Differential Effects of n-3 and n-6 Polyunsaturated Fatty Acids on Placental and Embryonic Growth and Development in Diabetic Pregnant Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
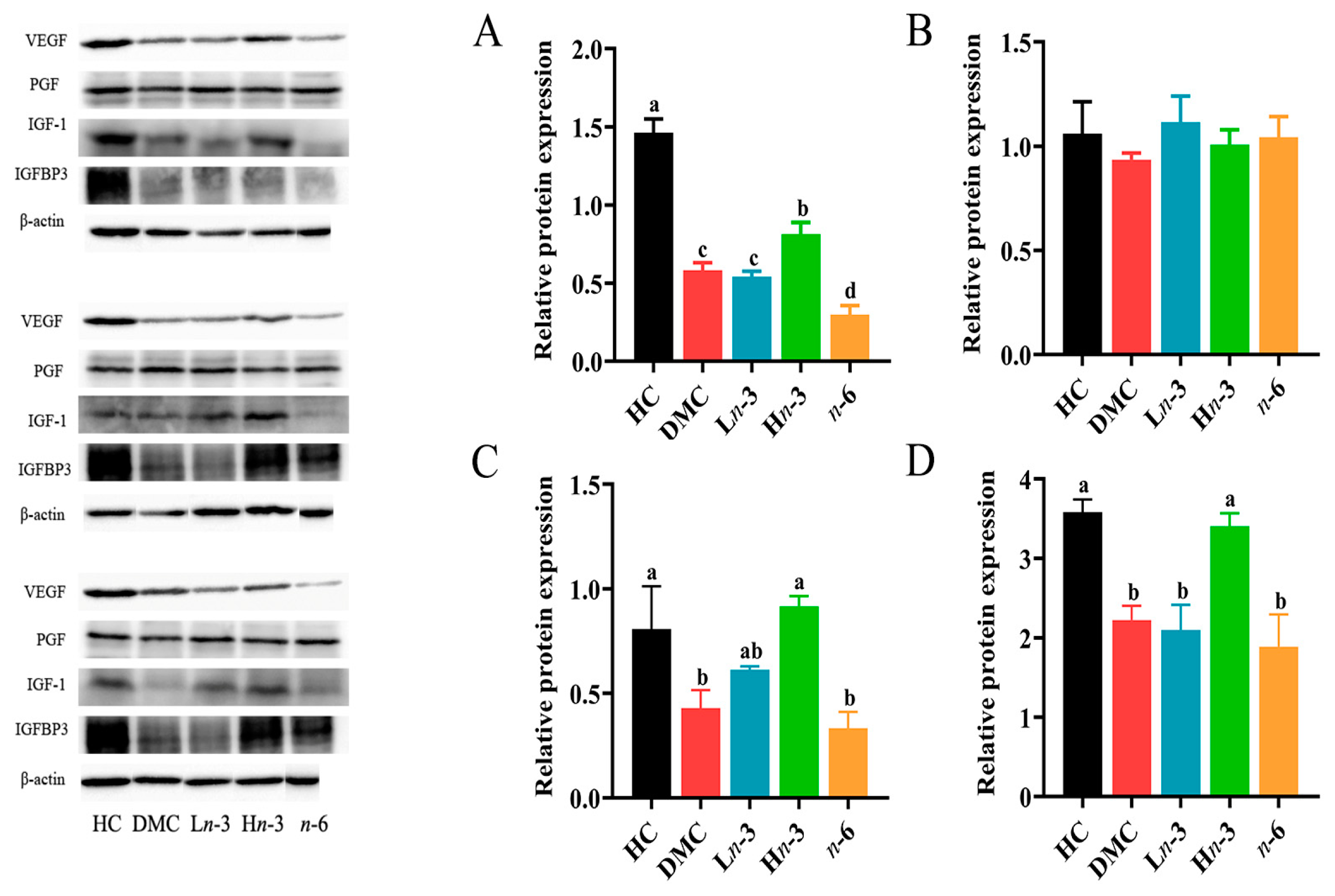
2.2. Western Blotting Analysis
2.3. Embryonic Metabolomic Analysis
2.3.1. Embryo Sample Preparation
2.3.2. LC/MS Analysis
2.3.3. Data Preprocessing
2.4. Statistical Analysis
3. Results
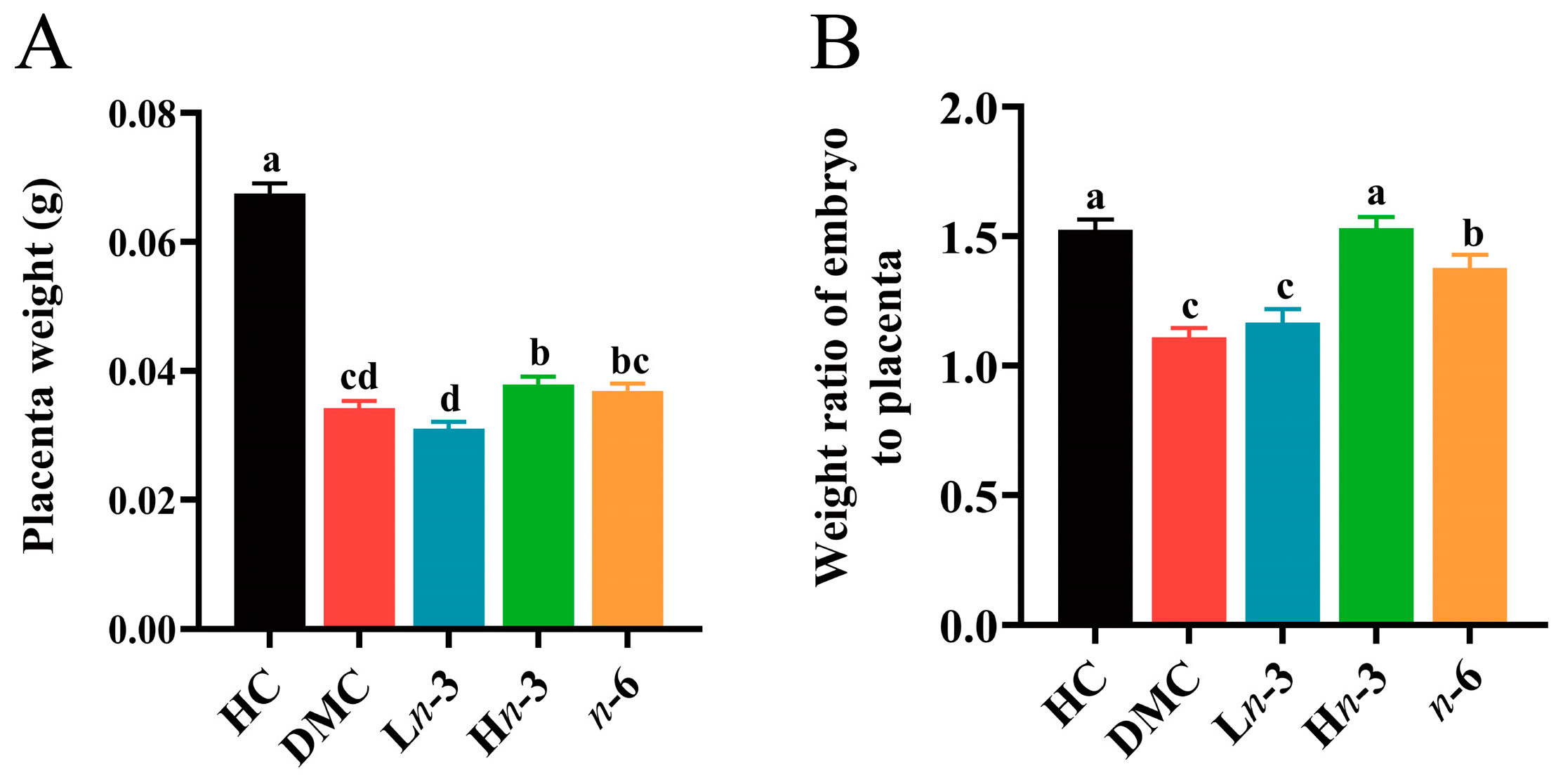
3.1. Effect of Polyunsaturated Fatty Acids on Embryonic and Placental Growth
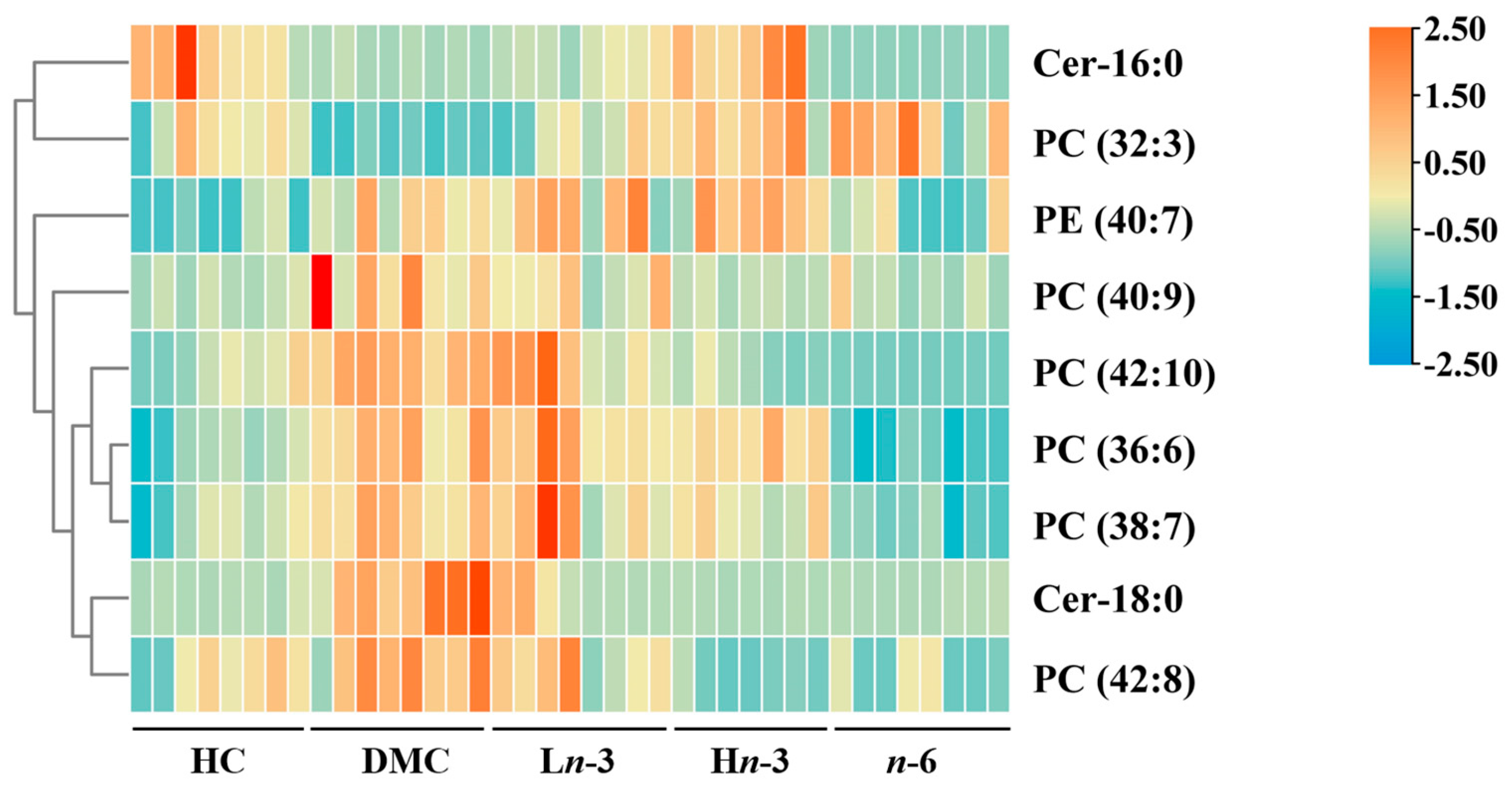
3.2. Effects of Polyunsaturated Fatty Acids on Embryonic Metabolites
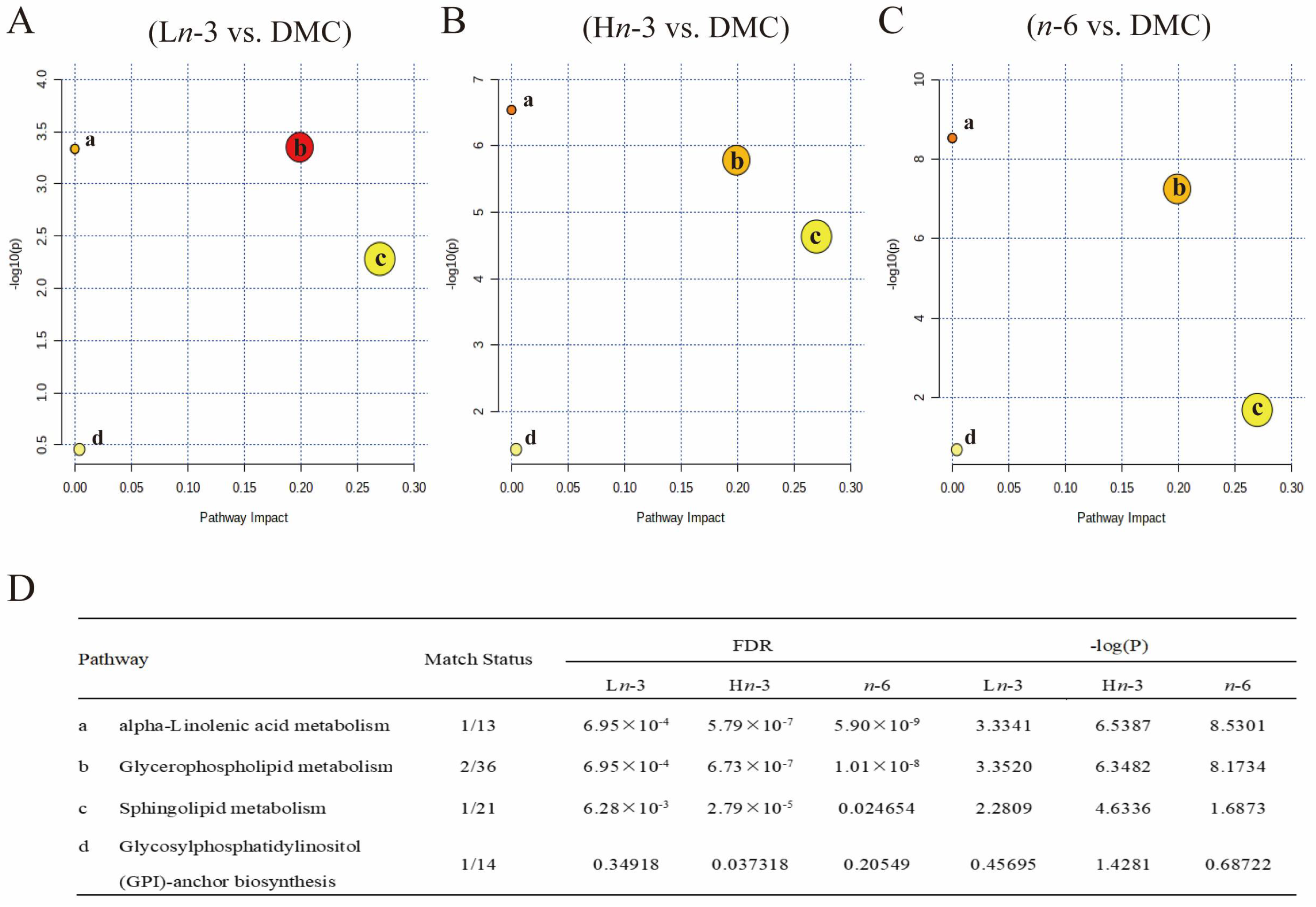
3.3. Metabolic Pathway Analysis
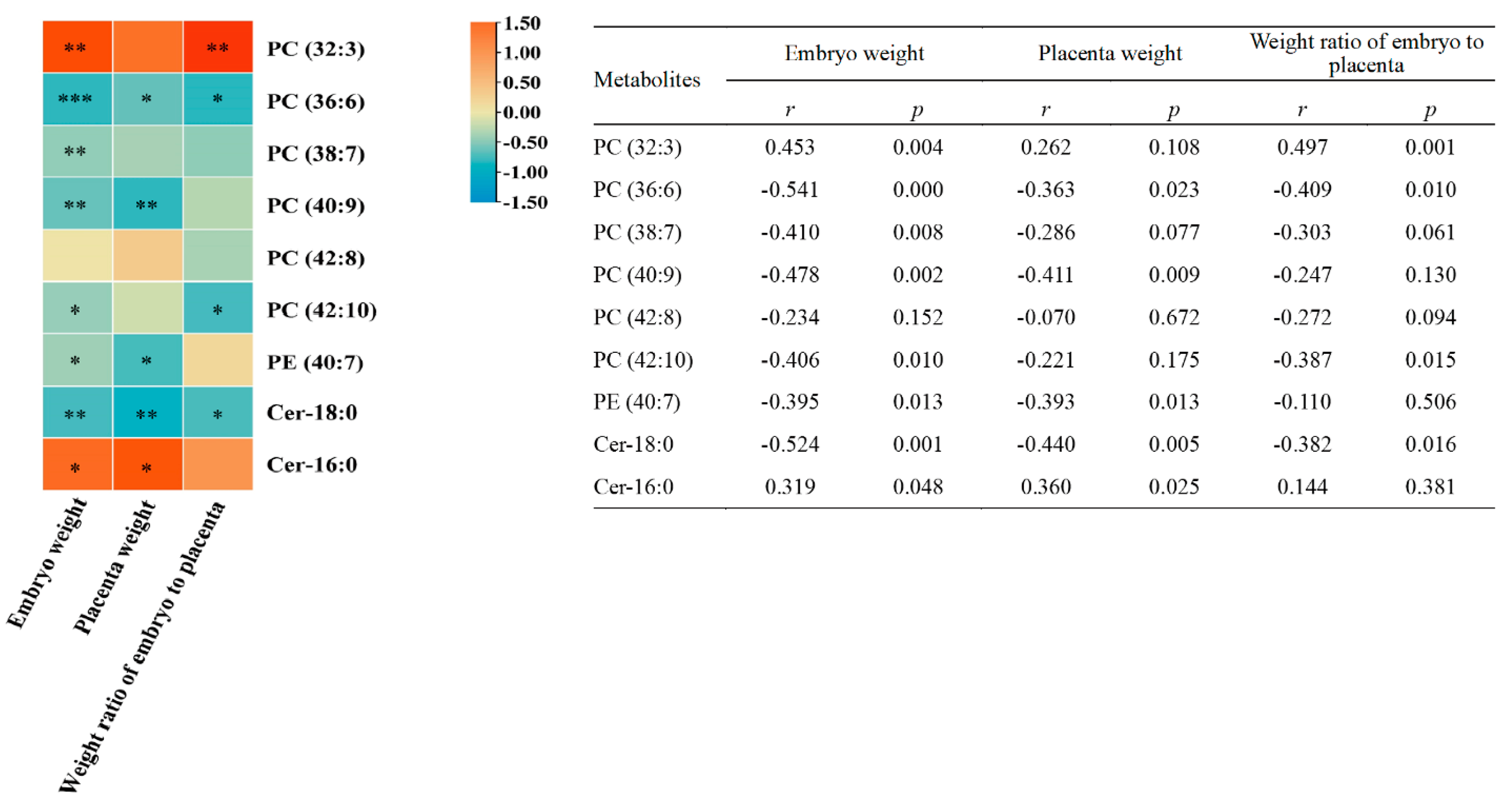
3.4. Correlation Analysis of Embryonic Metabolites with Embryo Weight, Placenta Weight, and Weight Ratio of Embryo to Placenta
3.5. Effect of Polyunsaturated Fatty Acids on Placental Growth Factors
3.6. Correlation Analysis of Placental Growth Factors with Embryo Weight, Placenta Weight, and Weight Ratio of Embryo to Placenta
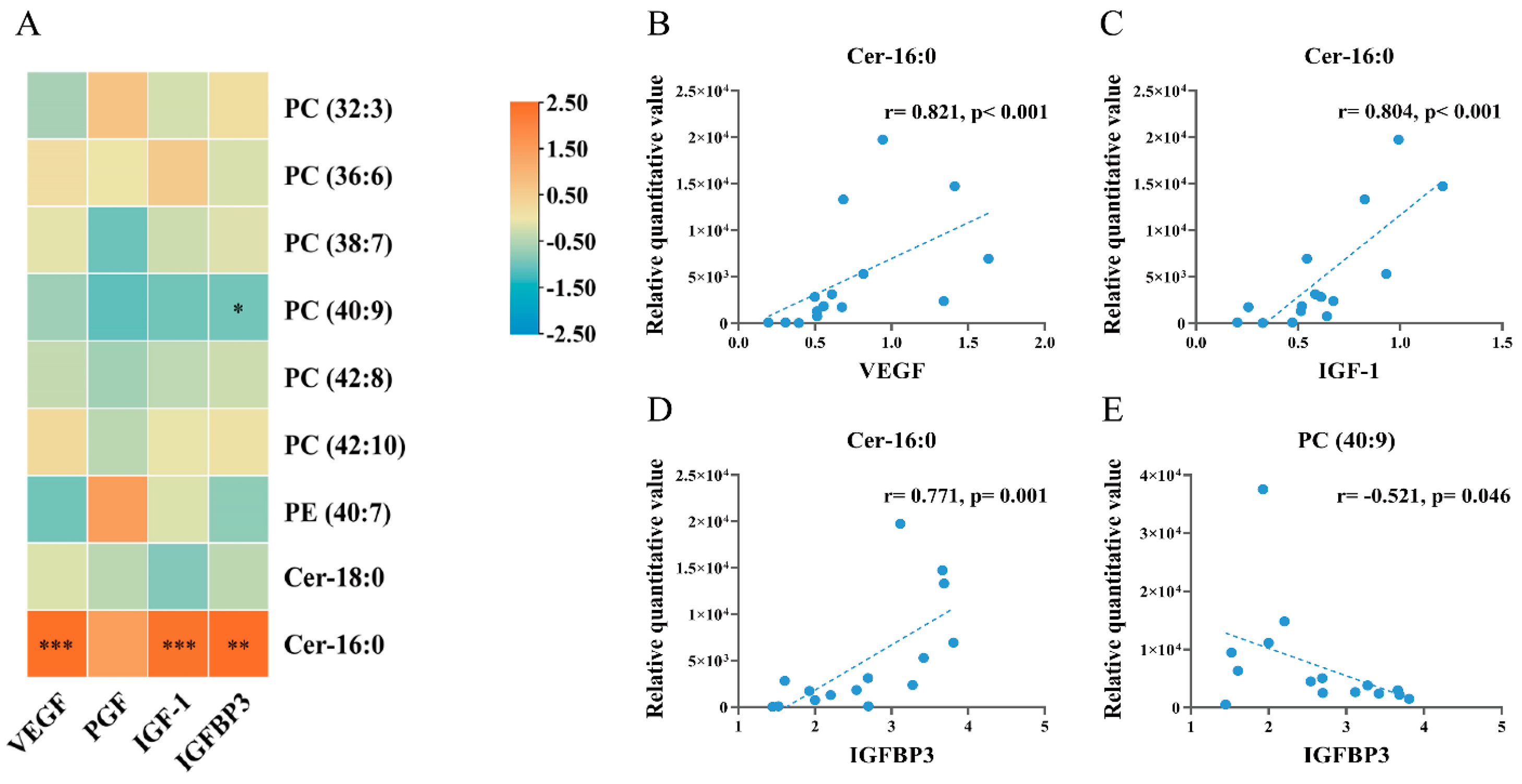
3.7. Correlation Analysis of Embryo Differential Metabolites with Placental Growth Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 6. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 25, 2021–067946. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, Y.; Zhu, S.; Shao, X.; Li, H.; Kuang, X.; Li, S.; Guo, X.F.; Li, D. N-3 polyunsaturated fatty acids effectively protect against neural tube defects in diabetic mice induced by streptozotocin. Food Funct. 2021, 12, 9188–9196. [Google Scholar] [CrossRef] [PubMed]
