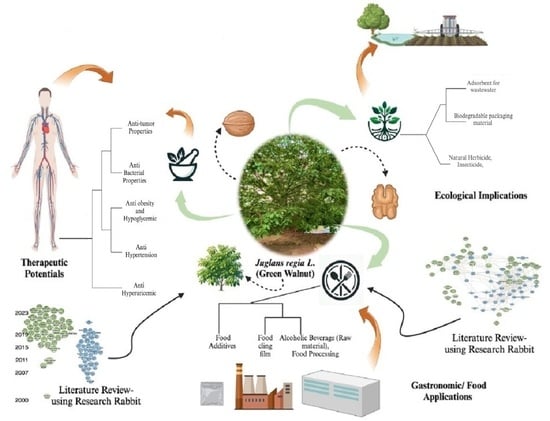
Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review
Abstract
1. Introduction
2. Current Research on Green Walnut and Its Availability
3. Botanical Profile and Impact
4. Best Practices for Harvesting of Green Walnuts
4.1. Harvesting Time
4.2. Selecting the Right Nuts
4.3. Wearing Gloves
4.4. Picking Method
4.5. Processing
4.6. Storage
4.7. Effect of Geographical Origin of Walnuts on Their Functionality and Composition
| Part of the Plant | General Uses | Bioactive Compounds | Medicinal Benefits | Availability of Nutrients | References | |
|---|---|---|---|---|---|---|
| Kernel | Used as a traditional remedy for the treatment of stomachache and cough. | Flavonoids | (+)-catechin Hydrate; (+)-catechin; (−)-epicatechin; Rutin;gallate (+)-catechin hydrate quercetin; syringol; chrysin; myricetin | Hypolipidemic effect; antioxidant activity; antibacterial properties; antiproliferative properties; chemo-preventive properties | A rich source of mono- and polyunsaturated fatty acids; also contains a high number of phenolic compounds. | [3,22,24,25,26,27] |
| Tannins | Quercetin-3-ß-d- glucoside 1;3;6- trigalloylglucose penta-o-galloyl-ß-d- glucose | |||||
| Phenolic acids | Neochlorogenic acid; ferulic acid;vanillic acid; syringic acid; caffeic acid; chlorogenic acid; ellagic acid; p-coumaric acid; cinnamic acid; quinic acid; gallic acid p-hydroxybenzoic acid | |||||
| Quinones | - | |||||
| Husk | The green husk is used in the preparation of liqueur; the husk extract as used as a source of antioxidants to suppress the level of LDL cholesterol and to treat and relieve pain related to skin disorders. | Flavonoids | Apigenin; apigenin; 7-o-b-d-glucuronide; rutin; kaempferol; (+)-catechin; (−)-epicatechin; Myricetin; sudachitin; cirsilineol; 5;6;4′-trihydroxy-7;3-dimethoxy-flavone; eriodictyol | Liver- and kidney-protective; antioxidant, anti-inflammatory, and anticancer properties; improves immune function | Contains gallic acid, ellagic acid, and flavonoids. | [28,29,30,31] |
| Tannins | tannic acid;ellagic acid | |||||
| Phenolic acids | n-hexadecanoic acid; octadecanoic acid; acetic acid; propanoic acid; formic acid; hydroxy-butanoic acid; pentanoic acid; crotonic acid; oxo-pentanoicacid;tetradecanoic acid;2-methyl-propanoic acid; butanoic acid | |||||
| Quinones | 2-methoxy juglone (26.75) juglone (113.63) 8-dihydroxy-1;4- naphthoquinone; 2-hydroxy-14 Naphthoquinone; 8-hydroxyquinoline; 1;4- Naphthoquinone; juglone1-naphtho | |||||
| Shell | The shell is biodegradable in nature and helps improve soil health; it is used as mulching material to control weed growth and regulate the temperature of soil, and a powdered form of the shell is used as a filter medium. | Juglone; tannins; phenolic compounds; pyroligneous acids | Antioxidant, anticancer, and anti-inflammatory properties | Good source of micronutrients including vitamins and minerals; also exhibits oleophilic (oil-attracting) and hydrophobic (water-repelling) properties. | [19,32,33,34,35,36,37] | |
| Bark | Walnut bark is used for cleaning teeth; bark paste is useful in the treatment of arthritis, skin diseases, and toothache and the growth of hairs. | Polyphenols; flavonoids | Prevents tooth ache and tooth decay; antimicrobial activity; antioxidant activity; antifungal activity; platelet aggregation; antiseptic | Walnut bark contains potassium hydroxide which has whitening properties, it is also rich in phenolic antioxidants. | [38,39,40,41,42] | |
| Root | Root extracts of walnut havie inhibitory activities against pathogenic bacteria (Escherichia coli, S. aureus, Staphylococcus saprophyticus, Klebsiella pneumonia, and Pseudomonas aeruginosa). | Alkaloids; flavonoids; polyphenolic compounds | Hair loss; dandruff; skin disorders | Ethyl acetate extract, methanolic extract, and hexane extract have bacterial inhibition activity (phenolic compounds; saponins). | [41,43,44,45] | |
| Leaves | Walnut leaves are utilized in traditional medicine as remedies to treat venous insufficiency and hemorrhoidal symptomatology, and for their anthelmintic, depurative, and antidiarrheal effects. Dried walnut leaves are used as an infusion. | Phenolic acids; tannins; essential fatty acids; ascorbic acid; flavonoids; caffeic acid; para-coumaric acid; Juglone; galactosidase; arabinoside; xyloside; rhamnoside; Naphthoquinone | Antioxidant activity; lipid-lowering effect; antihypertensive effect; antimicrobial effects; gastroprotective activity; hypercholesteraemic activity; antidiabetic effect; anticancer effect; hepato-protective activity and aanti-aging activity | Juglone is a natural phenolic compound is found in fresh walnut leaves. Walnut leaves are also good source of flavonoids, ascorbic acid, and p-coumaric acid. | [46,47,48,49,50,51] | |
