; Parisi, A.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship of vitamin D status with testosterone levels: A systematic review and meta-analysis. Endocrine 2021, 72, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Wehr, E.; Pilz, S.; Boehm, B.O.; März, W.; Obermayer-Pietsch, B. Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. 2010, 73, 243–248. [Google Scholar] [CrossRef]
- Sircar, S. Principles of Medical Physiology; Georg Thieme Verlag: Stuttgard, Germany, 2008; pp. 146–153. [Google Scholar]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, J. Iron deficiency: A concise review. Am. J. Hematol. 2005, 78, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594S–597S. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D. The effects of iron deficiency on physical performance. In Mineral Requirements for Military Personnel: Levels Needed for Cognitive and Physical Performance during Garrison Training; The National Academies Press: Washington, DC, USA, 2006; pp. 451–461. [Google Scholar] [CrossRef]
- Moran, D.S.; Heled, Y.; Arbel, Y.; Israeli, E.; Finestone, A.S.; Evans, R.K.; Yanovich, R. Dietary intake and stress fractures among elite male combat recruits. J. Int. Soc. Sports Nutr. 2012, 9, 6. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Jackson, S.; Izard, R.M.; Walsh, N.P.; Coombs, C.V.; Carswell, A.T.; Oliver, S.J.; Tang, J.C.Y.; Fraser, W.D.; Greeves, J.P. Sex differences in iron status during military training: A prospective cohort study of longitudinal changes and associations with endurance performance and musculoskeletal outcomes. Br. J. Nutr. 2024, 131, 581592. [Google Scholar] [CrossRef] [PubMed]