- Giabicani, E.; Pham, A.; Brioude, F.; Mitanchez, D.; Netchine, I. Diagnosis and management of postnatal fetal growth restriction. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 3, 50–58. [Google Scholar] [CrossRef]
- Innis, S.M. Palmitic Acid in Early Human Development. Crit. Rev. Food Sci. Nutr. 2016, 56, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579. [Google Scholar] [CrossRef]
- Cetin, I.; Giovannini, N.; Alvino, G.; Agostoni, C.; Riva, E.; Giovannini, M.; Pardi, G. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatr. Res. 2002, 52, 750–755. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Englund-Ögge, L.; Haugen, M.; Birgisdottir, B.E.; Knutsen, H.K.; Sengpiel, V.; Myhre, R.; Alexander, J.; Nilsen, R.M.; Jacobsson, B.; et al. Maternal intake of seafood and supplementary long chain n-3 poly-unsaturated fatty acids and preterm delivery. BMC Pregnancy Childbirth 2017, 17, 41. [Google Scholar] [CrossRef]
- Carlson, S.E.; Werkman, S.H.; Peeples, J.M.; Cooke, R.J.; Tolley, E.A. Arachidonic acid status correlates with first year growth in preterm infants. Proc. Natl. Acad. Sci. USA 1993, 90, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Simões, R.V.; Paules, C.; Cañueto, D.; Pardo-Cea, M.A.; García-Martín, M.L.; Crovetto, F.; Fuertes-Martin, R.; Domenech, M.; Gómez-Roig, M.D.; et al. Metabolic profiling and targeted lipidomics reveals a disturbed lipid profile in mothers and fetuses with intrauterine growth restriction. Sci. Rep. 2018, 8, 13614. [Google Scholar] [CrossRef] [PubMed]
- Karaer, A.; Mumcu, A.; Arda Düz, S.; Tuncay, G.; Doğan, B. Metabolomics analysis of placental tissue obtained from patients with fetal growth restriction. J. Obstet. Gynaecol. Res. 2022, 48, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, S.; Yinon, Y.; Xu, J.; Ermini, L.; Sallais, J.