Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women
Abstract
1. Introduction
2. Methods
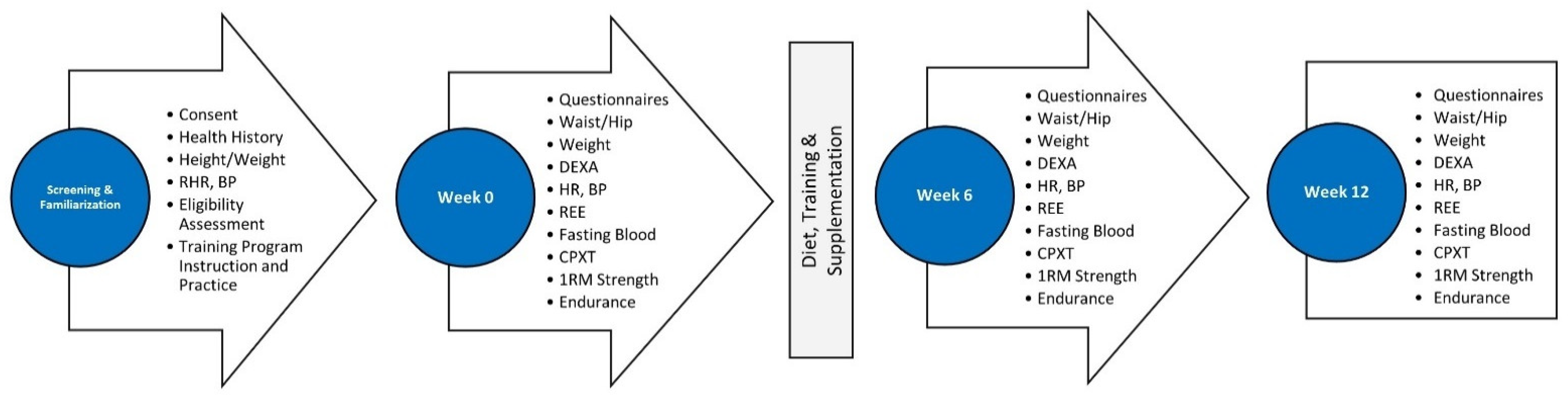
2.1. Research Design
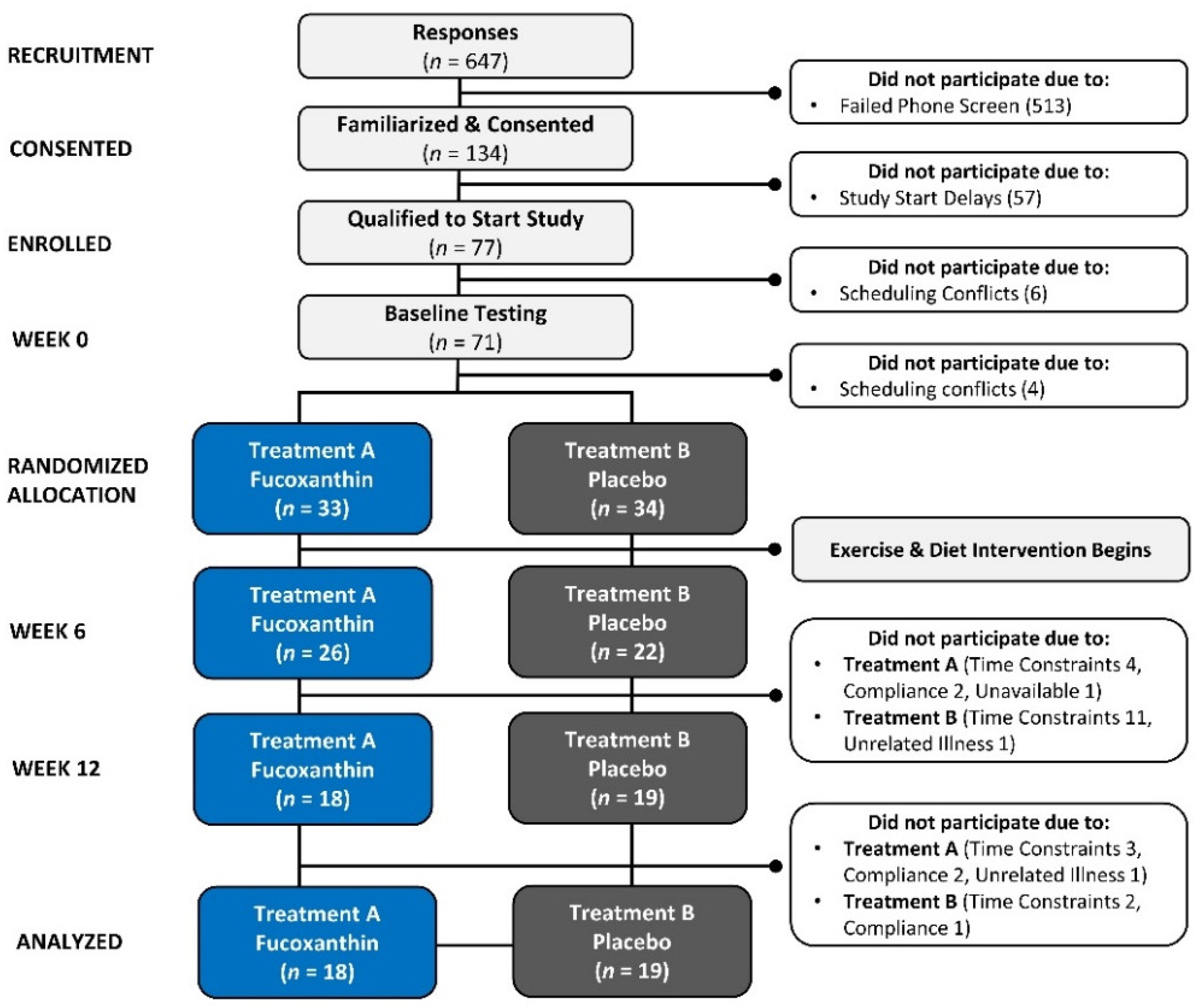
2.2. Study Participants
2.3. Testing Protocol
2.4. Familiarization
2.5. Randomization
2.6. Training Intervention
2.7. Diet Intervention
2.8. Supplementation Protocol
3. Procedures
3.1. Diet Assessment
3.2. Anthropometrics and Hemodynamics
3.3. Body Composition
3.4. Resting Energy Expenditure Assessment
3.5. Exercise Assessment
3.6. Blood Collection and Analysis
3.7. Quality of Life
3.8. Side Effects
3.9. Statistical Analysis
4. Results
4.1. Participant Demographics
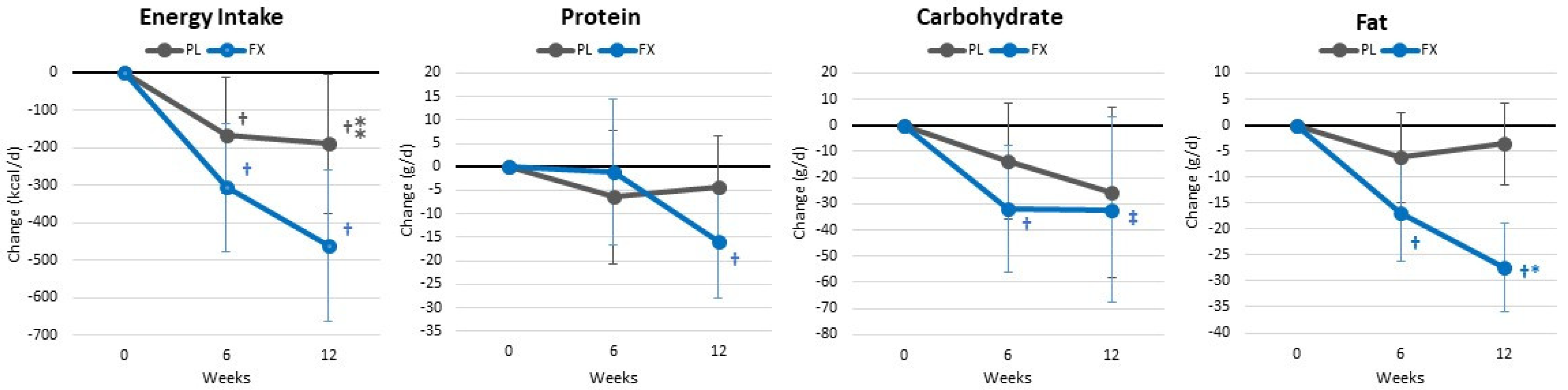
4.2. Energy and Macronutrient Intake
4.3. Training Volume
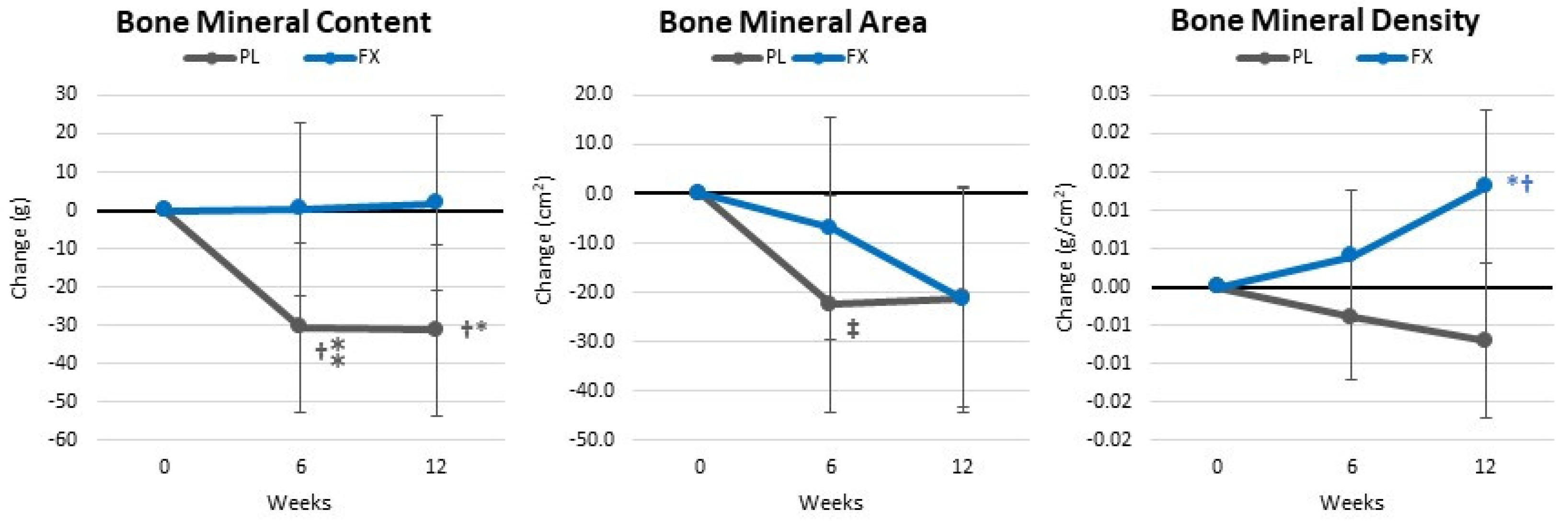
4.4. Body Composition and Anthropometric Measures
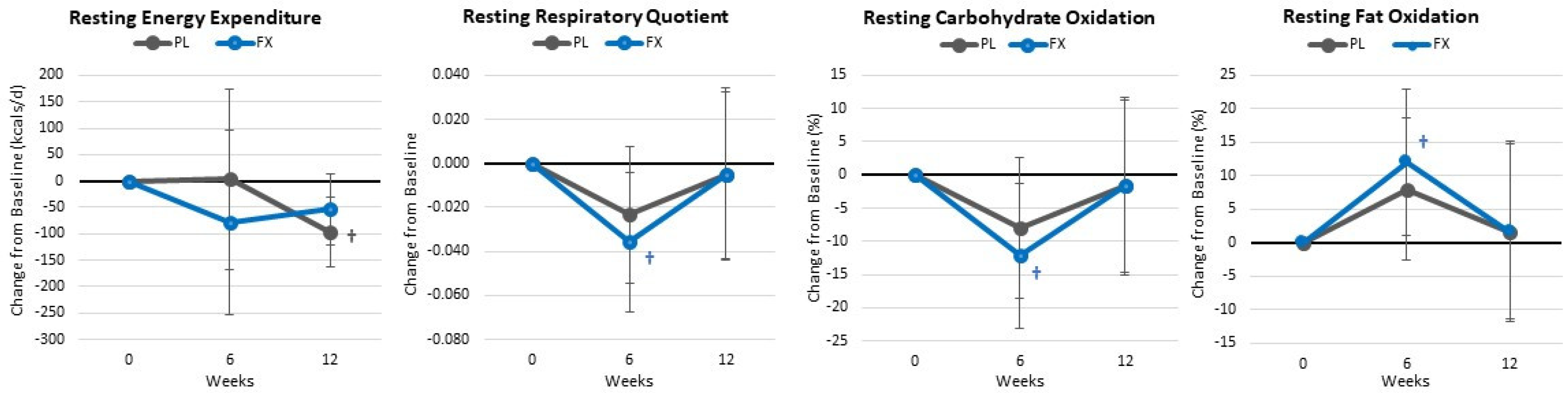
4.5. Resting Energy Expenditure and Metabolism
4.6. Exercise and Functional Capacity Assessment
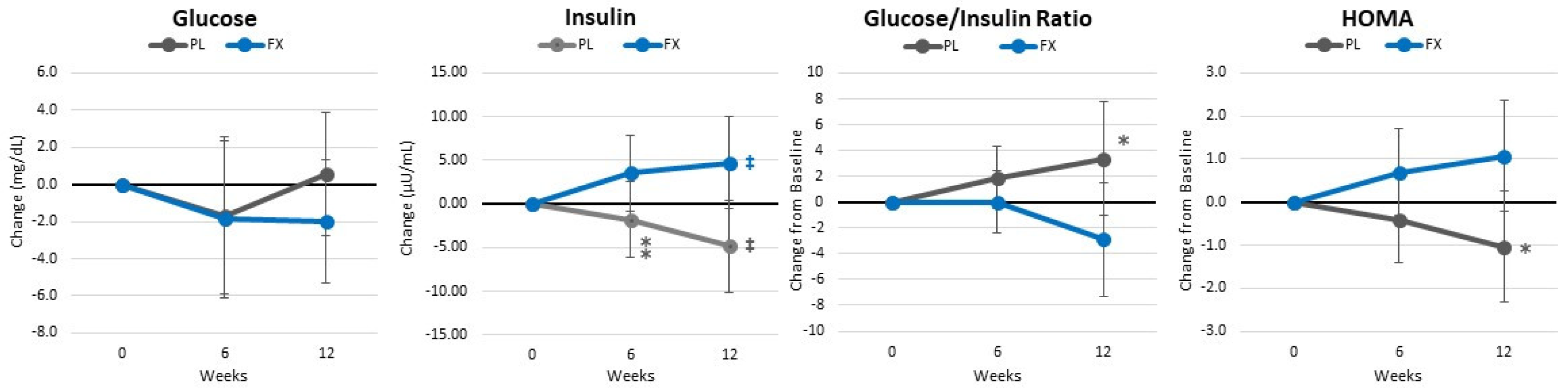
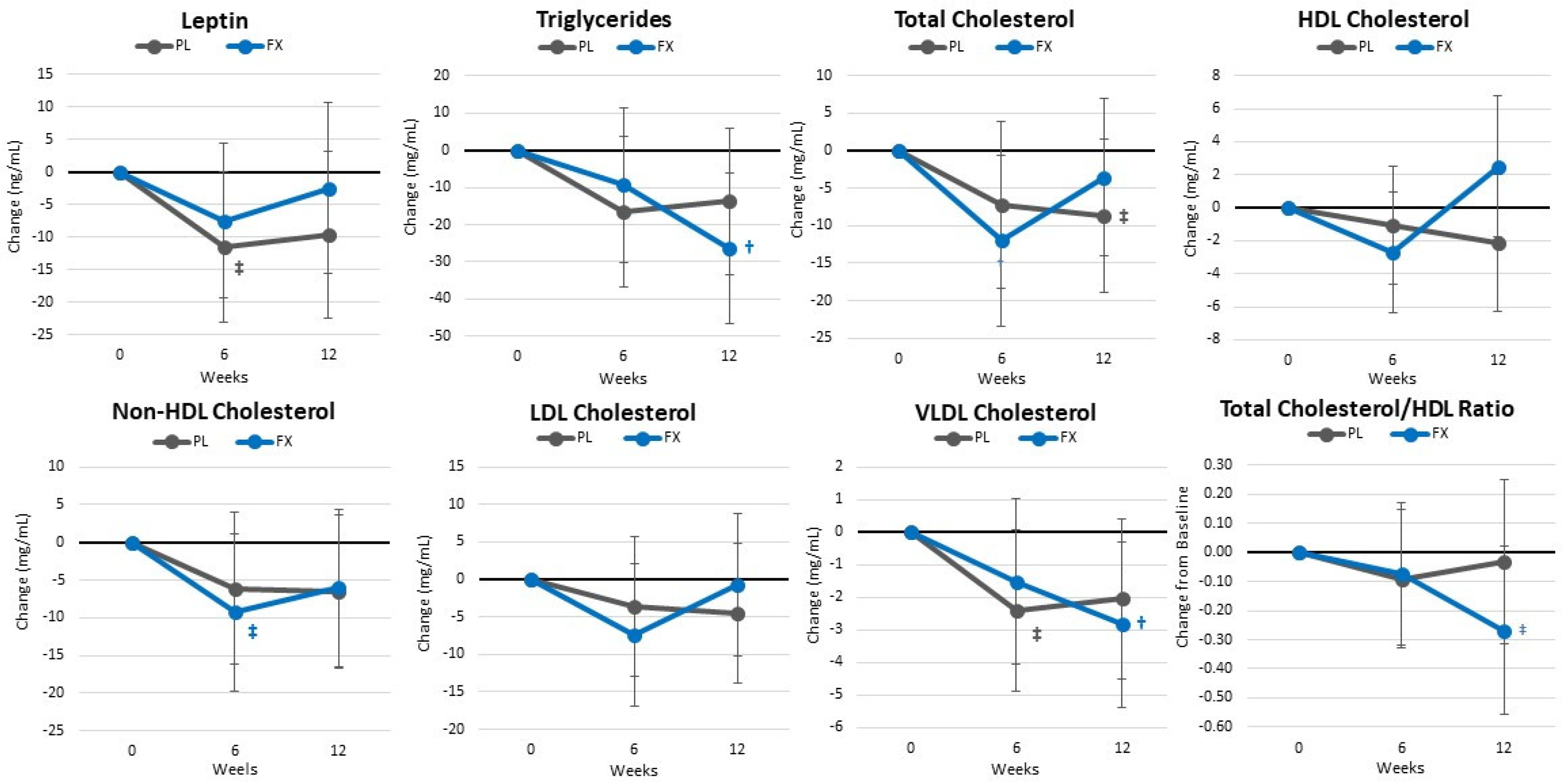
4.7. Blood Sample Analysis
4.8. Quality of Life
4.9. Resting Heart Rate and Blood Pressure
4.10. Side Effects
5. Discussion
5.1. Primary Outcomes
5.2. Secondary Outcomes
5.3. Limitation Considerations
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Fallahzadeh, M.K.; Hegazi, R.M. Nutritional Deficiencies and Sarcopenia in Heart Failure: A Therapeutic Opportunity to Reduce Hospitalization and Death. Rev. Cardiovasc. Med. 2016, 17 (Suppl. 1), S30–S39. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Jeon, B.H.; Reid Storm, S.N.; Jho, S.; No, J.K. The most effective factors to offset sarcopenia and obesity in the older Korean: Physical activity, vitamin D, and protein intake. Nutrition 2017, 33, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.; Beckham, J.; Campbell, B.; Harvey, T.; Galbreath, M.; La Bounty, P.; Nassar, E.; Wismann, J.; Kreider, R. Obesity: Prevalence, theories, medical consequences, management, and research directions. J. Int. Soc. Sports Nutr. 2005, 2, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kanter, R.; Caballero, B. Global gender disparities in obesity: A review. Adv. Nutr. 2012, 3, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewska, M.; Pikala, M.; Kaczmarczyk-Chalas, K.; Piwonnska, A.; Tykarski, A.; Kozakiewicz, K.; Pajak, A.; Zdrojewski, T.; Drygas, W. Smoking status, the menopausal transition, and metabolic syndrome in women. Menopause 2012, 19, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Polotsky, H.N.; Polotsky, A.J. Metabolic implications of menopause. Semin. Reprod. Med. 2010, 28, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Song, F.; Song, Y.; Dai, H. Ages at menarche and menopause, and mortality among postmenopausal women. Maturitas 2019, 130, 50–56. [Google Scholar] [CrossRef]