| Nutritional Component | Percentage | Pharmaceutical Potential | Impact on Human Health | Reference |
|---|---|---|---|---|
| Vitamin C | 8.7 mg/100 g | Antioxidant; immune system support | Helps protect cells from damage; boosts immune system | [52] |
| Vitamin E | 2.6 mg/100 g | Antioxidant; anti-inflammatory | Helps protect against oxidative stress; reduces inflammation | [53] |
| Omega-3 Fatty Acids | -- | Anti-inflammatory; cardiovascular support | Supports heart health; reduces inflammation | [54] |
| Polyphenols | -- | Antioxidant; anti-inflammatory | May help reduce inflammation; protect against chronic diseases | [55] |
| Melatonin | -- | Sleep regulation; antioxidant | Helps regulate sleep; acts as an antioxidant | [53] |
| Fiber | 3.4 g/100 g | Digestive health; weight management | Aids in digestion; helps maintain a healthy weight | [56] |
| Minerals (e.g., Magnesium, Potassium) | -- | Muscle function; bone health | Important for muscle and nerve function and bone health | [57] |
5. Different Applications of Walnut in Medicine and Gastronomy
5.1. Antitumor Activity
5.2. Improving Memory Loss/Improving Cells/Antihypertension
5.3. Regulation of Blood Sugar/Anti-Obesity and Hypoglycemic Effect/Antihyperuricemia
5.4. Antibacterial Agents
5.5. Enzyme Inhibitors
5.6. Antioxidant Activity
5.7. Culinary Uses
5.7.1. Nocino and Vin de Noix
5.7.2. Pickles and Preserves
5.7.3. Food Additives
5.7.4. Food Films/Alcohol
| Therapeutic Uses | Food/Culinary Uses | Ecological/Industrial Uses | References |
|---|---|---|---|
| Antibacterial agent | Application as a food additive | Contributes to increasing soil carbon | [78,79,90,91,118] |
| Antitumor activities | Preparation of bioactive packaging film and nano-chitosan coating | Helps in reducing pollution | [58,59,92,95,96,97] |
| Improves memory loss and protection of nerves | Formulation of beverages | Improves soil health | [19,56,63,87,93,98,99] |
| Anti-Obesity and hypoglycemia | To improve product texture | Acts as a natural insecticide | [99,100,101,102] |
| Neuroprotection | Dietary fiber as a source of prebiotic | Acts as a natural herbicide | [64,103,104] |
| Anti-inflammatory properties | Food fortification | Preparation of polymer composites | [103,105,106,107,108,109] |
| Improves cells | Confectionery applications | Natural dye source | [10,87,95,110] |
| Prevention of chronic diseases | Production of sugars | Removal of hazardous materials | [111,112,113,114,115,116] |
6. Ecological/Industrial Applications
6.1. Selective Harvesting
6.2. Organic and Fair-Trade Certification
6.3. Environmental Stewardship
6.4. Carbon Sequestration
6.5. Reduced Resource Consumption
6.6. Use of Walnut Husk As Biomass Material
6.7. Walnut Cultivation for Farmers and Producers
6.8. Role of Regulatory Approvals for Use of Green Walnuts
7. Industrial Applications
8. Challenges and Future Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization |
| WGH | Walnut green husk |
| WSP | Walnut septum |
| WMBPs | Walnut meal bioactive peptides |
| WM | Walnut meal |
References
- Taha, N.A.; Al-Wadaan, M.A. Significance and use of walnut, Juglans regia Linn: A review. Adv. J. Microbiol. Res. 2021, 15, 1–10. [Google Scholar]
- Fernandez-Lopez, J.; Aleta, N.; Alıas, R. Forest Genetic Resources Conservation of Juglans regia L.; IPGRI Publishers: Rome, Italy, 2000. [Google Scholar]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. (Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.; Kaiser, F.; Schmidt, K.; Ammann, B.; Carraro, G.; Grisa, E.; Tinner, W. Vegetation history of the walnut forests in Kyrgyzstan (Central Asia): Natural or anthropogenic origin. Quat. Sci. Rev. 2008, 27, 621–632. [Google Scholar] [CrossRef]
- Hassan, G.A.; Bilal, A.T.; Ahmad, B.T.; Sameena, W.; Irshad, A.N. Economic and ethno-medicinal uses of Juglans regia L. in Kashmir Himalaya. Unique J. Ayurvedic Herb. Med. 2013, 1, 64–67. [Google Scholar]