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Aberrant TGFβ Signalling Contributes to Dysregulation of Sphingolipid Metabolism in Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2015, 100, E986–E996. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Hamilton, J.A. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Owens, J.A.; Pringle, K.G.; Roberts, C.T. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J. Physiol. 2011, 589, 7–20. [Google Scholar] [CrossRef]
- Rajiv, P.; Cade, T.; Dean, J.; Jones, G.D.; Brennecke, S.P. Maternal serum soluble fms-like tyrosine kinase-1-to-placental growth factor ratio distinguishes growth-restricted from non-growth-restricted small-for-gestational-age fetuses. AJOG Glob. Rep. 2024, 4, 100302. [Google Scholar] [CrossRef]
- Ostlund, E.; Bang, P.; Hagenäs, L.; Fried, G. Insulin-like growth factor I in fetal serum obtained by cordocentesis is correlated with intrauterine growth retardation. Hum. Reprod. 1997, 12, 840–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nithya, M.N.; Krishnappa, J.; Sheela, S.R.; Raavi, V. The Role of Insulin-like Growth Factor-Axis and Mitotic Index in South Indian Neonates with Small for Gestational Age. Fetal Pediatr. Pathol. 2023, 42, 216–226. [Google Scholar]
- Pei, J.; Li, Y.; Min, Z.; Dong, Q.; Ruan, J.; Wu, J.; Hua, X. MiR-590-3p and its targets VEGF, PIGF, and MMP9 in early, middle, and late pregnancy: Their longitudinal changes and correlations with risk of fetal growth restriction. Ir. J. Med. Sci. 2022, 191, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Ferrari, S.L. Effects of long-term supplementation with omega-3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J. Nutr. Biochem. 2011, 22, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-K.; Zhou, Y.; Jiang, S.; Tao, Y.