Feasibility, Acceptability, and Potential Efficacy of a Mobile Health Application for Community-Dwelling Older Adults with Frailty and Pre-Frailty: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
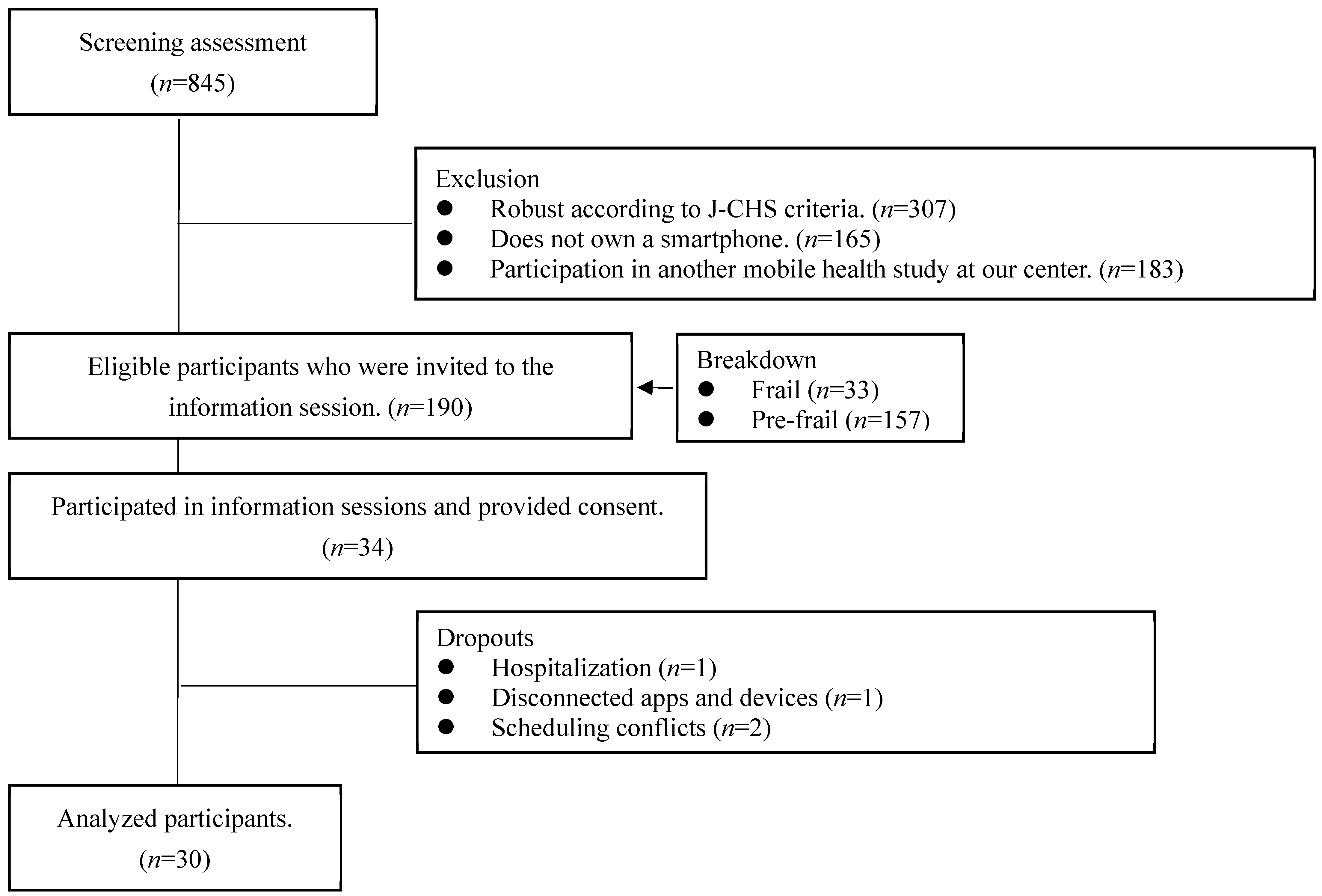
2.1. Design and Participants
2.2. Intervention Program
2.3. Sample Size
2.4. Feasibility of mHealth Apps
2.5. Acceptability
2.6. Potential Efficacy
- Frailty Phenotype Score:
- 2.
