Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity—A Pilot Study
Abstract
1. Introduction
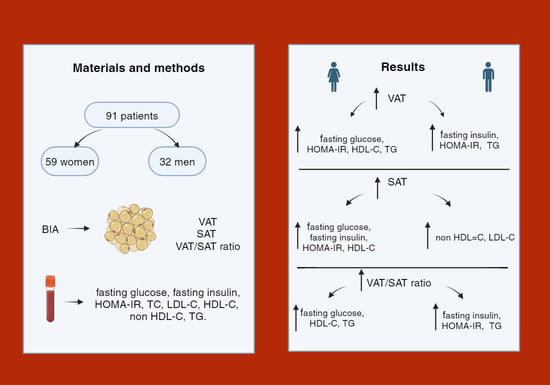
2. Materials and Methods
2.1. Ethics
2.2. Study Principants
2.3. Antropometric Parameters
2.4. Body Analysis
- Visceral fat tissue area (cm2) at the height of the measurement (at the navel);
- Subcutaneous fat tissue area (cm2) at the height of the measurement (at the navel);
- VAT/SAT ratio—the ratio of visceral fat tissue to subcutaneous fat tissue.
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group of Men and Women in Terms of Anthropometric Parameters
3.2. Estimation of Cut-Off Points for the Area of Visceral and/or Subcutaneous Adipose Tissue at Which Selected Parameters of Carbohydrate and/or Lipid Metabolism Occurred in Obese Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [CrossRef]
- Bąk-Sosnowska, M.; Białkowska, M.; Bogdański, P.; Chomiuk, T.; Gałązka-Sobotka, M.; Holecki, M.; Jarosińska, A.; Jezierska, M.; Kamiński, P.; Kłoda, K.; et al. Zalecenia kliniczne dotyczące postępowania u chorych na otyłość 2022—Stanowisko Polskiego Towarzystwa Leczenia Otyłości. Med. Prakt. Wyd. Specj. 2022, 5, 1–87. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011.
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-G.; Lee, H.-J.; Jin, B.-Y.; Gutierrez-Aguilar, R.; Shin, K.-H.; Choi, S.-H.; Um, S.H.; Kim, D.-H. Depot-specific differences in angiogenic capacity of adipose tissue in differential susceptibility to diet-induced obesity. Mol. Metab. 2016, 5, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Sci. Rep. 2017, 7, 10955. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2018, 38, e99291. [Google Scholar] [CrossRef] [PubMed]
- Marinou, K.; Hodson, L.; Vasan, S.K.; Fielding, B.A.; Banerjee, R.; Brismar, K.; Koutsilieris, M.; Clark, A.; Neville, M.J.; Karpe, F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014, 37, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; McCarthy, J.; Fitzpatrick, J. The TOFI Phenotype. Obes. Facts 2009, 2, 1–256. [Google Scholar]
- Brończyk-Puzoń, A.; Koszowska, A.; Bieniek, J. Podstawowe pomiary antropometryczne i pochodne wskaźniki w poradnictwie dietetycznym–część pierwsza Basic anthropometric measurements and derived ratios in dietary counseling: Part one. Pielęgniarstwo 2018, 8, 217–222. [Google Scholar] [CrossRef][Green Version]
- Walczyk, T. Anthropometric indicators of obesity. Are the new indicators a better predictor of body fat content than BMI? J. Educ. Health Sport 2021, 11, 11–23. [Google Scholar] [CrossRef]
- Podręcznik Użytkowania; BioScan 920; Maltron International Ltd.: Essex, UK, 2014.