-X.; Sun, H.; Peng, J.; Jiang, S. Feeding a DHA-enriched diet increases skeletal muscle protein synthesis in growing pigs: Association with increased skeletal muscle insulin action and local mRNA expression of insulin-like growth factor 1. Br. J. Nutr. 2013, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, G.M.; Basak, S.; Weedon-Fekjær, M.S.; Staff, A.C.; Duttaroy, A.K. Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta 2011, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.N.; Lonergan, P.; Diskin, M.G.; Pierce, K.M.; Kelly, A.K.; Stanton, C.; Waters, S.M.; Parr, M.H.; Kenny, D.A. Effect of dietary n-3 polyunsaturated fatty acid supplementation and post-insemination plane of nutrition on systemic concentrations of metabolic analytes, progesterone, hepatic gene expression and embryo development and survival in beef heifers. Theriogenology 2019, 127, 102–113. [Google Scholar] [CrossRef]
- Gaccioli, F.; Lager, S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front. Physiol. 2016, 7, 40. [Google Scholar] [CrossRef]
- Charnock-Jones, D.S.; Kaufmann, P.; Mayhew, T.M. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta 2004, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.H.; de Vrijer, B.; Galan, H.L.; Davidsen, M.L.; Trembler, K.A.; Battaglia, F.C.; Wilkening, R.B.; Anthony, R.V. The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J. Physiol. 2003, 550, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Lagerlöf, O.; Wennergren, M.; Powell, T.L.; Jansson, T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am. J. Physiol. Cell Physiol. 2009, 297, C723–C731. [Google Scholar] [CrossRef] [PubMed]