- Physical Fitness Test:
- 3.
- Questionnaire Survey:
- 4.
- Step Counts, Physical Activity, and Sedentary Behavior:
- 5.
- Adverse Events:
2.7. Data Analysis
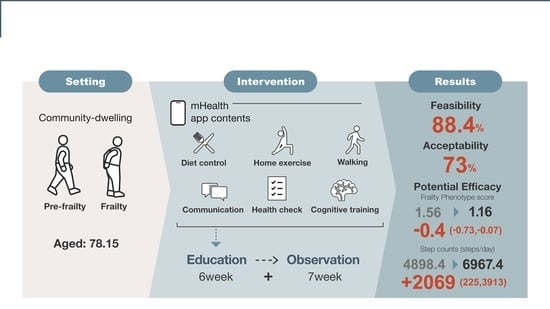
3. Results
3.1. Participants Recruited
3.2. Baseline Characteristics
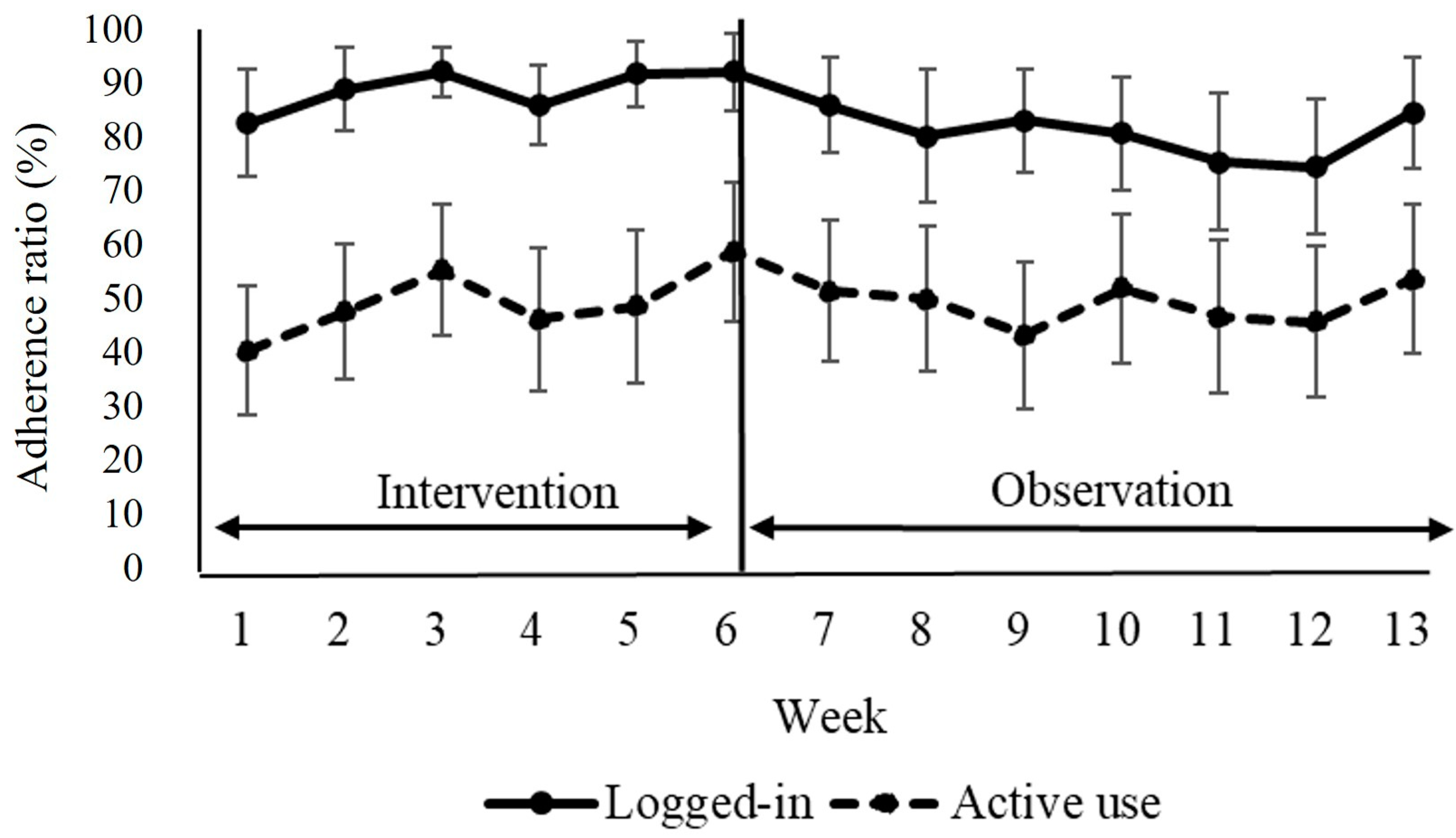
3.3. Attendance and Adverse Events
3.4. Feasibility and Acceptability
3.5. Potential Efficacy
3.6. Step Counts, Physical Activity, and Sedentary Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, 146–156. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kikuchi, T.; Kusumi, H.; Arai, H. The Influence of the COVID-19 Pandemic on Physical Activity and New Incidence of Frailty among Initially Non-Frail Older Adults in Japan: A Follow-Up Online Survey. J. Nutr. Health Aging 2021, 25, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Division of the Heath for the Elderly H and WB for the E. Fact-Finding Survey on Project of Long-Term Care; Ministry of Health Labor and Welfare: Tokyo, Japan, 2020. [Google Scholar]
- Ejiri, M.; Kawai, H.; Yasunaga, M.; Shirobe, M.; Ito, K.; Ueda, T.; Obuchi, S. Effective support based on length of participation for community-based activities led by older residents. Nihon Koshu Eisei Zasshi 2021, 68, 459–467. [Google Scholar]
- Soto-Bagaria, L.; Eis, S.; Pérez, L.M.; Villa-García, L.; de Solà-Morales, O.; Carrion, C.; Giné-Garriga, M.; Inzitari, M. Mobile applications to prescribe physical exercise in frail older adults: Review of the available tools in app stores. Age Ageing 2023, 52, afad227. [Google Scholar] [CrossRef]
- Shimada, H.; Lee, S.; Harada, K.; Bae, S.; Makino, K.; Chiba, I.; Katayama, O.; Arai, H. Study Protocol of a Comprehensive Activity Promotion Program for the Prevention of Dementia: A Randomized Controlled Trial Protocol. J. Prev. Alzheimer’s Dis. 2022, 9, 376–384. [Google Scholar]