- Vladu, I.M.; Fortofoiu, M.; Clenciu, D.; Fortofoiu, M.C.; Padureanu, R.; Radu, L.; Cojan, S.T.T.; Radulescu, P.M.; Padureanu, V. Insulin resistance quantified by the value of HOMA-IR and cardiovascular risk in patients with type 2 diabetes. Exp. Ther. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, H.E.; Miller, W.G.; Myers, G.L.; Sakurabayashi, I.; Bachmann, L.M.; Caudill, S.P.; Dziekonski, A.; Edwards, S.; Kimberly, M.M.; Korzun, W.J.; et al. Non-HDL cholesterol shows improved accuracy for cardiovascular risk score classification compared to direct or calculated LDL cholesterol in a dyslipidemic population. Clin. Chem. 2011, 57, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. QJM 2018, 111, 437–443. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Pesce, C.; De Vito, G.; Boreham, C.A. The Body Fat-Cognition Relationship in Healthy Older Individuals: Does Gynoid vs Android Distribution Matter? J. Nutr. Health Aging 2017, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Johnson Stoklossa, C.A.; Forhan, M.; Padwal, R.S.; Gonzalez, M.C.; Prado, C.M. Practical Considerations for Body Composition Assessment of Adults with Class II/III Obesity Using Bioelectrical Impedance Analysis or Dual-Energy X-Ray Absorptiometry. Curr. Obes. Rep. 2016, 5, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nat. Cell Biol. 