- Zhang, Y.-Y.; Ni, Z.-J.; Elam, E.; Zhang, F.; Thakur, K.; Wang, S.; Zhang, J.-G.; Wei, Z.-J. Juglone, a novel activator of ferroptosis, induces cell death in endometrial carcinoma Ishikawa cells. Food Funct. 2021, 12, 4947–4959. [Google Scholar] [CrossRef] [PubMed]
- Muzaffer, U.; Paul, V.I. Phytochemical analysis, invitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018, 21, 345–356. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits—A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar] [CrossRef]
- Khattak, P.; Khalil, T.F.; Bibi, S.; Jabeen, H.; Muhammad, N.; Khan, M.A.; Liaqat, S. Juglans regia (walnut tree) bark in dentistry. Pak. Biomed. J. 2022, 5, 152–156. [Google Scholar]
- Beiki, T.; Najafpour, G.D.; Hosseini, M. Evaluation of antimicrobial and dyeing properties of walnut (Juglans regia L.) green husk extract for cosmetics. Color. Technol. 2018, 134, 71–81. [Google Scholar] [CrossRef]
- Thakur, A. Juglone: A therapeutic phytochemical from Juglans regia L. J. Med. Plants Res. 2011, 5, 5324–5330. [Google Scholar]
- Sharma, M.; Sharma, M.; Sharma, M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Žalac, H.; Burgess, P.; Graves, A.; Giannitsopoulos, M.; Paponja, I.; Popović, B.; Ivezić, V. Modelling the yield and profitability of intercropped walnut systems in Croatia. Agroforest Syst. 2023, 97, 279–290. [Google Scholar] [CrossRef]
- Chand, L.; Singh, D.B.; Sharma, O.C.; Mir, J.I.; Kumawat, K.L.; Rai, K.M.; Rather, S.A.; Qureshi, I.; Lal, S.; Dev, I. Lateral Bearing Trait in Indian Walnut (Juglans regia L.) germplam: A potential yield contributing trait in early age of the tree. Int. J. Bio-Resour. Stress Manag. 2017, 8, 605–610. [Google Scholar] [CrossRef]
- Germain, E. Inheritance of late leafing and lateral bud fruitfulness inwalnut (Juglans regia L.), phenotypic correlations among some traits of the trees. Acta Hortic. 1990, 284, 125–134. [Google Scholar] [CrossRef]
- Solar, A.; Hudina, M.; Štampar, F. Fruiting Habit and Branching Pattern Affect Vegetative Growth and Reproductive Ability In Walnut (Juglans regia L.). Acta Hortic. 2004, 663, 387–392. [Google Scholar] [CrossRef]
- Laurens, F.; Audergon, J.M.; Claverie, J.; Duval, H.; Germain, E.; Kervella, J.; Le Lezec, M.; Lauri, P.E.; Lespinasse, J.M. Integration of architectural types in French programmes of ligneous fruit species genetic improvement. Fruits 2000, 55, 141–152. [Google Scholar]
- Akca, Y.; Ozongun, S. Selection of late leafing, late flowering, laterally fruitful walnut (Juglans regia) types in Turkey. New Zealand J. Crop Hortic. Sci. 2004, 32, 337–342. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Martín, D.; Gallo, S.; Fuente, G.; Bodoira, R.; Martínez, M.; Maestri, D. Evaluation of hazelnut and walnut oil chemical traits from conventional cultivars and native genetic resources in a non-traditional crop environment from Argentina. Eur. Food Res. Technol. 2020, 246, 833–843. [Google Scholar] [CrossRef]
- Fordos, S.; Abid, N.; Gulzar, M.; Pasha, I.; Oz, F.; Shahid, A.; Aadil, R.M. Recent development in the application of walnut processing by-products (walnut shell and walnut husk). Biomass Convers. Biorefinery 2023, 13, 14389–14411. [Google Scholar] [CrossRef]
- Martínez, M.L.; Mattea, M.A.; Maestri, D.M. Varietal and crop year effects on lipid composition of walnut (Juglans regia) genotypes. J. Am. Oil Chem. Soc. 2006, 83, 791–796. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six wal- nut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced recovery of phenolic and tocopherolic compounds from walnut (Juglans regia L.) male flowers based on process optimization of ultrasonic assisted-extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, P.Z.; Mohammadi, A.; Feyzi, P.; Alesheikh, P. In vitro antioxidant and antibacterial activity of various extracts from exocarps and endocarps of walnut. Pak. J. Pharm. Sci. 2017, 30, 1725–1731. [Google Scholar]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Greve, L.C.; McGranahan, G.; Hasey, J.; Snyder, R.; Kelly, K.; Goldhamer, D.; Labavitch, J.M. Variation in polyunsaturated fatty acids composition of Persian walnut. J. Am. Soc. Hortic. Sci. 1992, 117, 518–522. [Google Scholar] [CrossRef]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut Polyphenolics Inhibit In Vitro Human Plasma and LDL Oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crop. Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Meshkini, A.; Tahmasbi, M. Anti-platelet aggregation activity of walnut hull extract via suppression of ROS generation and caspase activation. J. Acupunct. Meridian Stud. 2017, 10, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2655–2661. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sial, T.A.; Shaheen, S.M.; Lan, Z.; Korai, P.K.; Ghani, M.I.; Khan, M.N.; Syed, A.-U.; Ali, M.N.H.A.; Rajpar, I.; Memon, M.; et al. Addition of walnut shells biochar to alkaline arable soil caused contradictory effects on CO2 and N2O emissions, nutrients availability, and enzymes activity. Chemosphere 2022, 293, 133476. [Google Scholar] [CrossRef]