- Linn, N.; Goetzinger, C.; Regnaux, J.-P.; Schmitz, S.; Dessenne, C.; Fagherazzi, G.; Aguayo, G.A. Digital Health Interventions among People Living with Frailty: A Scoping Review. J. Am. Med. Dir. Assoc. 2021, 22, 1802–1812.e21. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Lancaster, G.A.; Campbell, M.J.; Thabane, L.; Hopewell, S.; Coleman, C.L.; Bond, C.M. Defining feasibility and pilot studies in preparation for randomised controlled trials: Development of a conceptual framework. PLoS ONE 2016, 11, e0150205. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, S.; Didino, D.; Baez, M.; Casati, F. Feasibility of virtual tablet-based group exercise among older adults in Siberia: Findings from two pilot trials. JMIR Mhealth Uhealth 2018, 6, e40. [Google Scholar] [CrossRef] [PubMed]
- Geraedts, H.A.E.; Zijlstra, W.; Zhang, W.; Spoorenberg, S.L.W.; Báez, M.; Far, I.K.; Baldus, H.; Stevens, M. A home-based exercise program driven by tablet application and mobility monitoring for frail older adults: Feasibility and practical implications. Prev. Chronic Dis. 2017, 14, 160227. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Gianoudis, J.; Hall, T.; Mundell, N.L.; Maddison, R. Feasibility, usability, and enjoyment of a home-based exercise program delivered via an exercise app for musculoskeletal health in community-dwelling older adults: Short-term prospective pilot study. JMIR Mhealth Uhealth 2021, 9, e21094. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okusa, S.; Sato, M.; Miura, H.; Morimoto, M.; Tsutsumi, A. mHealth Intervention to Promote Physical Activity Among Employees Using a Deep Learning Model for Passive Monitoring of Depression and Anxiety: Single-Arm Feasibility Trial. JMIR Form. Res. 2023, 7, e51334. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Wilson, H. Development and validation of the user version of the mobile application rating scale (uMARS). JMIR Mhealth Uhealth 2016, 4, e5849. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Arita, N.; Takenaka, K.; Shimazaki, T. Development of a Home-Exercise Barrier Self-Efficacy Scale for Elderly People Requiring Support and Care. J. Jpn. Phys. Ther. Assoc. 2015, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Bird, V.; Rizzo, M.; Meader, N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: A meta analysis of GDS30 and GDS 15. J. Affect. Disord. 2010, 125, 10–17. [Google Scholar] [CrossRef]