- Lockard, B.; Mardock, M.; Oliver, J.M.; Byrd, M.; Simbo, S.; Jagim, A.R.; Kresta, J.; Baetge, C.C.; Jung, Y.P.; Koozehchian, M.S.; et al. Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women. Int. J. Environ. Res. Public Health 2022, 19, 4877. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P.; Behavioural Weight Management Review, G. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Gillison, F.; Stathi, A.; Reddy, P.; Perry, R.; Taylor, G.; Bennett, P.; Dunbar, J.; Greaves, C. Processes of behavior change and weight loss in a theory-based weight loss intervention program: A test of the process model for lifestyle behavior change. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 2. [Google Scholar] [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Arent, S.M.; Walker, A.J.; Pellegrino, J.K.; Sanders, D.J.; McFadden, B.A.; Ziegenfuss, T.N.; Lopez, H.L. The Combined Effects of Exercise, Diet, and a Multi-Ingredient Dietary Supplement on Body Composition and Adipokine Changes in Overweight Adults. J. Am. Coll. Nutr. 2018, 37, 111–120. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.A.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Hernández-Torres, R.P.; Wall-Medrano, A.; Ramos-Jiménez, A. Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program on the Body Composition and Cardiorespiratory Fitness of Overweight or Obese Subjects: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs 2018, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Li, Y.; Li, C.; Li, D. Natural products and body weight control. N. Am. J. Med. Sci. 2011, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef]