- Owens, N.; Lee, D. The use of micro bubble flotation technology in secondary and tertiary produced water treatment—A technical comparison with other separation technologies. In Proceedings of the TUV NEL, 5th Produced Water Workshop, Aberdeen, UK, 30–31 May 2007. [Google Scholar]
- Nowicki, P.; Pietrzak, R.; Wachowska, H. Sorption properties of active carbons obtained from walnut shells by chemical and physical activation. Catal. Today 2010, 150, 107–114. [Google Scholar] [CrossRef]
- Zare, H.; Najafpour, G.; Rahimnejad, M.; Tardast, A.; Gilani, S. Biofiltration of ethyl acetate by Pseudomonas putida immobilized on walnut shell. Bioresour. Technol. 2012, 123, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Light, D.M.; Knight, A.L.; Henrick, C.A.; Rajapaska, D.; Lingren, B.; Dickens, J.C.; Reynolds, K.M.; Buttery, R.G.; Merrill, G.; Roitman, J.; et al. A pear-derived kairomone with pheromonal potency that attracts male and female codling moth. Cydia pomonella (L.). Naturwissenschaften 2001, 88, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, J.; Saadipour, K.; Delaviz, H.; Mohammadi, B. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turk. J. Med. Sci. 2011, 41, 685–691. [Google Scholar] [CrossRef]
- Bhatia, K.; Rahman, S.; Ali, M.; Raisuddin, S. In vitro antioxidant activity of Juglans regia L. bark extract and its protective effect on cyclophosphamide-induced urotoxicity in mice. Redox Rep. 2006, 11, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, M.; Hussain, F.; Sultan, A. Ethnobotanical studies on plant resources of Ranyal Hills, District Shangla, Pakistan. Pak. J. Bot. 2007, 39, 329–337. [Google Scholar]
- Hasan, T.N.; Grace, B.L.; Shafi, G.; Al-Hazzani, A.A.; Alshatwi, A.A. Anti-proliferative effects of organic extracts from root bark of Juglans regia L. (RBJR) on MDA-MB-231 human breast cancer cells: Role of Bcl-2/Bax, caspases and Tp53. Asian Pac. J. Cancer Prev. 2011, 12, 525–530. [Google Scholar]
- Zakavi, F.; Hagh, L.G.; Sheikh, A.F.; Daraeighadikolaei, A.; Shooshtari, Z.L. Antibacterial effect of Juglans regia bark against oral pathologic bacteria. Int. J. Dent. 2013, 2013, 854765. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Panichayupakaranan, P. Review of phytochem- istry and pharmacology profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Allaie, A.H.; Singh, R.; Ganaie, A.A.; Mishra, R.P.; Ali, S. Phytochemically analyzed Juglans regia root extracts and their bactericidal facets against some urinary tract infection causing bacteria. Int. J. Biol. Med. Res. 2018, 9, 6304–6308. [Google Scholar]
- Raja, V.; Ahmad, S.I.; Irshad, M.; Wani, W.A.; Siddiqi, W.A.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. Mycol. MéDicale 2017, 27, 476–486. [Google Scholar] [CrossRef]
- Rahimipanah, M.; Hamedi, M.; Mirzapour, M. Antioxidant activity and phenolic contents of Persian walnut (Juglans regia L.) green husk extract. Afr. J. Food Sci. Technol 2010, 1, 105–111. [Google Scholar]
- Al-Snai, A. Iraqi medicinal plants with antifungal effect-A review. IOSR J. Pharm. 2019, 9, 16–56. [Google Scholar]
- Noumi, E.; Snoussi, M.; Trabelsi, N.; Hajlaoui, H.; Ksouri, R.; Valentin, E.; Bakhrouf, A. Antibacterial, anticandidal and antioxidant activities of Salvadora persica and Juglans regia L. extracts. J. Med. Plants Res. 2011, 5, 4138–4146. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Thul, S.T. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crop. Prod. 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Boukhari, F.; Tigrine-Kordjani, N.; Youcef Meklati, B. Phytochemical Investigation by Microwave-Assisted Extraction of Essential Oil of the Leaves of Walnut Cultivated in Algeria. Helv. Chim. Acta 2013, 96, 1168–1175. [Google Scholar] [CrossRef]