- Friemel, T.N. The digital divide has grown old: Determinants of a digital divide among seniors. New Media Soc. 2016, 18, 313–331. [Google Scholar] [CrossRef]
- Lee, H.; Lim, J.A.; Nam, H.K. Effect of a Digital Literacy Program on Older Adults’ Digital Social Behavior: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 12404. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.A.; Olds, T.; Vandelanotte, C.; Plotnikoff, R.; Edney, S.M.; Ryan, J.C.; DeSmet, A.; Curtis, R.G. Gamification in a Physical Activity App: What Gamification Features Are Being Used, by Whom, and Does It Make a Difference? Games Health J. 2022, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, H.; Shen, M.; Ni, Y.; Zhang, X.; Pang, Y.; Yu, T.; Lian, X.; Yu, T.; Yang, X.; et al. The Effects of mHealth-Based Gamification Interventions on Participation in Physical Activity: Systematic Review. JMIR Mhealth Uhealth 2022, 10, e27794. [Google Scholar] [CrossRef] [PubMed]
- Vikberg, S.; Björk, S.; Nordström, A.; Nordström, P.; Hult, A. Feasibility of an Online Delivered, Home-Based Resistance Training Program for Older Adults—A Mixed Methods Approach. Front. Psychol. 2022, 13, 869573. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.; Yan, R.J.; Harkin, L.J.; Katz, D.; Stevenson, C.; Mehta, V.; Giles, E.; Talbot, C.; Gooch, D.; Bennasar, M.; et al. Digital Intervention in Loneliness in Older Adults: Qualitative Analysis of User Studies. JMIR Form. Res. 2023, 7, e42172. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, F.; Suomi, R. Elderly Forgotten? Digital Exclusion in the Information Age and the Rising Grey Digital Divide. Inquiry 2022, 59, 00469580221096272. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Kamp, A.; Fersch, B. Detached co-involvement in interactional care: Transcending temporality and spatiality through mHealth in a social psychiatry out-patient setting. Soc. Sci. Med. 2021, 285, 114297. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Brothers, T.D.; Peña, F.G.; Mitnitski, A.; Rockwood, K. Identifying common characteristics of frailty across seven scales. J. Am. Geriatr. Soc. 2014, 62, 901–906. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare. Active Guide—Japanese Official Physical Activity Guidelines for Health Promotion 2023; Ministry of Health Labour and Welfare: Tokyo, Japan, 2023. [Google Scholar]