- Muradian, K.; Vaiserman, A.; Min, K.J.; Fraifeld, V.E. Fucoxanthin and lipid metabolism: A minireview. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Zheng, J.W.; Manabe, Y.; Hirata, T.; Sugawara, T. Anti-Obesity Properties of the Dietary Green Alga, Codium cylindricum, in High-Fat Diet-Induced Obese Mice. J. Nutr. Sci. Vitaminol. 2018, 64, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Dordevic, A.L.; Cox, K.H.M.; Scholey, A.; Ryan, L.; Bonham, M.P. Study protocol for a double-blind randomised controlled trial investigating the impact of 12 weeks supplementation with a Fucus vesiculosus extract on cholesterol levels in adults with elevated fasting LDL cholesterol who are overweight or have obesity. BMJ Open 2018, 8, e022195. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Seo, Y.J.; Pan, C.H.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Fucoxanthin Suppresses Lipid Accumulation and ROS Production During Differentiation in 3T3-L1 Adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef]
- Woo, M.-N.; Jeon, S.-M.; Shin, Y.C.; Lee, M.-K.; Kang, M.A.; Choi, M.-S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Bermano, G.; Stoyanova, T.; Hennequart, F.; Wainwright, C.L. Seaweed-derived bioactives as potential energy regulators in obesity and type 2 diabetes. Adv. Pharmacol. 2020, 87, 205–256. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Human. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Maury, J.; Delbrut, A.; Villard, V.; Pradelles, R. A Standardized Extract of Microalgae Phaeodactylum tricornutum (Mi136) Inhibit D-Gal Induced Cognitive Dysfunction in Mice. Mar. Drugs 