Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents for the Experiments
2.2. Cell Lines
2.2.1. C2C12
2.2.2. C26
2.3. MTT Assay
2.4. Treatments
- Control (Co): DM;
- CM-C26: diluted CM-C26 (1:3 in DM);
- TNF-α: DM containing 100 ng/mL of TNF-α;
- OC: DM containing 10 µM of OC;
- CM-C26 + OC: diluted CM-C26 + 10 µM of OC.
- TNF + OC: DM + TNF-α and OC with final concentrations of 100 ng/mL and 10 µM, respectively.
2.5. Immunofluorescence
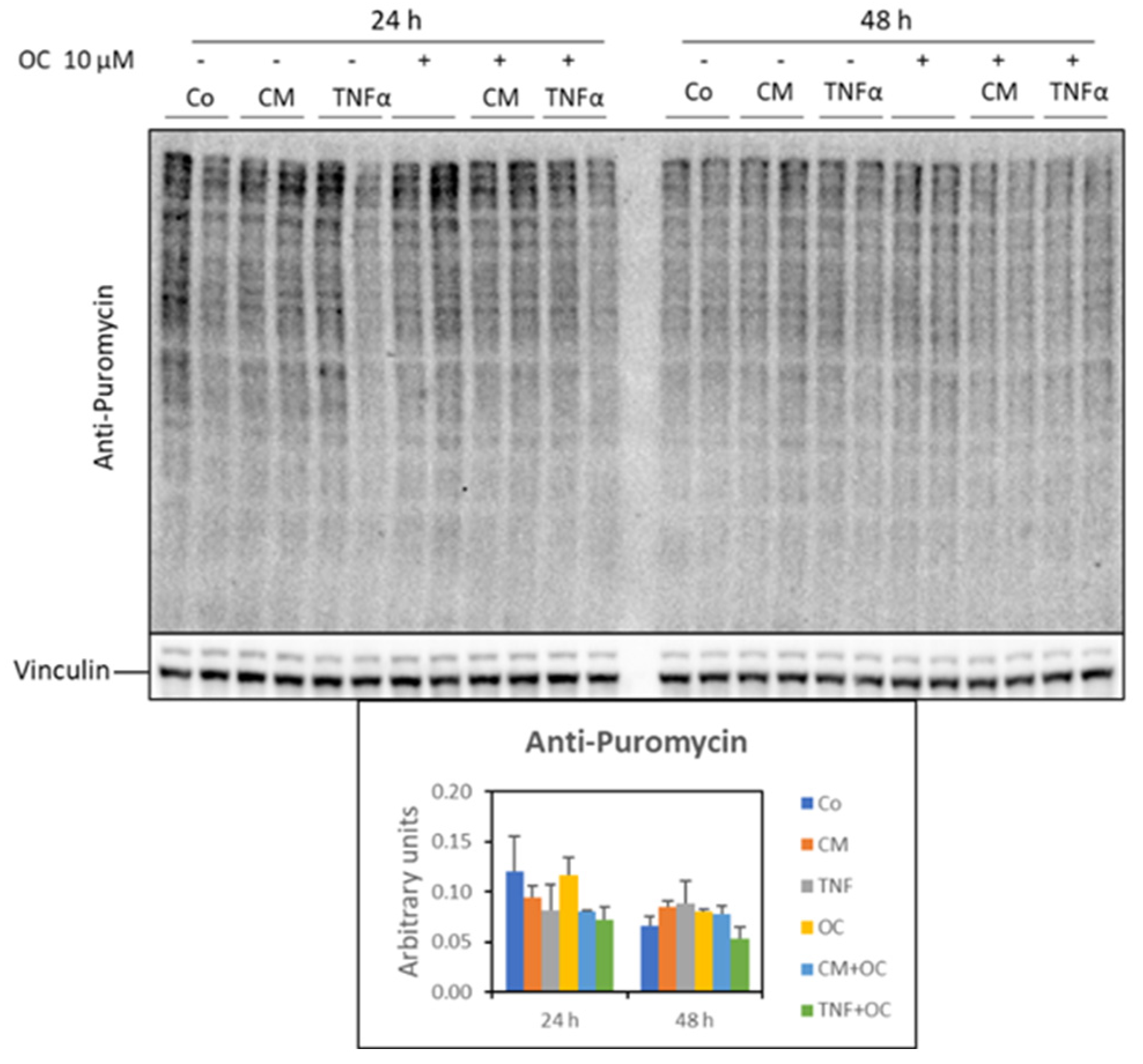
2.6. Protein Synthesis
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
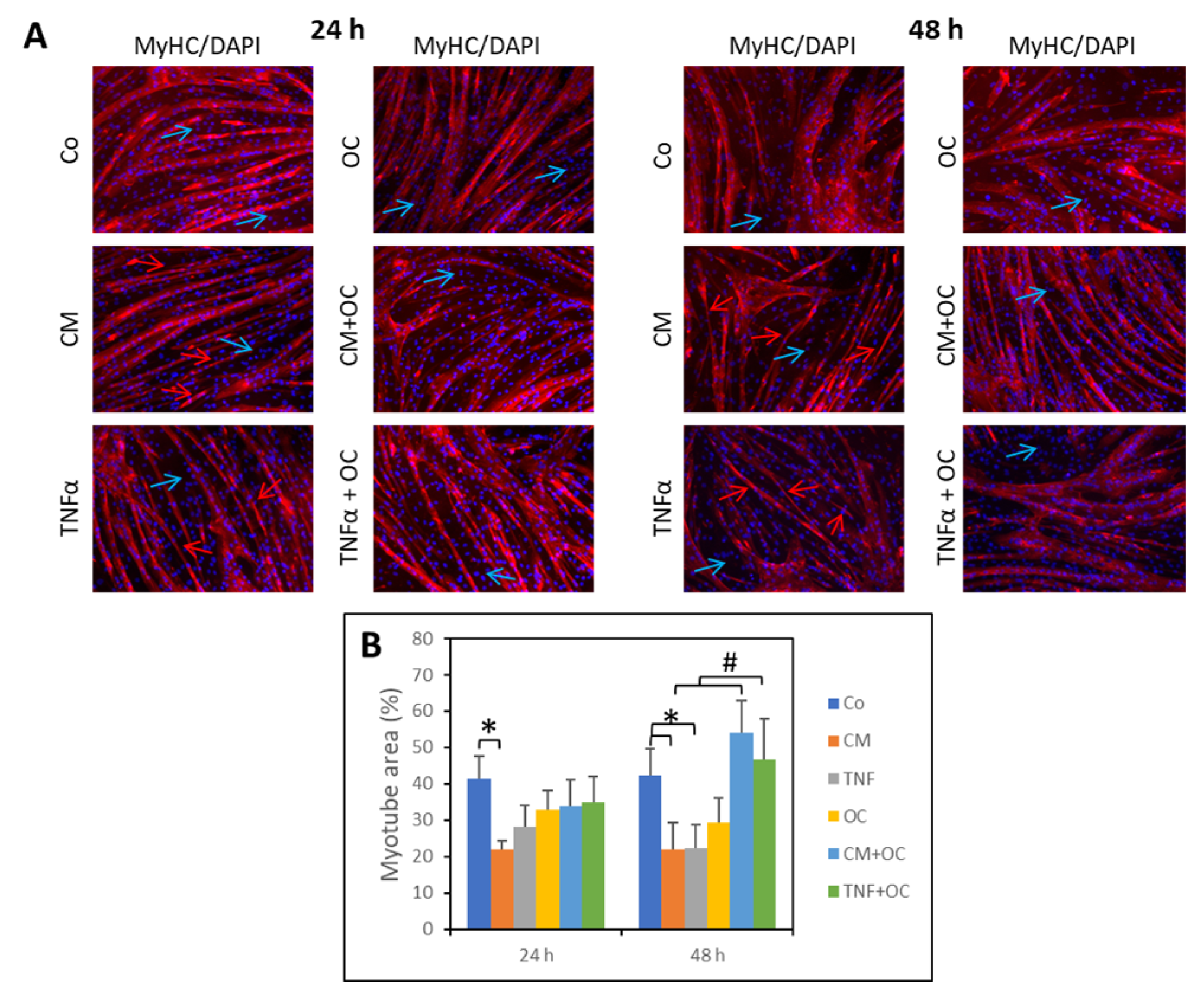
3.1. OC Partially Restores Myotube Morphology
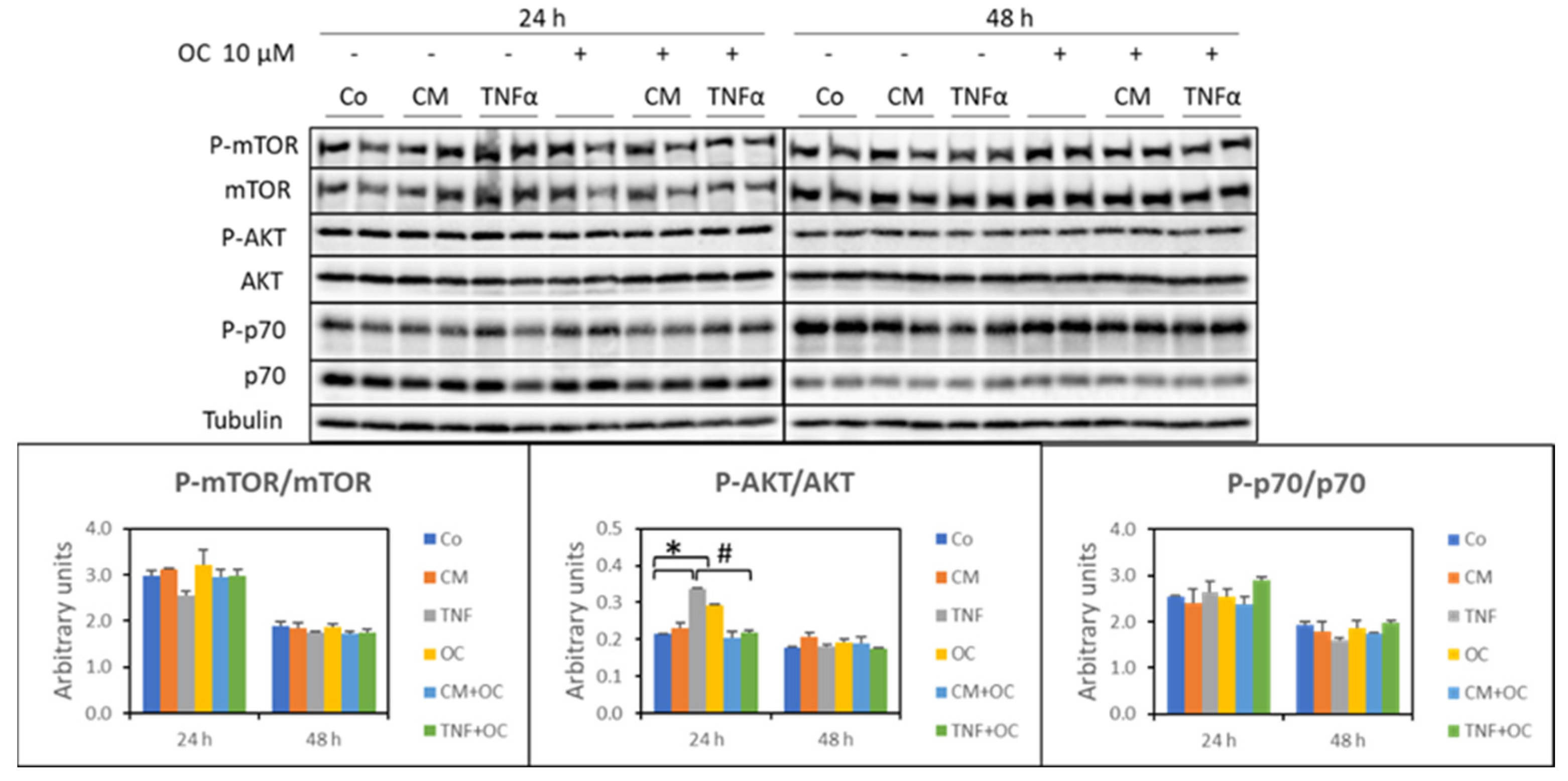
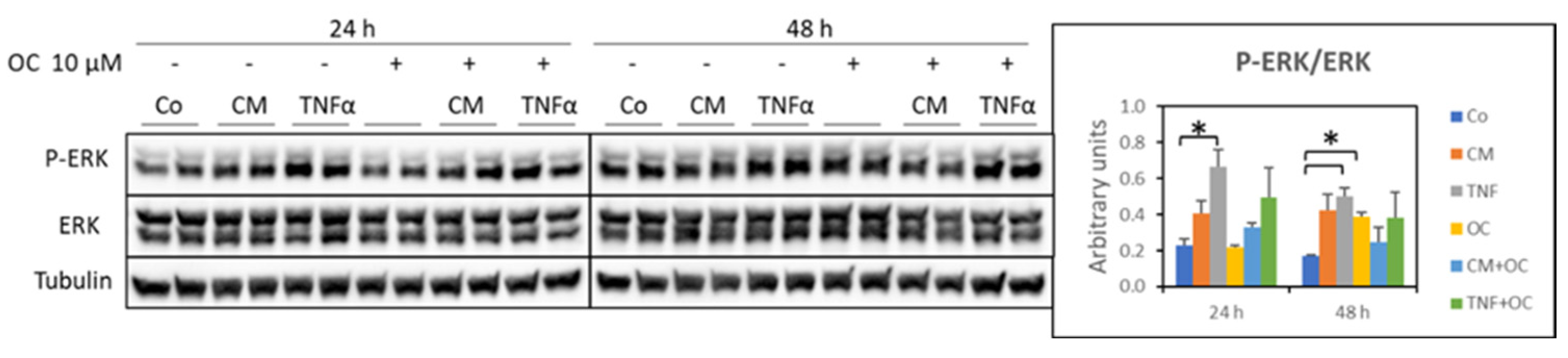
3.2. Molecular Markers of Protein Catabolism Are DownRegulated by OC
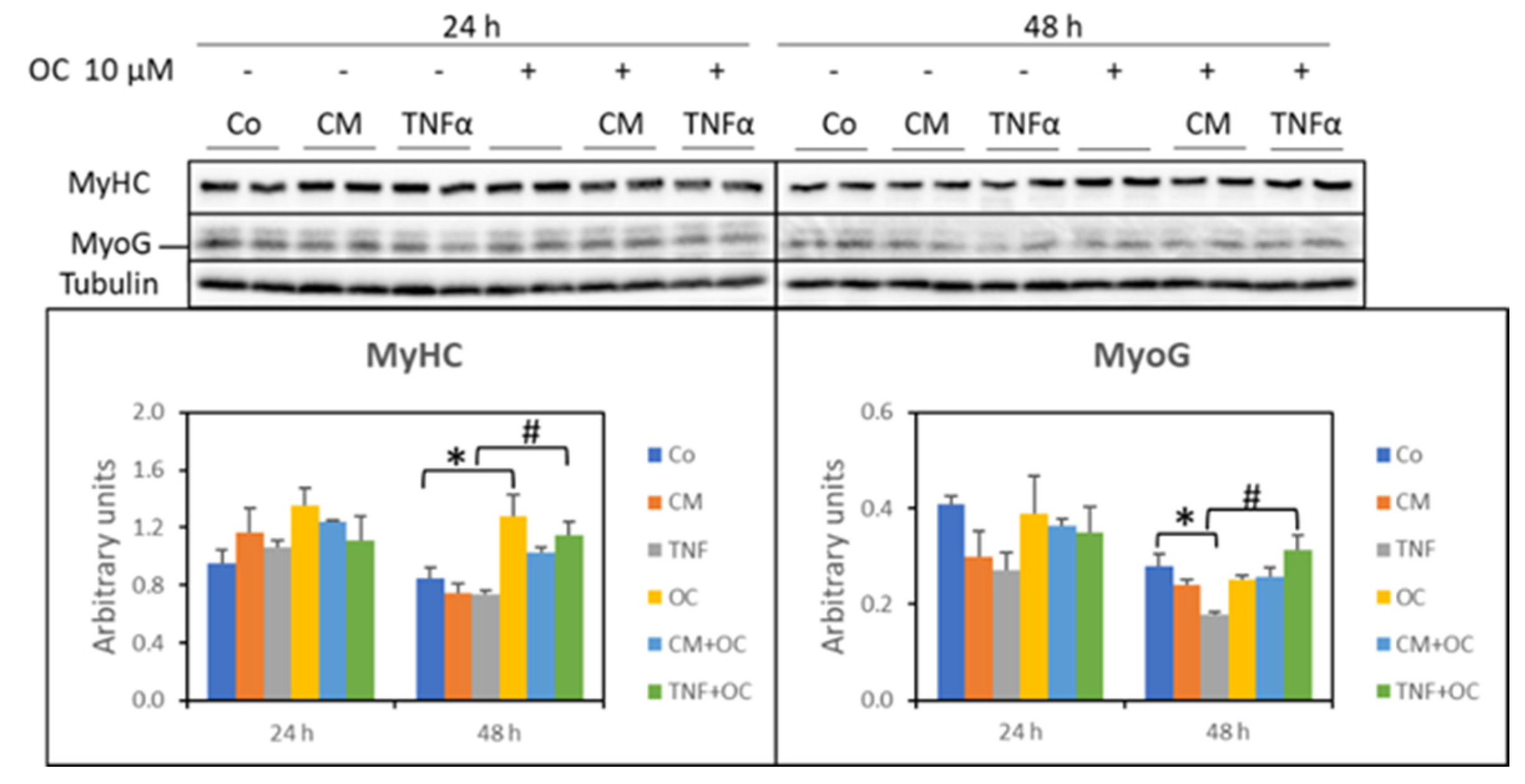
3.3. Effects of OC on Myogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, K.L.; Chin, K.Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Filardo, S.; Mattioli, R.; Di Risola, D.; Mosca, L.; Di Pietro, M.; Sessa, R. Olea europaea L-derived secoiridoids: Beneficial health effects and potential therapeutic approaches. Pharmacol. Ther. 2024, 254, 108595. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Quintero, J.E.; Rosado, J.A. Anticancer molecular mechanisms of oleocanthal. Phytother. Res. 2020, 34, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, A.; Koumarianou, P.; Diamantakos, P.; Melliou, E.; Magiatis, P.; Boleti, H. A systematic ex-vivo study of the anti-proliferative/cytotoxic bioactivity of major olive secoiridoids’ double combinations and of total olive oil phenolic extracts on multiple cell-culture based cancer models highlights synergistic effects. Nutrients 2023, 15, 2538. [Google Scholar] [CrossRef]