- Hino, K.; Lee, J.S.; Asami, Y. Associations between seasonal meteorological conditions and the daily step count of adults in Yokohama, Japan: Results of year-round pedometer measurements in a large population. Prev. Med. Rep. 2017, 8, 15–17. [Google Scholar] [CrossRef]
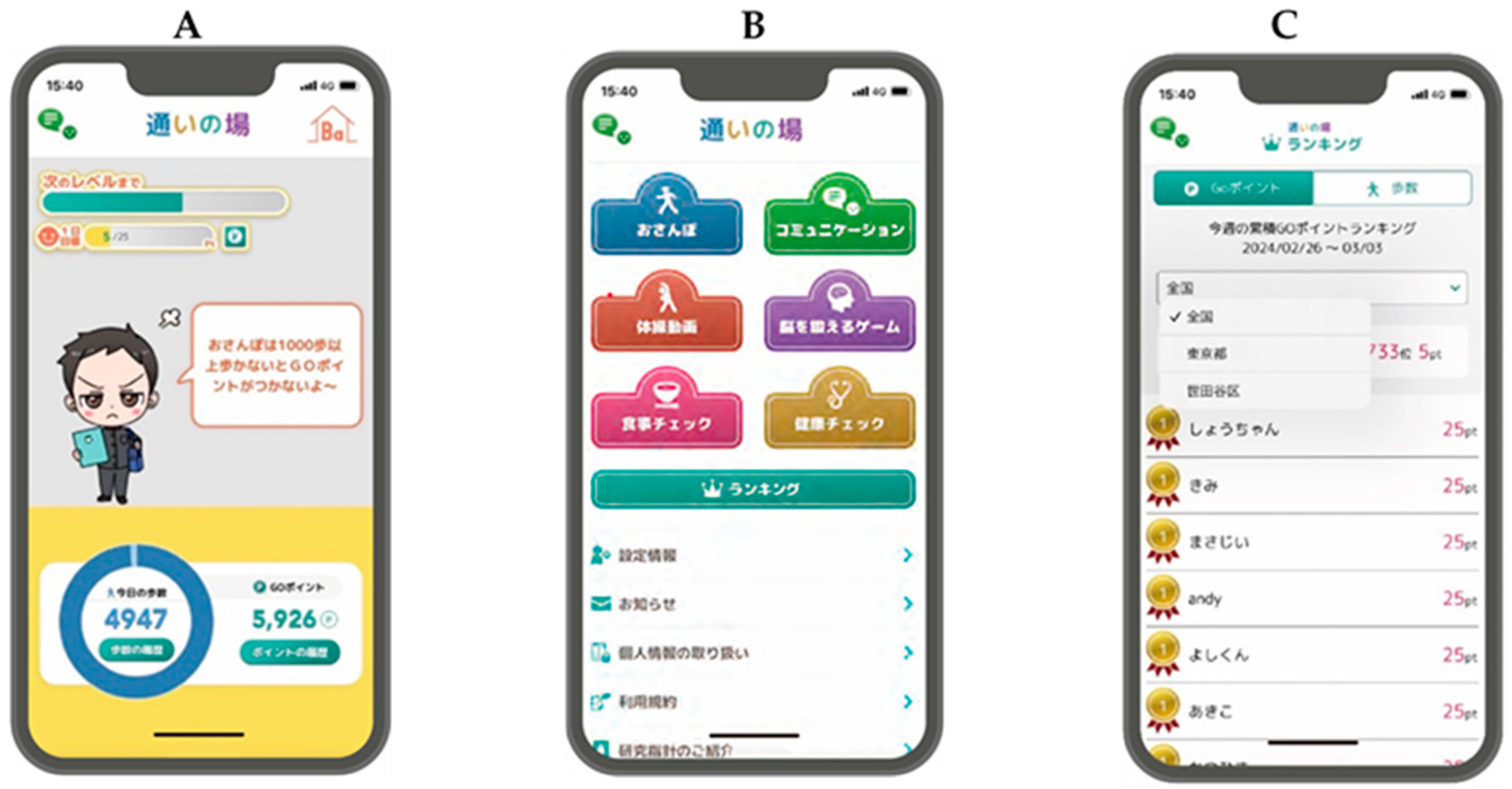
| Visit | Contents of Lesson | Summary |
|---|---|---|
| 1 | Let us take a walk using the walking function | Installation of the application and explanation of GO points. Start the walking function and try to generate a walking course automatically. |
| 2 | 1. Let us communicate within the application 2. Review of the walking function | Post, like, and comment on photos using communication tools. |
| 3 | 1. Enter today’s meal in the diet diary 2. Review of communication tools | Register what you ate this morning (or for lunch) and check your nutritional intake. |
| 4 | 1. Let us try some local exercises 2. Review the diet check | Participants can access and try more than 1000 different local gymnastics exercises through the app |
| 5 | 1. Play games using your brain 2. Review of local exercises | Activate and try playing the cognitive game. |
| 6 | General review section | Reconfirm understanding of previous functions as a general review of 1–5. |
| Overall (n = 34) | Pre-Frail (n = 29) | Frail (n = 5) | |
|---|---|---|---|
| Age, year | 78.15 (4.61) | 77.72 (3.97) | 80.60 (7.50) |
| Body mass index, kg/m2 | 21.75 (3.45) | 21.72 (3.61) | 21.94 (2.62) |
| Systolic blood pressure, mmHg | 125.29 (16.28) | 124.97 (15.74) | 127.20 (21.15) |
| Frail phenotype score | 1.56 (0.82) | 1.28 (0.45) | 3.20 (0.45) |
| Grip strength, kg | 18.01 (3.89) | 18.49 (3.62) | 15.24 (4.65) |
| Gait speed, m/s | 1.32 (0.20) | 1.35 (0.20) | 1.18 (0.17) |
| Chair stand 30 s, times | 15.41 (5.73) | 15.69 (5.41) | 13.80 (7.85) |
| 8-foot up and go, s | 6.45 (1.71) | 6.25 (1.56) | 7.62 (2.23) |