2024, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen™ in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hitoe, S.; Shimoda, H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Funct. Foods Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Liguori, G. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: New York, NY, USA, 2020. [Google Scholar]
- Kerksick, C.; Thomas, A.; Campbell, B.; Taylor, L.; Wilborn, C.; Marcello, B.; Roberts, M.; Pfau, E.; Grimstvedt, M.; Opusunju, J.; et al. Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr. Metab. 2009, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.W.; Morgan, N.; Ward, D.; Tangney, C.; Alshurafa, N.; Van Horn, L.; Spring, B. Comparative Validity of Mostly Unprocessed and Minimally Processed Food Items Differs Among Popular Commercial Nutrition Apps Compared with a Research Food Database. J. Acad. Nutr. Diet. 2022, 122, 825–832.e821. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am. J. Epidemiol. 2002, 156, 78–85. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Lohman, T.G.; Harris, M.; Teixeira, P.J.; Weiss, L. Assessing body composition and changes in body composition. Another look at dual-energy X-ray absorptiometry. Ann. N. Y. Acad. Sci. 2000, 904, 45–54. [Google Scholar] [CrossRef]
- Klesges, R.C.; Ward, K.D.; Shelton, M.L.; Applegate, W.B.; Cantler, E.D.; Palmieri, G.M.; Harmon, K.; Davis, J. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA 1996, 276, 226–230. [Google Scholar] [CrossRef]