- Rivero-Pino, F. Oleocanthal—Characterization, production, safety, functionality and in vivo evidences. Food Chem. 2023, 42, 5136504. [Google Scholar] [CrossRef] [PubMed]
- De Stefanis, D.; Scimè, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-proliferative effects of an extra-virgin olive oil extract enriched in ligstroside aglycone and oleocanthal on human liver cancer cell lines. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef]
- Tajmim, A.; Cuevas-Ocampo, A.K.; Siddique, A.B.; Qusa, M.H.; King, J.A.; Abdelwahed, K.S.; Sonju, J.J.; El Sayed, K.A. (-)-Oleocanthal nutraceuticals for Alzheimer’s disease amyloid pathology: Novel oral formulations, therapeutic, and molecular insights in 5xFAD transgenic mice model. Nutrients 2021, 13, 1702. [Google Scholar] [CrossRef]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Rosillo, M.Á.; González-Benjumea, A.; Alarcón-de-la-Lastra, C. Dietary oleocanthal supplementation prevents inflammation and oxidative stress in collagen-induced arthritis in mice. Antioxidants 2021, 10, 650. [Google Scholar] [CrossRef]
- Ruiz-García, I.; Ortíz-Flores, R.; Badía, R.; García-Borrego, A.; García-Fernández, M.; Lara, E.; Martín-Montañez, E.; García-Serrano, S.; Valdés, S.; Gonzalo, M.; et al. Rich oleocanthal and oleacein extra virgin olive oil and inflammatory and antioxidant status in people with obesity and prediabetes. The APRIL study: A randomised, controlled crossover study. Clin. Nutr. 