- Salimi, M.; Majd, A.; Sepahdar, Z.; Azadmanesh, K.; Irian, S.; Ardestaniyan, M.H.; Hedayati, M.H.; Rastkari, N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm. Biol. 2012, 50, 1416–1422. [Google Scholar] [CrossRef]
- Fu, M.; Qu, Q.; Yang, X.; Zhang, X. Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. LWT 2016, 65, 1126–1132. [Google Scholar] [CrossRef]
- Raja, G.; Shaker, I.A.; Sailaja, I.; Swaminathan, R.; Babu, K.S.; Basha, S.S. Nutritional analysis of nuts extract of Juglans regia L. Int. J. Bioassays 2012, 1, 68–73. [Google Scholar] [CrossRef][Green Version]
- Al-Snafi, A.E. Chemical constituents, nutritional, pharmacological and therapeutic importance of Juglans regia—A review. IOSR J. Pharm. 2018, 8, 1–21. [Google Scholar]
- Mohammed Junaidh, K.; Muhammed Sajid, E.K.; Mohammed Ashif, A. A review on walnut: Its pharmacological properties and role in human life. Energy 2022, 2809, 3. [Google Scholar]
- Petrović, M.; Pastor, F.; Đurović, S.; Veljović, S.; Gorjanović, S.; Sredojević, M.; Vukosavljević, P. Evaluation of novel green walnut liqueur as a source of antioxidants: Multi-method approach. J. Food Sci. Technol. 2020, 58, 2160–2169. [Google Scholar] [CrossRef]
- Kithi, L.; Lengyel-Kónya, É.; Berki, M.; Bujdosó, G. Role of the green husks of persian walnut (Juglans regia L.)—A Review. Horticulturae 2023, 9, 782. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Hara, H.; Taib, S.H.M. Cytotoxicity assay of biosynthesis gold nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Mol. Struct. 2018, 1151, 97–105. [Google Scholar] [CrossRef]
- Dzidziguri, D.; Rukhadze, M.; Modebadze, I.; Bakuradze, E.; Kurtanidze, M.; Giqoshvili, V. The Study of the Immune Corrective Properties of Greek Walnut (Juglans regia L.) septa on the experimental model of leukopenia. Georgian Med. News 2016, 252, 84–89. [Google Scholar]
- Ren, D.; Zhao, F.; Liu, C.; Wang, J.; Guo, Y.; Liu, J.; Min, W. Antioxidant hydrolyzed peptides from Manchurian walnut (Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food Agric. 2018, 98, 5142–5152. [Google Scholar] [CrossRef]
- Zheng, X.; Li, D.S.; Ding, K. Purification and identification of angiotensin I-converting enzyme inhibitory peptides from fermented walnut residues. Int. J. Food Prop. 2017, 20, S3326–S3333. [Google Scholar] [CrossRef]
- Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent studies on protective effects of walnuts against neuroinflammation. Nutrients 2022, 14 (Suppl. 3), 4360. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, D.; Liu, H.; Yang, T.; Liang, Q.; Wang, J.; Zhang, R.; Zhang, Y. Water soluble dietary fiber from walnut meal as a prebiotic in preventing metabolic syndrome. J. Funct. Foods 2021, 78, 104358. [Google Scholar] [CrossRef]
- Wang, J.; Du, K.; Fang, L.; Liu, C.; Min, W.; Liu, J. Evaluation of the antidiabetic activity of hydrolyzed peptides derived from Juglans mandshurica Maxim. fruits in insulin-resistant HepG2 cells and type 2 diabetic mice. J. Food Biochem. 2018, 42, e12518. [Google Scholar]
- Feng, L.; Wang, X.; Peng, F.; Liao, J.; Nai, Y.; Lei, H.; Li, M.; Xu, H. Walnut protein hydrolysates play a protective role on neurotoxicity induced by d-galactose and aluminum chloride in mice. Molecules 2018, 23, 2308. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.; Tan, D.-X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Serrano, A.; Cofrades, S.; Ruiz-Capillas, C.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Jiménez-Colmenero, F. Nutritional profile of restructured beef steak with added walnuts. Meat Sci. 2005, 70, 647–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shukla, P.; Chopada, K.; Sakure, A.; Hati, S. Current Trends and Applications of Food-derived Antihypertensive Peptides for the Management of Cardiovascular Disease. Protein Pept. Lett. 2022, 29, 408–428. [Google Scholar] [CrossRef]
- Wang, F.-J.; Yin, X.-Y.; Regenstein, J.M.; Wang, J.-Z. Separation and purification of angiotensin-I-converting enzyme (ACE) inhibitory peptides from walnuts (Juglans regia L.) meal. Eur. Food Res. Technol. 2015, 242, 911–918. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Li, S.; Zhou, J.; Xu, J. Physico-chemical properties, antioxidant activities and antihypertensive effects of walnut protein and its hydrolysate. J. Sci. Food Agric. 2015, 96, 2579–2587. [Google Scholar] [CrossRef]