- Chacińska, M.; Zabielski, P.; Książek, M.; Szałaj, P.; Jarząbek, K.; Kojta, I.; Chabowski, A.; Błachnio-Zabielska, A.U. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.-E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared with Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Godhamgaonkar, A.A.; Wadhwani, N.S.; Joshi, S.R. Exploring the role of LC-PUFA metabolism in pregnancy complications. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102203. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.L.; Innis, S.M. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am. J. Clin. Nutr. 2001, 73, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Mohrhauer, H.; Holman, R.T. The effect of dose level of essential fatty acids upon fatty acid composition of the rat liver. J. Lipid Res. 1963, 4, 151–159. [Google Scholar] [CrossRef]
- Koletzko, B.; Braun, M. Arachidonic acid and early human growth: Is there a relation? Ann. Nutr. Metab. 1991, 35, 128–131. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Nie, P.; Zhong, L.; Feng, L.; Guan, Q.; Song, L. Changes in the serum metabolomic profiles of subjects with NAFLD in response to n-3 PUFAs and phytosterol ester: A double-blind randomized controlled trial. Food Funct. 2022, 13, 5189–5201. [Google Scholar] [CrossRef]
- Naoe, S.; Tsugawa, H.; Takahashi, M.; Ikeda, K.; Arita, M. Characterization of Lipid Profiles after Dietary Intake of Polyunsaturated Fatty Acids Using Integrated Untargeted and Targeted Lipidomics. Metabolites 2019, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Taltavull, N.; Ras, R.; Marine, S.; Romeu, M.; Giralt, M.; Mendez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L.; Nogues, M.R. Protective effects of fish oil on pre-diabetes: A lipidomic analysis of liver ceramides in rats. Food Funct. 