2023, 42, 1389–1398. [Google Scholar] [CrossRef]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a mediterranean diet component, in the management of muscle mass and function preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Man-Yau Szeto, I.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, J.; Feng, Z.; Shi, W.; Qu, L.; Li, Y.; Liu, J.; Long, J. Reloading functionally ameliorates disuse-induced muscle atrophy by reversing mitochondrial dysfunction, and similar benefits are gained by administering a combination of mitochondrial nutrients. Free Radic. Biol. Med. 2014, 69, 116–128. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zheng, A.; Yang, L.; Liu, J.; Chen, C.; Tang, Y.; Zou, X.; Li, Y.; Long, J.; et al. Mitochondrial dysfunction-associated OPA1 cleavage contributes to muscle degeneration: Preventative effect of hydroxytyrosol acetate. Cell Death Dis. 2014, 5, e1521. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef]
- Kikusato, M.; Muroi, H.; Uwabe, Y.; Furukawa, K.; Toyomizu, M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome proliferator-activated receptor γ coactivator-1α. Anim. Sci. J. 2016, 87, 1371–1378. [Google Scholar] [CrossRef]
- Zhang, J.; Nugrahaningrum, D.A.; Marcelina, O.; Ariyanti, A.D.; Wang, G.; Liu, C.; Wu, S.; Kasim, V. Tyrosol facilitates neovascularization by enhancing skeletal muscle cells viability and paracrine function in diabetic hindlimb ischemia mice. Front. Pharmacol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 carcinoma tumor-bearing mouse as a model for the study of cancer cachexia. J. Vis. Exp. 2016, 117, 54893. [Google Scholar] [CrossRef]