- Yanovich, R.; Karl, J.P.; Yanovich, E.; Lutz, L.J.; Williams, K.W.; Cable, S.J.; Young, A.J.; Pasiakos, S.M.; McClung, J.P. Effects of basic combat training on iron status in male and female soldiers: A comparative study. US Army Med. Dep. J. 2015, 57–63. [Google Scholar]
- Martin, N.M.; Conlon, C.A.; Smeele, R.J.M.; Mugridge, O.A.R.; von Hurst, P.R.; McClung, J.P.; Beck, K.L. Iron status and associations with physical performance during basic combat training in female New Zealand Army recruits. Br. J. Nutr. 2019, 121, 887–893. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.P.; Murray-Kolb, L.E. Iron nutrition and premenopausal women: Effects of poor iron status on physical and neuropsychological performance. Annu. Rev. Nutr. 2013, 33, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Brownlie, T. 4th. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [PubMed]
- Eichner, E.R. Minerals: Iron. In Nutrition in Sport; Maughan, R.J., Ed.; Blackwell Science: Oxford, UK, 2001; pp. 326–338. [Google Scholar]
- Masuda, K.; Okazaki, K.; Kuno, S.; Asano, K.; Shimojo, H.; Katsuta, S. Endurance training under 2500-m hypoxia does not increase myoglobin content in human skeletal muscle. Eur. J. Appl. Physiol. 2001, 85, 486–490. [Google Scholar] [CrossRef]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
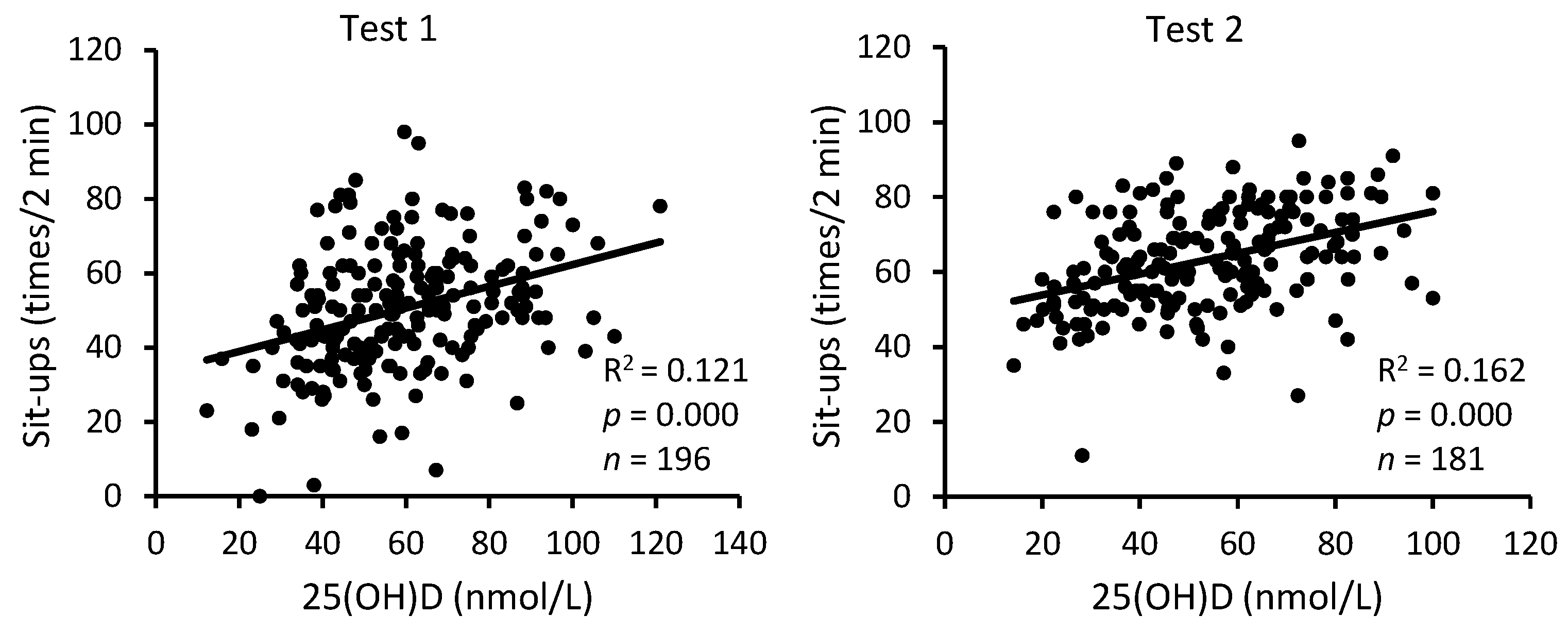
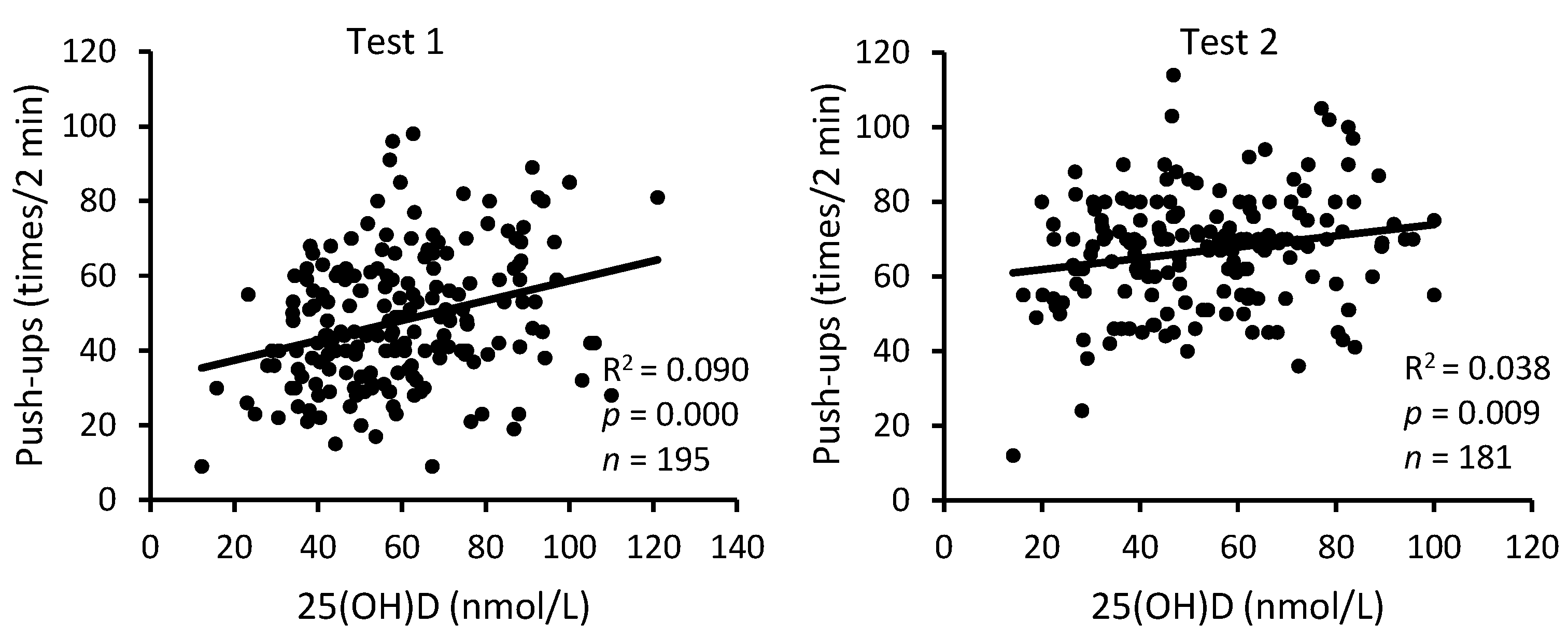
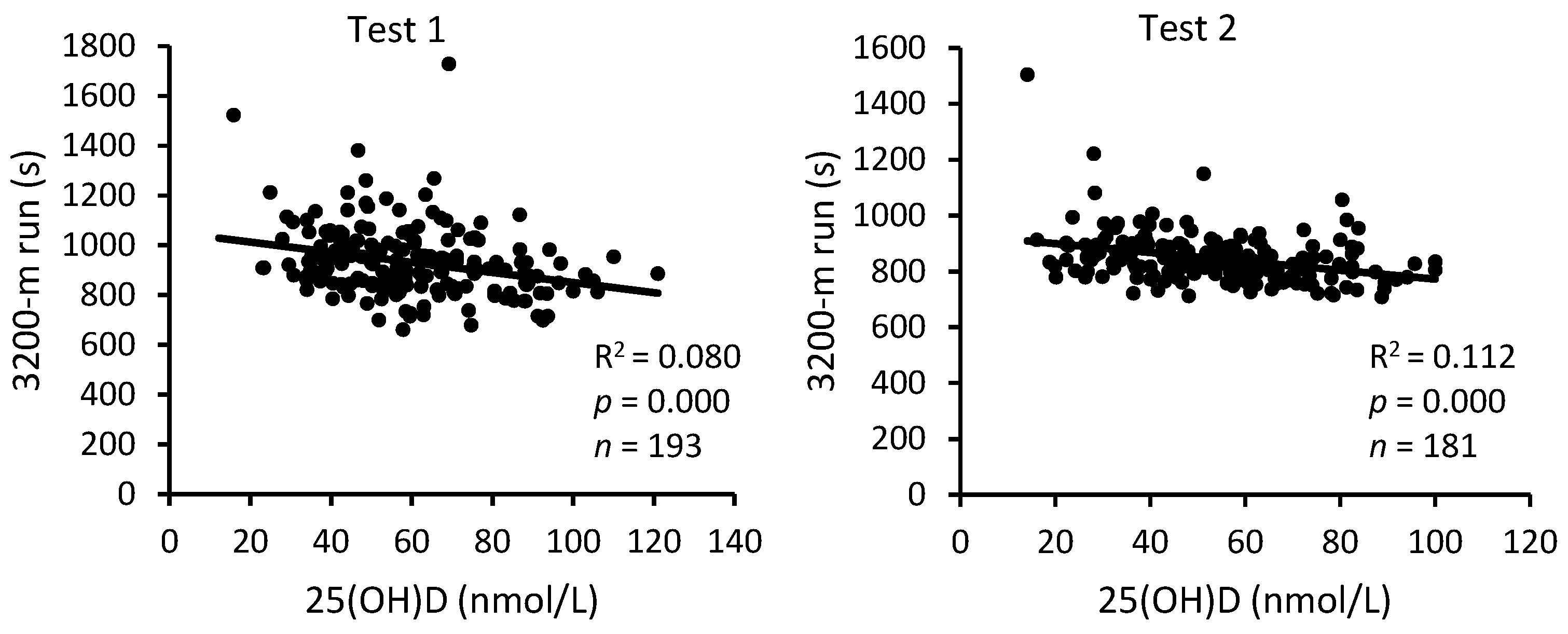