2016, 7, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; McKenzie, J.H.; Wang, B.; Strassburg, K.; Doneanu, A.; Johnson, J.; Baker, A.; Hankemeier, T.; Murphy, J.; Vreeken, R.J.; et al. A protective lipidomic biosignature associated with a balanced omega-6/omega-3 ratio in fat-1 transgenic mice. PLoS ONE 2014, 9, e96221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, Y.; Lin, Z.; Chen, R.; Niu, Y.; Kan, H. Mechanistic insights into the health benefits of fish-oil supplementation against fine particulate matter air pollution: A randomized controlled trial. Environ. Health 2022, 21, 104. [Google Scholar] [CrossRef]
- Austdal, M.; Thomsen, L.C.V.; Tangerås, L.H.; Skei, B.; Mathew, S.; Bjørge, L.; Austgulen, R.; Bathen, T.F.; Iversen, A.-C. Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta 2015, 36, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Gu, J.; Guo, X.; Gao, T.; Li, D. The protective effect of polyunsaturated fatty acid intake during pregnancy against embryotoxicity of sodium valproate in mice. Food Funct. 2018, 9, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Wahabi, H.; Elmorshedy, H.; Amer, Y.S.; Saeed, E.; Razak, A.; Hamama, I.A.; Hadid, A.; Ahmed, S.; Aleban, S.A.; Aldawish, R.A.; et al. Neonatal Birthweight Spectrum: Maternal Risk Factors and Pregnancy Outcomes in Saudi Arabia. Medicina 2024, 60, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hakvoort, T.B.M.; Ruijter, J.M.; Jongejan, A.; Koster, J.; Swagemakers, S.M.A.; Sokolovic, A.; Lamers, W.H. Maternal diabetes causes developmental delay and death in early-somite mouse embryos. Sci. Rep. 2017, 7, 11714. [Google Scholar] [CrossRef]