- Jackman, R.W.; Floro, J.; Yoshimine, R.; Zitin, B.; Eiampikul, M.; El-Jack, K.; Seto, D.N.; Kandarian, S.C. Continuous Release of Tumor-Derived Factors Improves the Modeling of Cachexia in Muscle Cell Culture. Front. Physiol. 2017, 8, 738. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef]
- Li, Y.P.; Chen, Y.; John, J.; Moylan, J.; Jin, B.; Mann, D.L.; Reid, M.B. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005, 3, 362–370. [Google Scholar] [CrossRef]
- Bonetto, A.; Penna, F.; Minero, V.G.; Reffo, P.; Costamagna, D.; Bonelli, G.; Baccino, F.M.; Costelli, P. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-α. Amino Acids 2011, 2, 585–594. [Google Scholar] [CrossRef]
- Penna, F.; Costamagna, D.; Fanzani, A.; Bonelli, G.; Baccino, F.M.; Costelli, P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS ONE 2010, 5, e13604. [Google Scholar] [CrossRef]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef]
- Chen, S.L.; Wu, C.C.; Li, N.; Weng, T.H. Post-transcriptional regulation of myogenic transcription factors during muscle development and pathogenesis. J. Muscle Res. Cell Motil. 2024, 1, 21–39. [Google Scholar] [CrossRef]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol. Cell Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef]
- Oelkrug, C.; Horn, K.; Makert, R.; Schubert, A. Novel in vitro platform to investigate myotube atrophy. Anticancer Res. 2015, 35, 2085–2091. [Google Scholar]
- Chang, Y.C.; Liu, H.W.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. The green tea polyphenol epigallocatechin-3-gallate attenuates age-associated muscle loss via regulation of miR-486-5p and myostatin. Arch. Biochem. Biophys. 2020, 692, 108511. [Google Scholar] [CrossRef]