- Prokisch, J.; El-Ramady, H.; Daróczi, L.; Nagy, É; Badgar, K.; Kiss, A.; Shaikh, A.M.; Gilányi, I.; Oláh, C. Functional Yogurt Fortified with Honey Produced by Feeding Bees Natural Plant Extracts for Controlling Human Blood Sugar Level. Plants 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Milind, P.; Deepa, K. Walnut: Not a hard nut to crack. Int. Res. J. Pharm. 2011, 2, 8–17. [Google Scholar]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.; Costa, P.C.; Bahia, M.F. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008, 106, 1014–1020. [Google Scholar] [CrossRef]
- Girzu, M.; Carnat, A.; Privat, A.M.; Fialip, J.; Carnat, A.P.; Lamaison, J.L. Sedative Effect of Walnut Leaf Extract and Juglone on Isoleted Contituent. Pharm. Biol. 1998, 36, 280–286. [Google Scholar] [CrossRef]
- Yabalak, E.; Eliuz, E.A.E. Green synthesis of walnut shell hydrochar, its antimicrobial activity and mechanism on some pathogens as a natural sanitizer. Food Chem. 2021, 366, 130608. [Google Scholar] [CrossRef] [PubMed]
- Soto-Madrid, D.; Gutiérrez-Cutiño, M.; Pozo-Martínez, J.; Zúñiga-López, M.C.; Olea-Azar, C.; Matiacevich, S. Dependence of the ripeness stage on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts from industrial by-products. Molecules 2021, 26, 2878. [Google Scholar] [CrossRef]
- Abedi, P.; Yaralizadeh, M.; Fatahinia, M.; Namjoyan, F.; Nezamivand-Chegini, S.; Yaralizadeh, M. Comparison of the Effects of Juglans nigra Green Husk and Clotrimazole on Candida albicans in Rats. Jundishapur J. Microbiol. 2017, 11, e58151. [Google Scholar] [CrossRef]
- Baghkheirati, E.K.; Bagherieh-Najjar, M.B.; Fadafan, H.K.; Abdolzadeh, A. Synthesis and antibacterial activity of stable bio-conjugated nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Exp. Nanosci. 2015, 11, 512–517. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeleye, S.I.; Ogunsuyi, O.B.; Oboh, G. Phenolic analysis and erectogenic function of African Walnut (Tetracarpidium conophorum) seeds: The impact of the seed shell on biological activity. J. Food Biochem. 2018, 43, e12815. [Google Scholar] [CrossRef]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential valorization of edible nuts by-products: Exploring the immune-modulatory and antioxidants effects of selected nut shells extracts in relation to their metabolic profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Zambón, D.; Sabaté, J.; Muñoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. Substituting Walnuts for Monounsaturated Fat Improves the Serum Lipid Profile of Hypercholesterolemic Men and Women. Ann. Intern. Med. 2000, 132, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.M. Walnut, Cultivation, Nutritional Value, Folklore, 4th ed.; CC Publication: Ankara, Turkey, 2011; p. 220. (In Turkish) [Google Scholar]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and Exploitation of Bioactive Compounds of Walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur—Cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Serdar Uğurlu, E.O.; Bakkalbaş, E. Reduction of bitterness in green walnuts by conventional and ultrasound-assisted maceration. Ultrason. Sonochemistry 2020, 66, 105094. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Fasihi, H.; Moradipayam, A. Study on the Antioxidant Effects of Extract and Powder of Green Walnut Hulls on the Oxidation of Sunflower Oil. Iran. J. Nutr. Sci. Food Technol. 2015, 10, 79–90. [Google Scholar]
- Salejda, A.M.; Janiewicz, U.; Korzeniowska, M.; Kolniak-Ostek, J.; Krasnowska, G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT 2016, 65, 751–757. [Google Scholar] [CrossRef]
- Chatrabnous, N.; Yazdani, N.; Tavallali, V.; Vahdati, K. Preserving quality of fresh walnuts using plant extracts. LWT 2018, 91, 1–7. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Xie, X.; Xie, C.; Li, A.; Xia, N.; Gong, X.; Zhang, H. Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int. J. Biol. Macromol. 2022, 209, 1307–1318. [Google Scholar] [CrossRef]
- Egea, T.; Signorini, M.A.; Bruschi, P.; Rivera, D.; Obón, C.; Alcaraz, F.; Palazón, J.A. Spirits and liqueurs in European traditional medicine: Their history and ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 2015, 175, 241–255. [Google Scholar] [CrossRef]