- Almada, A.; Kreider, R.; Ransom, J.; Rasmussen, C. Comparison of the reliability of repeated whole body dexa scans to repeated spine and hip scans. J. Bone Miner. Res. 1999, 14, S369. [Google Scholar]
- Matarese, L.E. Indirect calorimetry: Technical aspects. J. Am. Diet. Assoc. 1997, 97, S154–S160. [Google Scholar] [CrossRef]
- Feurer, I.D.; Crosby, L.O.; Mullen, J. Measured and predicted resting energy expenditure in clinically stable patients. Clinical Nutrition 1984, 3, 27–34. [Google Scholar] [CrossRef]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport. Sci. 1991, 16, 23–29. [Google Scholar]
- Gupta, R.D.; Ramachandran, R.; Venkatesan, P.; Anoop, S.; Joseph, M.; Thomas, N. Indirect Calorimetry: From Bench to Bedside. Indian. J. Endocrinol. Metab. 2017, 21, 594–599. [Google Scholar] [CrossRef]
- Mackay, K.J.; Schofield, K.L.; Sims, S.T.; McQuillan, J.A.; Driller, M.W. The Validity of Resting Metabolic Rate-Prediction Equations and Reliability of Measured RMR in Female Athletes. Int. J. Exerc. Sci. 2019, 12, 886–897. [Google Scholar]
- Haff, G.; Triplett, N.T.; National, S.; Conditioning, A. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kauffman, R.P.; Castracane, V.D. Assessing insulin sensitivity.(Controlling PCOS, part 1). Contemp. OB/GYN 2003, 48, 30–39. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Taft, C.; Karlsson, J.; Sullivan, M. Performance of the Swedish SF-36 version 2.0. Qual. Life Res. 2004, 13, 251–256. [Google Scholar] [CrossRef]