- Asami, Y.; Aizawa, M.; Kinoshita, M.; Ishikawa, J.; Sakuma, K. Resveratrol attenuates denervation-induced muscle atrophy due to the blockade of atrogin-1 and p62 accumulation. Int. J. Med. Sci. 2018, 15, 628–637. [Google Scholar] [CrossRef]
- Liu, H.W.; Chen, Y.J.; Chang, Y.C.; Chang, S.J. Oligonol, a low-molecular weight polyphenol derived from Lychee, alleviates muscle loss in diabetes by suppressing Atrogin-1 and MuRF1. Nutrients 2017, 9, 1040. [Google Scholar] [CrossRef]
- Cardaci, T.D.; Machek, S.B.; Wilburn, D.T.; Hwang, P.S.; Willoughby, D.S. Ubiquitin Proteasome System activity is suppressed by curcumin following exercise-induced muscle damage in human skeletal muscle. J. Am. Coll. Nutr. 2021, 40, 401–411. [Google Scholar] [CrossRef]
- Murata, M.; Shimizu, Y.; Marugame, Y.; Nezu, A.; Fujino, K.; Yamada, S.; Kumazoe, M.; Fujimura, Y.; Tachibana, H.I. EGCG down-regulates MuRF1 expression through 67-kDa laminin receptor and the receptor signaling is amplified by eriodictyol. J. Nat. Med. 2020, 74, 673–679. [Google Scholar] [CrossRef]
- González-Hedström, D.; Moreno-Rupérez, Á.; de la Fuente-Fernández, M.; de la Fuente-Muñoz, M.; Román-Carmena, M.; Amor, S.; García-Villalón, Á.L.; López-Calderón, A.; Martín, A.I.; Priego, T.; et al. A nutraceutical product based on a mixture of algae and Extra Virgin Olive Oils and Olive Leaf Extract attenuates sepsis-induced cardiovascular and muscle alterations in rats. Front. Nutr. 2022, 20, 918841–918855. [Google Scholar] [CrossRef]