- Luan, H.; Liu, Y.; Huang, S.; Qiao, W.; Chen, J.; Guo, T.; Zhang, X.; Guo, S.; Zhang, X.; Qi, G. Successive walnut plantations alter soil carbon quantity and quality by modifying microbial communities and enzyme activities. Front. Microbiol. 2022, 13, 953552. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, P.; Pashangeh, S.; Berizi, E.; Majlesi, M.; Hosseinzadeh, S.; Salehi, S.O.; Derakhshan, Z.; Giannakis, S. Potential of nanochitosan coating combined with walnut green husk to improve the preservation of rainbow trout (Oncorhynchus mykiss) during refrigerated storage. Environ. Res. 2022, 214, 114019. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, S.; Jia, J.; Cui, J. Resource efficiency and environmental impact of juglone in Pericarpium Juglandis: A review. Front. Environ. Sci. 2022, 10, 999059. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Luo, W.; Božović, S.; Gong, C.; Ren, J. Characterization and analysis of antioxidant activity of walnut-derived pentapeptide PW5 via nuclear magnetic resonance spectroscopy. Food Chem. 2020, 339, 128047. [Google Scholar] [CrossRef]
- Zhu, K.; Ma, J.; Cong, J.; Zhang, T.; Lei, H.; Xu, H.; Luo, Z.; Li, M. The road to reuse of walnut by-products: A comprehensive review of bioactive compounds, extraction and identification methods, biomedical and industrial applications. Trends Food Sci. Technol. 2024, 143, 104264. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Barbut, S.; Marangoni, A.G. Food-grade filler particles as an alternative method to modify the texture and stability of myofibrillar gels. Sci. Rep. 2017, 7, 11544. [Google Scholar] [CrossRef]
- Kavdir, Y.; Gozel, U.; Sahiner, N. Nematicidal effects of olive pomace and green walnut husk on root-knot nematode Meloidogyne incognita on tomato. Allelopath. J. 2019, 46, 3–16. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, H.X.; Shen, Z.J.; Zhao, L.L.; Clarke, S.R.; Sun, J.H.; Du, Y.Y.; Shi, G.L. Methyl palmitate, an Acaricidal compound occurring in green walnut husks. J. Econ. Entomol. 2009, 102, 196–202. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.S. Phytochemicals and biological activities of walnut septum: A systematic review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Leszczyński, B.; Czerniewicz, P.; Sytykiewicz, H.; Matok, H.; Krzyżanowski, R.; Sempruch, C. Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop. Prot. 2012, 35, 71–77. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Gabaston, J.; Ortiz-Somovilla, V.; Cantos-Villar, E. Wood Waste from Fruit Trees: Biomolecules and Their Applications in Agri-Food Industry. Biomolecules 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Almoraie, N.M. The effect of walnut flour on the physical and sensory characteristics of wheat bread. Int. J. Food Sci. 2019, 2019, 5676205. [Google Scholar] [CrossRef] [PubMed]
- Mirheidari, A.; Torbatinejad, N.M.; Shakeri, P.; Mokhtarpour, A. Effects of walnut shell and chicken manure biochar on in vitro fermentation and in vivo nutrient digestibility and performance of dairy ewes. Trop. Anim. Health Prod. 2019, 51, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Ayrilmis, N.; Kaymakci, A.; Ozdemir, F. Physical, mechanical, and thermal properties of polypropylene composites filled with walnut shell flour. J. Ind. Eng. Chem. 2013, 19, 908–914. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Quiles-Carrillo, L.; Richart, N.; Torres-Giner, S.; Montanes, N. Enhanced Interfacial Adhesion of Polylactide/Poly (ε-caprolactone)/Walnut Shell Flour Composites by Reactive Extrusion with Maleinized Linseed Oil. Polymers 2019, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Ramishvili, L.; Gordeziani, M.; Tavdishvili, E.; Bedineishvili, N.; Dzidziguri, D.; Kotrikadze, N. The effect of extract of greek walnut (Juglans regia L.) septa on some functional characteristics of erythrocytes. Georgian Med. News 2016, 261, 51–57. [Google Scholar]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Critical Reviews in Food Science and Nutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef]
- Nodeh, A.A.; Ardjmand, M.; Fanaei, M.; Safekordi, A. Neural network for modeling of chemical reaction systems: Kinetics of concentrated acid hydrolysis of walnut green skin. Asian J. Chem. 2013, 25, 1793–1799. [Google Scholar] [CrossRef]