| 25(OH)D | Sit-Ups | Push-Ups | 3200 m Run | |||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | |
| ≥75 nmol/L | r = 0.250 | r = 0.065 | r = 0.156 | r = –0.113 | r = –0.236 | r = –0.102 |
| R2 = 0.064 | R2 = 0.004 | R2 = 0.024 | R2 = 0.013 | R2 = 0.056 | R2 = 0.010 | |
| p = 0.108 | p = 0.750 | p = 0.323 | p = 0.582 | p = 0.133 | p = 0.621 | |
| n = 42 | n = 26 | n = 42 | n = 26 | n = 42 | n = 26 | |
| <75 nmol/L | r = 0.320 | r = 0.384 | r = 0.280 | r = 0.191 | r = –0.188 | r = –0.361 |
| R2 = 0.102 | R2 = 0.147 | R2 = 0.078 | R2 = 0.036 | R2 = 0.035 | R2 = 0.130 | |
| p = 0.000 | p = 0.000 | p = 0.000 | p = 0.017 | p = 0.021 | p = 0.000 | |
| n = 154 | n = 155 | n = 153 | n = 155 | n = 151 | n = 155 | |
| ≥50 nmol/L | r = 0.243 | r = 0.268 | r = 0.185 | r = 0.121 | r = –0.128 | r = –0.174 |
| R2 = 0.059 | R2 = 0.072 | R2 = 0.034 | R2 = 0.015 | R2 = 0.016 | R2 = 0.030 | |
| p = 0.006 | p = 0.009 | p = 0.038 | p = 0.244 | p = 0.145 | p = 0.092 | |
| n = 128 | n = 95 | n = 127 | n = 95 | n = 128 | n = 95 | |
| <50 nmol/L | r = 0.428 | r = 0.413 | r = 0.320 | r = 0.272 | r = –0.131 | r = –0.331 |
| R2 = 0.183 | R2 = 0.171 | R2 = 0.102 | R2 = 0.074 | R2 = 0.017 | R2 = 0.110 | |
| p = 0.000 | p = 0.000 | p = 0.008 | p = 0.011 | p = 0.298 | p = 0.002 | |
| n = 68 | n = 86 | n = 68 | n = 86 | n = 65 | n = 86 | |
| Variable | Cohort | Weeks | |||
|---|---|---|---|---|---|
| Week 1 | Week 2 or 3 | Week 6 or 7 | Week 10 or 11 | ||
| Hemoglobin (g/L) | Summer | 149.2 ± 9.7 | 150.4 ± 9.2 | 150.5 ± 10.7 | 149.9 ± 11.3 |
| Autumn | 147.3 ± 9.9 | 146.3 ± 9.8 | 149.2 ± 9.1 # | 152.7 ± 8.2 *# | |