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Bjorntorp, P. Metabolic implications of body fat distribution. Diabetes Care 1991, 14, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Mehta, J.L. Metabolic Syndrome: Does It Differ between Women and Men? Cardiovasc. Drugs Ther. 2015, 29, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; McDermott, R.A. Using anthropometric indices to predict cardio-metabolic risk factors in Australian indigenous populations. Diabetes Res. Clin. Pract. 2010, 87, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Roriz, A.K.C.; de Oliveira, C.C.; Moreira, P.A.; Eickemberg, M.; Medeiros, J.M.B.; Sampaio, L.R. Methods of Predicting Visceral Fat in Brazilian Adults and Older Adults: A Comparison between Anthropometry and Computerized Tomography. Arch. Latinoam. Nutr. 2011, 61, 5–12. [Google Scholar] [PubMed]
- da Rosa, S.E.; Costa, A.C.; Fortes, M.S.R.; Marson, R.A.; Neves, E.B.; Rodrigues, L.C.; Ferreira, P.F.; Filho, J.F. Cut-Off Points of Visceral Adipose Tissue Associated with Metabolic Syndrome in Military Men. Healthcare 2021, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ono, A.; Monter-Carreola, G.; Zamora-Gonzalez, J.; Cardoso-Saldana, G.; Posadas-Sanchez, R.; Torres-Tamayo, M.; Posadas-Romero, C. Association of visceral fat with coronary risk factors in a population-based sample of postmenopausal women. Int. J. Obes. 2002, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]