- Arasteh Nodeh, A.; Hemmati, H. Using response surface method for optimizing dilute acid hydrolysis of walnut green skin. J. Appl. Environ. Biol. Sci. 2015, 4, 209–212. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Bozkurt, H.; Geyik, F. Artificial neural network and genetic algorithms for modeling of removal of an azo dye on walnut husk. Desalination Water Treat. 2016, 57, 15580–15591. [Google Scholar] [CrossRef]
- Cantarello, E.; Lovegrove, A.; Orozumbekov, A.; Birch, J.; Brouwers, N.; Newton, A.C. Human Impacts on Forest Biodiversity in Protected Walnut-Fruit Forests in Kyrgyzstan. J. Sustain. For. 2014, 33, 454–481. [Google Scholar] [CrossRef]
- Popa, R.G.; Bălăcescu, A.; Popescu, L.G. Organic Walnut Cultivation in Intensive and Super-Intensive System-Sustainable Investment. Case Study: Gorj County, Romania. Sustainability 2023, 15, 1244. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Plastina, P.; Cione, E.; Fazio, A. Green husk of walnuts (Juglans regia L.) from southern Italy as a valuable source for the recovery of glucans and pectins. Fermentation 2021, 7, 305. [Google Scholar] [CrossRef]
- Yilmaz, S.; Akça, Y.; Saçlik, S. Green husk and inshell biomass production capabilities of six walnut cultivars. Agric Food 2017, 5, 389–397. [Google Scholar]
- Dong, N.; Hu, G.; Zhang, Y.; Qi, J.; Chen, Y.; Hao, Y. Effects of green-manure and tillage management on soil microbial community composition, nutrients and tree growth in a walnut orchard. Sci. Rep. 2021, 11, 16882. [Google Scholar] [CrossRef]
- Binbin, Y.; Peijun, T.; Yuechen, Z. Research Article Analysis on the Effect of Poverty Alleviation Based on Walnut Industry. Adv. J. Food Sci. Technol. 2015, 8, 499–504. [Google Scholar] [CrossRef]
- Ros, E.M. Izquierdo-Pulido, and A. Sala-Vila, Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Tehrani-Bagha, A.R.; Gharanjig, K.; Hashemi, E. Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J. Clean. Prod. 2019, 231, 1463–1473. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption: What You Need to Know About the FDA Regulation: Guidance for Industry Small Entity Compliance Guide; U.S. Department of Health and Human Services Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Latos, M.; Masek, A.; Zaborski, M. The potential of juglone as natural dye and indicator for biodegradable polye-sters. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 276–285. [Google Scholar]
- Medic, A.; Baez Vega, C.B. Benefits of Cover Cropping Systems in Walnut Orchards as Sustainable Agricultural Practice. J. Sustain. Clim. Chang. 2021, 1, 35–44. [Google Scholar] [CrossRef]
- Pardon, P.; Mertens, J.; Reubens, B.; Reheul, D.; Coussement, T.; Elsen, A.; Nelissen, V.; Verheyen, K. Juglans regia (walnut) in temperate arable agroforestry systems: Effects on soil characteristics, arthropod diversity and crop yield. Renew. Agric. Food Syst. 2020, 35, 533–549. [Google Scholar] [CrossRef]
- Shaziya Gull, T.A.; Rasool, A. Studies on diversity indices and insect pest damage of walnuts in Kashmir, India. Acta Agric. Slov. 2019, 113, 121–135. [Google Scholar]
- Danielsen, F.; Burgess, N.D.; Balmford, A. Monitoring matters: Examining the potential of locally-based approaches. Biodivers. Conserv. 2005, 14, 2507–2542. [Google Scholar] [CrossRef]
- Rehnus, M.; Mamadzhanov, D.; Venglovsky, B.I.; Sorg, J.-P. The importance of agroforestry hay and walnut production in the walnut-fruit forests of southern Kyrgyzstan. Agrofor. Syst. 2012, 87, 1–12. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukarram, S.A.; Wandhekar, S.S.; Ahmed, A.E.M.; Pandey, V.K.; Csaba, O.; Lajos, D.; József, P.; Harsányi, E.; Bela, K. Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review. Nutrients 2024, 16, 1183. https://doi.org/10.3390/nu16081183
Mukarram SA, Wandhekar SS, Ahmed AEM, Pandey VK, Csaba O, Lajos D, József P, Harsányi E, Bela K. Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review. Nutrients. 2024; 16(8):1183. https://doi.org/10.3390/nu16081183
Chicago/Turabian StyleMukarram, Shaikh Ayaz, Sangram S. Wandhekar, Abdelhakam Esmaeil Mohamed Ahmed, Vinay Kumar Pandey, Oláh Csaba, Daróczi Lajos, Prokisch József, Endre Harsányi, and Kovács Bela. 2024. "Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review" Nutrients 16, no. 8: 1183. https://doi.org/10.3390/nu16081183
APA StyleMukarram, S. A., Wandhekar, S. S., Ahmed, A. E. M., Pandey, V. K., Csaba, O., Lajos, D., József, P., Harsányi, E., & Bela, K. (2024). Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review. Nutrients, 16(8), 1183. https://doi.org/10.3390/nu16081183