- Çelik, D.; Çoban, Ö. Short Form Health Survey version-2.0 Turkish (SF-36v2) is an efficient outcome parameter in musculoskeletal research. Acta Orthop. Et Traumatol. Turc. 2016, 50, 558–561. [Google Scholar] [CrossRef]
- Grubic, T.J.; Sowinski, R.J.; Nevares, B.E.; Jenkins, V.M.; Williamson, S.L.; Reyes, A.G.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Earnest, C.P.; et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: An open label, randomized, counterbalanced, crossover pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 34. [Google Scholar] [CrossRef]
- Sowinski, R.; Gonzalez, D.; Xing, D.; Yoo, C.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Humphries, M.; Leonard, M.; Ko, J.; et al. Effects of Inositol-Enhanced Bonded Arginine Silicate Ingestion on Cognitive and Executive Function in Gamers. Nutrients 2021, 13, 3758. [Google Scholar] [CrossRef]
- Sowinski, R.J.; Grubic, T.J.; Dalton, R.L.; Schlaffer, J.; Reyes-Elrod, A.G.; Jenkins, V.M.; Williamson, S.; Rasmussen, C.; Murano, P.S.; Earnest, C.P.; et al. An Examination of a Novel Weight Loss Supplement on Anthropometry and Indices of Cardiovascular Disease Risk. J. Diet. Suppl. 2021, 18, 478–506. [Google Scholar] [CrossRef]
- Farris, G.D.; Wismann, J.A.; Farris, R.W.; Gandy, N.; Long, L.; Pfau, E.; Kreider, R. Exercise Intensity and Energy Expenditure Analysis of Women Participating in the Curves® Exercise Program. FASEB J. 2006, 20, LB93–LB94. [Google Scholar] [CrossRef]