| n | Age Me (Q1–Q3) | Body Weight Me (Q1–Q3) | BMI Me (Q1–Q3) | WC Me (Q1–Q3) | BF (kg) Me (Q1–Q3) | PBF (%) Me (Q1–Q3) | VAT (cm2) Me (Q1–Q3) | SAT (cm2) Me (Q1–Q3) | VAT/SAT Me (Q1–Q3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Women | 59 | 47.0 (39.0–57.0) | 90.0 (84.5–96.7) | 33.6 (32.0–35.1) | 109.0 (104.0–115.0) | 39.9 (35.7–43.6) | 43.9 (42.2–46.2) | 198.0 (148.0–262.0) | 112.0 (101.0–136.0) | 1.70 (1.28–2.08) |
| Men | 32 | 42.0 (36.5–48.5) | 115.0 (103.5–124.0) | 35.2 (32.3–37.3) | 120.0 (113.2–123.5) | 40.2 (33.5–44.2) | 34.4 (32.5–37.0) | 231.5 (183.5–324.0) | 113.0 (101.5–134.5) | 2.09 (1.70–2.43) |
| Age | Body Weight | BMI | WC | BF (kg) | PBF (%) | VAT (cm2) | SAT (cm2) | VAT/SAT | ||
|---|---|---|---|---|---|---|---|---|---|---|
| WOMEN | ||||||||||
| Fasting glucose | r | 0.41 * | 0.25 * | 0.24 | 0.28 * | 0.28 * | 0.32 * | 0.26 * | 0.26 * | 0.16 |
| p | 0.001 | 0.04 | 0.06 | 0.02 | 0.03 | 0.01 | 0.04 | 0.04 | 0.19 | |
| Fasting insulin | r | 0.16 | 0.04 | 0.08 | 0.10 | 0.07 | 0.24 | 0.14 | 0.24 | 0.08 |
| p | 0.21 | 0.74 | 0.52 | 0.40 | 0.56 | 0.06 | 0.27 | 0.06 | 0.52 | |
| HOMA-IR | r | 0.23 | 0.08 | 0.11 | 0.14 | 0.12 | 0.29 * | 0.18 | 0.27 * | 0.11 |
| p | 0.07 | 0.55 | 0.37 | 0.28 | 0.35 | 0.02 | 0.16 | 0.03 | 0.38 | |
| MEN | ||||||||||
| Fasting glucose | r | 0.41 * | −0.26 | −0.12 | −0.14 | −0.15 | 0.00 | 0.10 | −0.10 | 0.07 |
| p | 0.01 | 0.13 | 0.48 | 0.41 | 0.40 | 0.98 | 0.95 | 0.56 | 0.69 | |