| 2-min step in place, times | 112.47 (14.16) | 112.45 (14.87) | 112.60 (10.33) |
| Comorbidities, yes | |||
| Hypertension | 13 (38.2) | 11 (37.9) | 2 (40.0) |
| Stroke | 3 (8.8) | 2 (6.9) | 1 (20.0) |
| Heart disease | 4 (11.8) | 4 (13.8) | 0 (0.0) |
| Diabetes | 2 (5.9) | 2 (6.9) | 0 (0.0) |
| Dyslipidemia | 8 (23.5) | 7 (24.1) | 1 (20.0) |
| Osteoporosis | 9 (26.5) | 7 (24.1) | 2 (40.0) |
| Chronic pain, yes | |||
| Low back | 5 (14.7) | 5 (17.2) | 0 (0.0) |
| Knee | 10 (29.4) | 9 (31.0) | 1 (20.0) |
| Falls, yes | 13 (38.2) | 10 (34.5) | 3 (60.0) |
| Home-Exercise Barrier Self-Efficacy Scale | 18.15 (3.95) | 18.31 (4.10) | 17.20 (3.11) |
| Social isolation, pts | 13.88 (4.74) | 14.28 (4.80) | 11.60 (4.10) |
| GDS-15 | 3.21 (2.53) | 3.28 (2.70) | 2.80 (1.30) |
| Dietary variety score | 4.91 (2.08) | 4.90 (1.86) | 5.00 (3.39) |
| SF-36 | |||
| PCS | 42.70 (9.07) | 43.34 (7.99) | 38.98 (14.53) |
| MCS | 56.06 (6.57) | 55.10 (6.18) | 61.66 (6.54) |
| RCS | 48.31 (11.11) | 49.79 (10.56) | 39.68 (11.35) |
| Living alone, yes | 14 (41.2) | 11 (37.9) | 3 (60.0) |
| Wearing time, min/day | 947.72 (130.46) | 937.83 (106.61) | 1003.11 (234.37) |
| Step counts, steps/day | 4898.38 (2596.04) | 4906.84 (2666.48) | 4851.06 (2432.86) |
| Light-intensity physical activity, min/day | 348.28 (75.99) | 341.59 (78.50) | 385.74 (50.43) |
| Moderate-to-vigorous intensity, min/day | 36.90 (31.94) | 39.35 (33.60) | 23.17 (16.36) |
| Sedentary time, min/day | 562.54 (130.24) | 556.89 (121.79) | 594.20 (184.68) |
| Baseline to 13 Weeks | |
|---|---|
| Frail phenotype score | −0.40 (−0.73, −0.07) |
| Grip strength, kg | −1.12 (−1.97, −0.27) |
| Gait speed, m/s | 0.25 (0.16, 0.34) |
| Chair stand 30 s, times | 1.67 (0.22, 3.12) |
| 8-foot up and go, s | −0.45 (−0.66, −0.24) |
| 2-min step in place, times | −4.93 (−9.03, −0.84) |
| Home-Exercise Barrier Self-Efficacy Scale | −1.63 (−3.41, 0.14) |
| Social isolation | 0.20 (−0.94, 1.34) |
| GDS-15 | −0.23 (−1.14, 0.67) |
| Dietary variety score | −0.43 (−0.91, 0.04) |
| SF-36 | |
| PCS | 0.89 (−1.51, 3.29) |
| MCS | 0.43 (−1.47, 2.33) |
| RCS | 0.54 (−3.04, 4.12) |
| Baseline to 6 Weeks | Baseline to 13 Weeks | |