- Hod, M.; Kapur, A.; McIntyre, H.D. Evidence in support of the International Association of Diabetes in Pregnancy study groups’ criteria for diagnosing gestational diabetes mellitus worldwide in 2019. Am. J. Obstet. Gynecol. 2019, 221, 109–116. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Molsted-Pedersen, L. Early fetal growth delay detected by ultrasound marks increased risk of congenital malformation in diabetic pregnancy. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Berezowsky, A.; Ardestani, S.; Hiersch, L.; Shah, B.R.; Berger, H.; Halperin, I.; Retnakaran, R.; Barrett, J.; Melamed, N. Glycemic control and neonatal outcomes in twin pregnancies with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2023, 229, 29. [Google Scholar] [CrossRef] [PubMed]
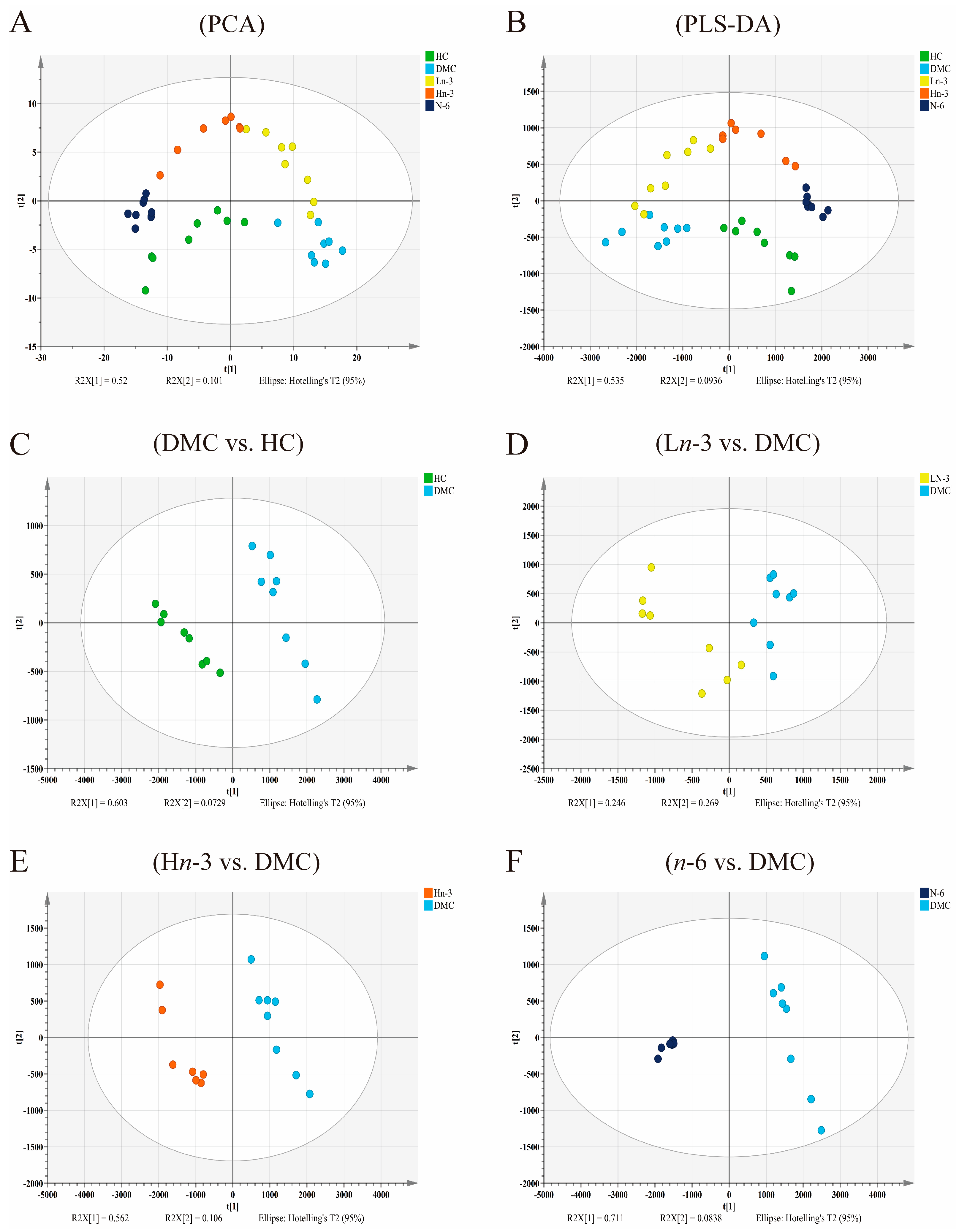
| R2Y | Q2Y | |
|---|---|---|
| DMC vs. HC | 0.92 | 0.88 |
| Ln-3 vs. DMC | 0.91 | 0.68 |
| Hn-3 vs. DMC | 0.96 | 0.95 |
| n-6 vs. DMC | 0.98 | 0.98 |
| Lipid Class | Compound | m/z | Formula | Changing Trend | ||||
|---|---|---|---|---|---|---|---|---|
| DMC vs. HC | Ln-3 vs. DMC | Hn-3 vs. DMC | n-6 vs. DMC | Hn-3 vs. n-6 | ||||
| Glycerophospholipids | ||||||||
| Phosphatidylcholine | ||||||||
| PC (32:3) | 728.5178 | C40H74NO8P | ↓ ** | ↑ * | ↑ *** | ↑ *** | - | |
| PC (36:6) | 778.5412 | C44H76NO8P | ↑ *** | - | - | ↓ *** | ↑ ### | |
| PC (38:7) | 804.5558 | C46H78NO8P | ↑ ** | - | - | ↓ *** | ↑ # | |
| PC (40:9) | 828.5541 | C48H78NO8P | ↑ ** | ↓ * | ↓ ** | ↓ ** | - | |
| PC (42:8) | 858.5974 | C50H84NO8P | ↑ ** | - | ↓ *** | ↓ *** | - | |
| PC (42:10) | 854.5692 | C50H80NO8P | ↑ *** | - | ↓ *** | ↓ *** | - | |
| Phosphatidylethanolamine | PE (40:7) | 790.554 | C45H76NO8P | ↑ ** | - | - | ↓ * | ↑ # |
| Sphingolipids | Cer-18:0 | 566.5499 | C36H71NO3 | ↑ *** | ↓ *** | ↓ *** | ↓ *** | - |