| Fasting insulin | r | 0.01 | 0.35 * | 0.37 * | 0.35 * | 0.36 * | 0.23 | 0.25 | 0.23 | 0.14 |
| p | 0.93 | 0.04 | 0.03 | 0.04 | 0.04 | 0.20 | 0.16 | 0.18 | 0.43 | |
| HOMA-IR | r | 0.13 | 0.18 | 0.23 | 0.23 | 0.22 | 0.19 | 0.26 | 0.21 | 0.15 |
| p | 0.45 | 0.31 | 0.19 | 0.18 | 0.22 | 0.29 | 0.14 | 0.22 | 0.38 | |
| Age | Body Weight | BMI | WC | BF (kg) | PBF (%) | VAT (cm2) | SAT (cm2) | VAT/SAT | ||
|---|---|---|---|---|---|---|---|---|---|---|
| WOMEN | ||||||||||
| Total cholesterol | r | 0.19 | −0.07 | −0.01 | 0.05 | 0.02 | 0.12 | 0.08 | 0.05 | 0.05 |
| p | 0.13 | 0.57 | 0.88 | 0.68 | 0.85 | 0.33 | 0.52 | 0.70 | 0.70 | |
| LDL-C | r | 0.05 | 0.00 | 0.02 | 0.02 | 0.05 | 0.07 | 0.11 | 0.08 | 0.04 |
| p | 0.69 | 0.95 | 0.83 | 0.84 | 0.69 | 0.57 | 0.39 | 0.54 | 0.73 | |
| HDL-C | r | −0.09 | −0.12 | −0.07 | −0.06 | −0.17 | −0.20 | −0.32 * | −0.12 | −0.32 * |
| p | 0.46 | 0.34 | 0.58 | 0.60 | 0.19 | 0.11 | 0.01 | 0.33 | 0.01 | |
| Non-HDL-C | r | 0.19 | −0.05 | 0.01 | 0.05 | 0.05 | 0.17 | 0.14 | 0.07 | 0.12 |
| p | 0.13 | 0.69 | 0.92 | 0.68 | 0.65 | 0.17 | 0.26 | 0.56 | 0.34 | |
| Triglycerydes (TGs) | r | 0.16 | 0.06 | 0.11 | 0.12 | 0.13 | 0.18 | 0.26 * | 0.09 | 0.23 |
| p | 0.20 | 0.61 | 0.38 | 0.32 | 0.32 | 0.15 | 0.04 | 0.48 | 0.07 | |
| MEN | ||||||||||
| Total cholesterol | r | 0.32 | −0.15 | −0.08 | −0.04 | 0.01 | 0.12 | −0.00 | 0.14 | −0.03 |
| p | 0.07 | 0.41 | 0.66 | 0.72 | 0.92 | 0.52 | 0.99 | 0.43 | 0.83 | |