- Perrone, L.; Squillaro, T.; Napolitano, F.; Terracciano, C.; Sampaolo, S.; Melone, M.A.B. The autophagy signaling pathway: A potential multifunctional therapeutic target of curcumin in neurological and neuromuscular diseases. Nutrients 2019, 11, 1881. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, D.; Shang, Y.; Li, Y.; Chen, S.Z. Magnolol inhibits myotube atrophy induced by cancer cachexia through myostatin signaling pathway in vitro. J. Nat. Med. 2020, 74, 741–749. [Google Scholar] [CrossRef]
- Wang, D.T.; Yin, Y.; Yang, Y.J.; Lv, P.J.; Shi, Y.; Lu, L.; Wei, L.B. Resveratrol prevents TNF-α-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Costamagna, D.; Duelen, R.; Penna, F.; Neumann, D.; Costelli, P.; Sampaolesi, M. Interleukin-4 administration improves muscle function, adult myogenesis, and lifespan of colon carcinoma-bearing mice. J. Cachexia Sarcopenia Muscle 2020, 11, 783–801. [Google Scholar] [CrossRef]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.; Oh, H.T.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. (-)-Epigallocatechin-3-gallate stimulates myogenic differentiation through TAZ activation. Biochem. Biophys. Res. Commun. 2017, 486, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Wang, Y.; Lin, Z.P.; Li, H.X. The protective effect of rosmarinic acid on myotube formation during myoblast differentiation under heat stress. Cell Dev. Biol. Anim. 2020, 56, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Beltrà, M.; Garcia-Castillo, L.; Pardini, B.; Birolo, G.; Matullo, G.; Penna, F.; Guttridge, D.; Costelli, P. Extracellular vesicles derived