| Cer-16:0 | 538.5176 | C34H67NO3 | ↓ *** | - | ↑ *** | - | ↑ ### | |
| Parameters | Embryo Weight | Placenta Weight | Weight Ratio of Embryo to Placenta | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| VEGF (relative protein level) | 0.579 | 0.024 | 0.686 | 0.005 | 0.039 | 0.889 |
| PGF (relative protein level) | 0.036 | 0.899 | −0.207 | 0.459 | 0.018 | 0.950 |
| IGF-1 (relative protein level) | 0.236 | 0.398 | 0.346 | 0.206 | −0.143 | 0.612 |
| IGFBP3 (relative protein level) | 0.646 | 0.009 | 0.800 | <0.001 | 0.089 | 0.752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, C.; Liu, S.; Shi, Y.; Kuang, X.; Song, X.; Li, D.; Li, K. Differential Effects of n-3 and n-6 Polyunsaturated Fatty Acids on Placental and Embryonic Growth and Development in Diabetic Pregnant Mice. Nutrients 2024, 16, 1182. https://doi.org/10.3390/nu16081182
Li H, Chen C, Liu S, Shi Y, Kuang X, Song X, Li D, Li K. Differential Effects of n-3 and n-6 Polyunsaturated Fatty Acids on Placental and Embryonic Growth and Development in Diabetic Pregnant Mice. Nutrients. 2024; 16(8):1182. https://doi.org/10.3390/nu16081182
Chicago/Turabian StyleLi, Huiying, Chuanjing Chen, Shiyi Liu, Yan Shi, Xiaotong Kuang, Xiaolei Song, Duo Li, and Kelei Li. 2024. "Differential Effects of n-3 and n-6 Polyunsaturated Fatty Acids on Placental and Embryonic Growth and Development in Diabetic Pregnant Mice" Nutrients 16, no. 8: 1182. https://doi.org/10.3390/nu16081182
APA StyleLi, H., Chen, C., Liu, S., Shi, Y., Kuang, X., Song, X., Li, D., & Li, K. (2024). Differential Effects of n-3 and n-6 Polyunsaturated Fatty Acids on Placental and Embryonic Growth and Development in Diabetic Pregnant Mice. Nutrients, 16(8), 1182. https://doi.org/10.3390/nu16081182