| Hematocrit (%) | Summer | 43.6 ± 2.5 | 44.5 ± 2.3 * | 44.2 ± 2.8 | 44.6 ± 2.9 * |
| Autumn | 44.6 ± 2.5 | 43.6 ± 2.4 * | 44.6 ± 2.2 # | 45.6 ± 2.1 *# | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timpmann, S.; Rips, L.; Olveti, I.; Mooses, M.; Mölder, H.; Varblane, A.; Lille, H.-R.; Gapeyeva, H.; Ööpik, V. Seasonal Variation in Vitamin D Status Does Not Interfere with Improvements in Aerobic and Muscular Endurance in Conscripts during Basic Military Training. Nutrients 2024, 16, 1306. https://doi.org/10.3390/nu16091306
Timpmann S, Rips L, Olveti I, Mooses M, Mölder H, Varblane A, Lille H-R, Gapeyeva H, Ööpik V. Seasonal Variation in Vitamin D Status Does Not Interfere with Improvements in Aerobic and Muscular Endurance in Conscripts during Basic Military Training. Nutrients. 2024; 16(9):1306. https://doi.org/10.3390/nu16091306
Chicago/Turabian StyleTimpmann, Saima, Leho Rips, Indrek Olveti, Martin Mooses, Hanno Mölder, Ahti Varblane, Hele-Reet Lille, Helena Gapeyeva, and Vahur Ööpik. 2024. "Seasonal Variation in Vitamin D Status Does Not Interfere with Improvements in Aerobic and Muscular Endurance in Conscripts during Basic Military Training" Nutrients 16, no. 9: 1306. https://doi.org/10.3390/nu16091306
APA StyleTimpmann, S., Rips, L., Olveti, I., Mooses, M., Mölder, H., Varblane, A., Lille, H.-R., Gapeyeva, H., & Ööpik, V. (2024). Seasonal Variation in Vitamin D Status Does Not Interfere with Improvements in Aerobic and Muscular Endurance in Conscripts during Basic Military Training. Nutrients, 16(9), 1306. https://doi.org/10.3390/nu16091306