from tumour cells as a trigger of energy crisis in the skeletal muscle. J. Cachexia Sarcopenia Muscle 2022, 1, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Cannella, L.; Colognesi, M.; Scaffidi, M.; Polini, B.; Digiacomo, M.; Salsano, J.E.; Manera, C.; et al. The Extra Virgin Olive Oil Polyphenol Oleocanthal Exerts Antifibrotic Effects in the Liver. Front. Nutr. 2021, 8, 715183. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Hoan, L.; Chu-Sook, K.; Taesun, P.; Jung Han, P.Y.; Mi-Kyung, S.; Dong Gun, L.; Sun-Myung, H.; Suck-Young, C.; Tsuyoshi, G.; Teruo, K.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef]
- Hemdan, D.I.I.; Hirasaka, K.; Nakao, R.; Kohno, S.; Kagawa, S.; Abe, T.; Harada-Sukeno, A.; Okumura, Y.; Nakaya, Y.; Terao, J.; et al. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J. Med. Investig. 2009, 56, 26–32. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Stefanis, D.; Balestrini, A.; Costelli, P. Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo. Nutrients 2024, 16, 1302. https://doi.org/10.3390/nu16091302
De Stefanis D, Balestrini A, Costelli P. Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo. Nutrients. 2024; 16(9):1302. https://doi.org/10.3390/nu16091302
Chicago/Turabian StyleDe Stefanis, Daniela, Andrea Balestrini, and Paola Costelli. 2024. "Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo" Nutrients 16, no. 9: 1302. https://doi.org/10.3390/nu16091302
APA StyleDe Stefanis, D., Balestrini, A., & Costelli, P. (2024). Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo. Nutrients, 16(9), 1302. https://doi.org/10.3390/nu16091302