| LDL-C | r | 0.20 | 0.01 | −0.05 | 0.03 | 0.09 | 0.09 | 0.00 | 0.13 | −0.11 |
| p | 0.27 | 0.92 | 0.77 | 0.82 | 0.61 | 0.62 | 0.99 | 0.45 | 0.51 | |
| HDL-C | r | −0.00 | −0.21 | −0.04 | −0.14 | −0.09 | −0.05 | −0.25 | −0.19 | −0.10 |
| p | 0.99 | 0.23 | 0.81 | 0.43 | 0.62 | 0.75 | 0.15 | 0.28 | 0.55 | |
| Non-HDL-C | r | 0.32 | −0.07 | −0.02 | 0.02 | 0.08 | 0.17 | 0.05 | 0.20 | −0.03 |
| p | 0.06 | 0.70 | 0.87 | 0.90 | 0.65 | 0.36 | 0.77 | 0.28 | 0.84 | |
| Triglycerydes (TGs) | r | 0.27 | −0.01 | 0.01 | 0.03 | 0.01 | 0.07 | 0.22 | 0.14 | 0.29 |
| p | 0.12 | 0.93 | 0.95 | 0.85 | 0.94 | 0.69 | 0.21 | 0.43 | 0.10 | |
| WOMEN | ||
| VAT | SAT | V/S ratio |
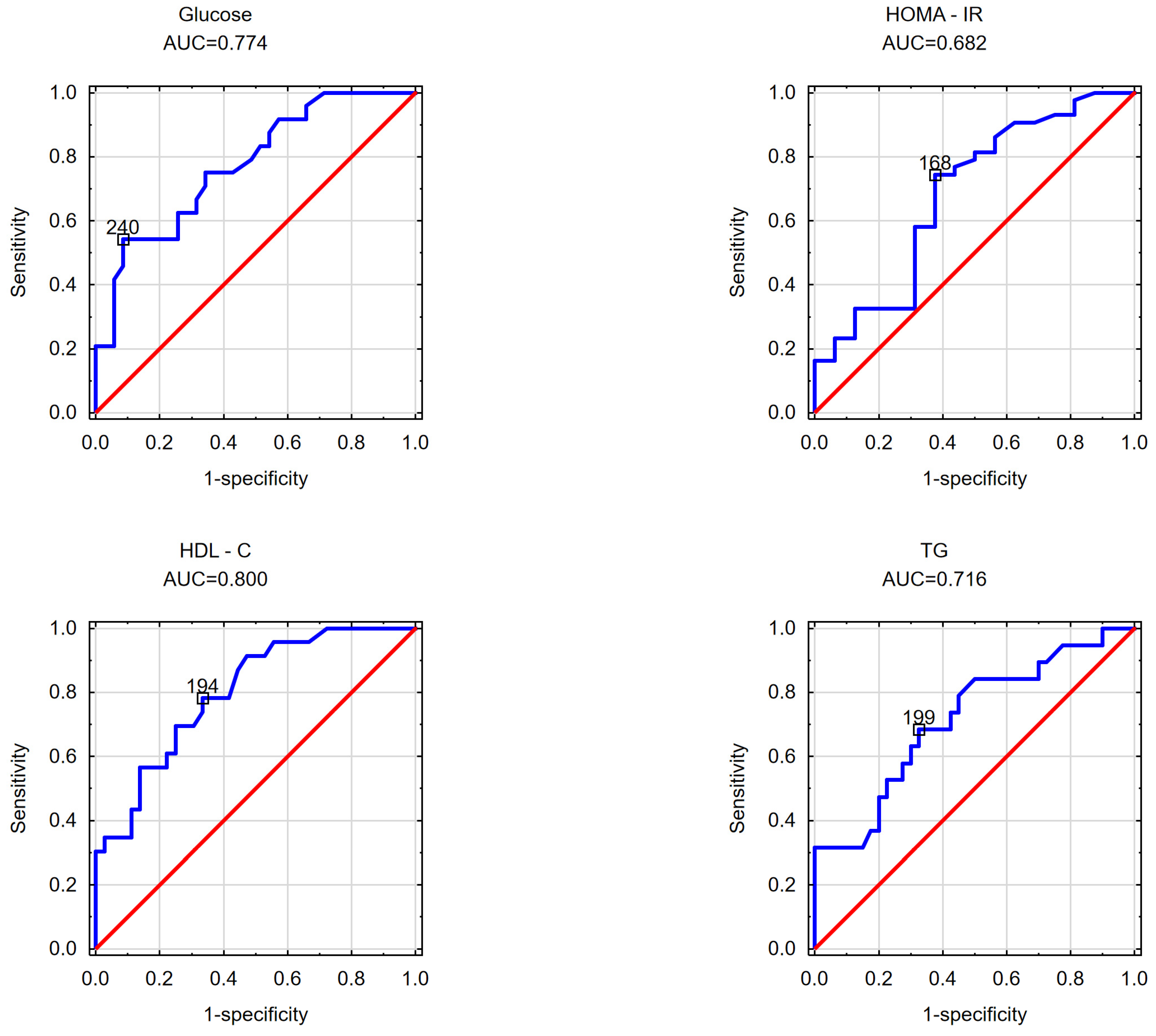
| glucose AUC = 0.774 cut-off point = 240 cm2 | glucose AUC = 0.686 cut-off point = 117 cm2 | glucose AUC = 0.695 cut-off point = 1.55 |
| HOMA-IR AUC = 0.682 cut-off point = 168 cm2 | insulin AUC = 0.675 cut-off point = 111 cm2 | |
| HOMA-IR AUC = 0.799 cut-off point = 109 cm2 | ||
| MEN | ||
| VAT | SAT | V/S ratio |
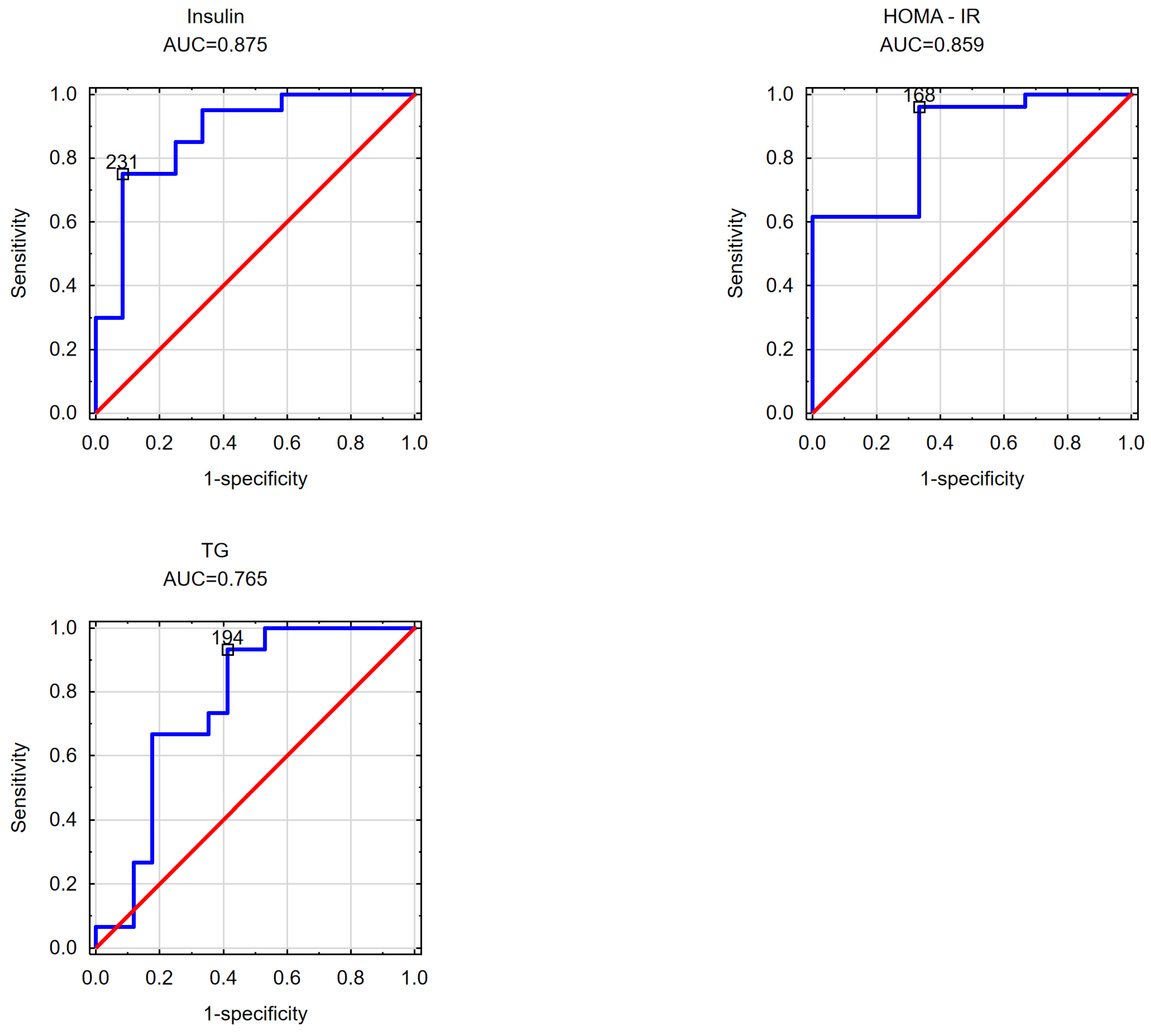
| insulin AUC = 0.875 cut-off point = 231 cm2 | insulin AUC = 0.850 cut-off point = 1.939 | |
| HOMA-IR AUC = 0.859 cut-off point = 168 cm2 | HOMA-IR AUC = 0.872 cut-off point = 1.876 | |
| WOMEN | ||
| VAT | SAT | V/S Ratio |
| HDL-C AUC = 0.800 cut-off point = 194 cm2 | HDL-C AUC = 0.664 cut-off point = 108 cm2 | HDL-C AUC = 0.745 cut-off point = 1.79 |