|---|---|---|
| Wearing time, min/day | −14 (−32, 4.2) | −23 (−41, −4.9) |
| Step counts, steps/day | 828 (−916, 2572) | 2069 (225, 3913) |
| Mean change per hour wearing time | 41 (−40, 123) | 132 (33, 231) |
| Light-intensity physical activity, min/day | −24 (−38, −10) | −13 (−35, 7.8) |
| Mean change per hour wearing time | −1.20 (−2.2, −0.24) | −0.2 (−1.7, 1.2) |
| Moderate-to-vigorous intensity, min/day | 8 (−7.1, 23) | 11 (−4.2, 27) |
| Mean change per hour wearing time | 0.41 (−0.26, 1.1) | 0.68 (−0.14, 1.5) |
| Sedentary time, min/day | 2 (−18, 23) | −21 (−50, 8.8) |
| Mean change per hour wearing time | 0.80 (−0.25, 1.8) | −0.5 (−2.1, 1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, T.; Osuka, Y.; Shida, T.; Daimaru, K.; Kojima, N.; Maruo, K.; Iizuka, A.; Kitago, M.; Fujiwara, Y.; Sasai, H. Feasibility, Acceptability, and Potential Efficacy of a Mobile Health Application for Community-Dwelling Older Adults with Frailty and Pre-Frailty: A Pilot Study. Nutrients 2024, 16, 1181. https://doi.org/10.3390/nu16081181
Ohta T, Osuka Y, Shida T, Daimaru K, Kojima N, Maruo K, Iizuka A, Kitago M, Fujiwara Y, Sasai H. Feasibility, Acceptability, and Potential Efficacy of a Mobile Health Application for Community-Dwelling Older Adults with Frailty and Pre-Frailty: A Pilot Study. Nutrients. 2024; 16(8):1181. https://doi.org/10.3390/nu16081181
Chicago/Turabian StyleOhta, Takahisa, Yosuke Osuka, Takashi Shida, Kaori Daimaru, Narumi Kojima, Kazushi Maruo, Ai Iizuka, Moe Kitago, Yoshinori Fujiwara, and Hiroyuki Sasai. 2024. "Feasibility, Acceptability, and Potential Efficacy of a Mobile Health Application for Community-Dwelling Older Adults with Frailty and Pre-Frailty: A Pilot Study" Nutrients 16, no. 8: 1181. https://doi.org/10.3390/nu16081181
APA StyleOhta, T., Osuka, Y., Shida, T., Daimaru, K., Kojima, N., Maruo, K., Iizuka, A., Kitago, M., Fujiwara, Y., & Sasai, H. (2024). Feasibility, Acceptability, and Potential Efficacy of a Mobile Health Application for Community-Dwelling Older Adults with Frailty and Pre-Frailty: A Pilot Study. Nutrients, 16(8), 1181. https://doi.org/10.3390/nu16081181