| TGs AUC = 0.716 cut-off point = 199 cm2 | TGs AUC = 0.715 cut-off point = 1.935 | |
| MEN | ||
| VAT | SAT | V/S ratio |
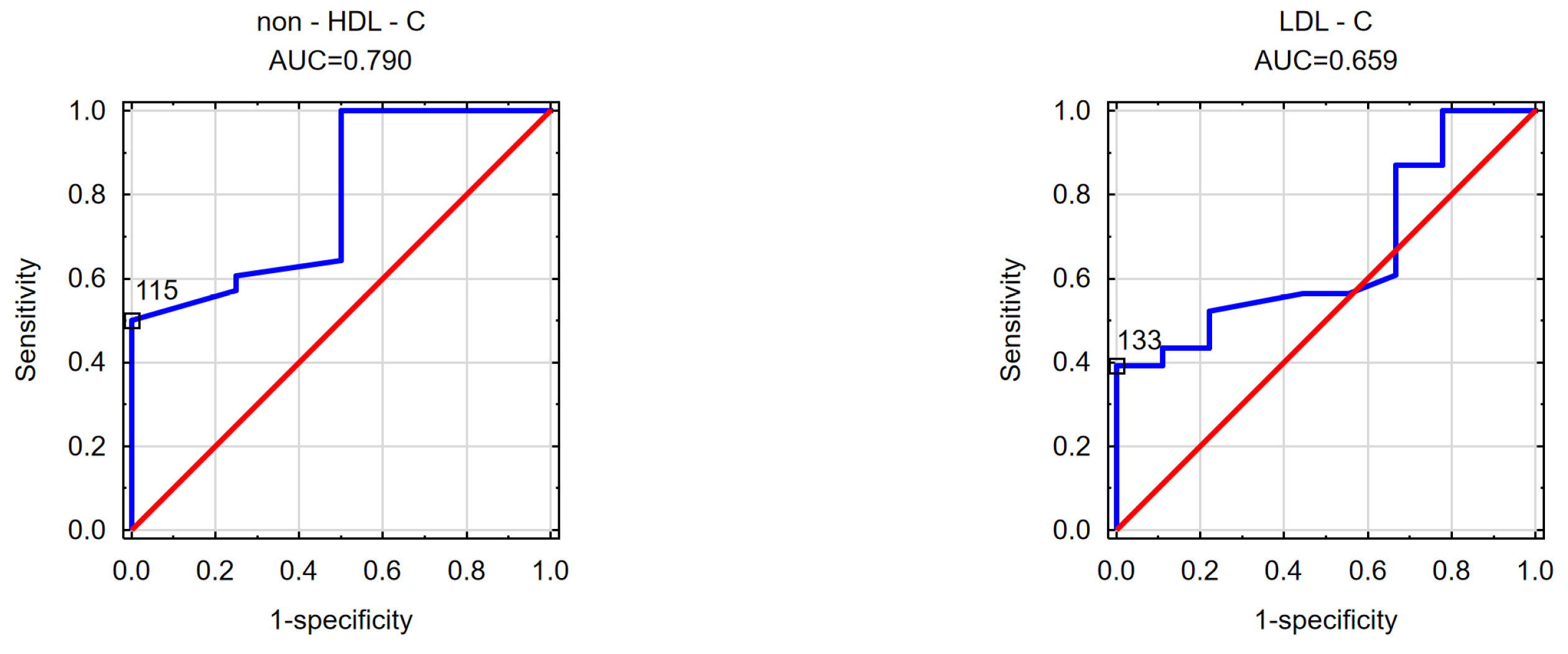
| TGs AUC = 0.765 cut-off point = 168.50 cm2 | non-HDL-C AUC = 0.790 cut-off point = 115 cm2 | TGs AUC = 0.753 cut-off point = 1.64 |
| LDL—C AUC = 0.659 cut-off point = 133 cm2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witczak-Sawczuk, K.; Ostrowska, L.; Cwalina, U.; Leszczyńska, J.; Jastrzębska-Mierzyńska, M.; Hładuński, M.K. Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity—A Pilot Study. Nutrients 2024, 16, 1301. https://doi.org/10.3390/nu16091301
Witczak-Sawczuk K, Ostrowska L, Cwalina U, Leszczyńska J, Jastrzębska-Mierzyńska M, Hładuński MK. Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity—A Pilot Study. Nutrients. 2024; 16(9):1301. https://doi.org/10.3390/nu16091301
Chicago/Turabian StyleWitczak-Sawczuk, Katarzyna, Lucyna Ostrowska, Urszula Cwalina, Joanna Leszczyńska, Marta Jastrzębska-Mierzyńska, and Marcin Krzysztof Hładuński. 2024. "Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity—A Pilot Study" Nutrients 16, no. 9: 1301. https://doi.org/10.3390/nu16091301
APA StyleWitczak-Sawczuk, K., Ostrowska, L., Cwalina, U., Leszczyńska, J., Jastrzębska-Mierzyńska, M., & Hładuński, M. K. (2024). Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity—A Pilot Study. Nutrients, 16(9), 1301. https://doi.org/10.3390/nu16091301