- Kreider, R.; Rasmussen, C.; Kerksick, C.; Campbell, B.; Baer, J.; Slonaker, B.; Pfau, E.; Grimstvedt, M.; Opusunju, J.; Wilborn, C. Effects of the Curves® Fitness & Weight Loss Program on Weight Loss and Resting Energy Expenditure. Med. Sci. Sport. Exerc. 2004, 36, S81. [Google Scholar]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Maeda, H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: A review. J. Oleo Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef]
- Miyashita, K. Function of marine carotenoids. Forum. Nutr. 2009, 61, 136–146. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 196–199. [Google Scholar]
- Okada, T.; Mizuno, Y.; Sibayama, S.; Hosokawa, M.; Miyashita, K. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipids. J. Food Sci. 2011, 76, H2–H6. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating AMP-activated protein kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of Fucoxanthin on the Inhibition of Dexamethasone-Induced Skeletal Muscle Loss in Mice. Nutrients 2021, 13, 79. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef]
- Takatani, N.; Taya, D.; Katsuki, A.; Beppu, F.; Yamano, Y.; Wada, A.; Miyashita, K.; Hosokawa, M. Identification of Paracentrone in Fucoxanthin-Fed Mice and Anti-Inflammatory Effect against Lipopolysaccharide-Stimulated Macrophages and Adipocytes. Mol. Nutr. Food Res. 2021, 65, e2000405. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Miyashita, K. Seaweed carotenoid, fucoxanthin, with highly bioactive and nutritional activities. J. Mar. Biosci. Biotechnol. 2006, 1, 48–58. [Google Scholar]
- Baetge, C.; Earnest, C.P.; Lockard, B.; Coletta, A.M.; Galvan, E.; Rasmussen, C.; Levers, K.; Simbo, S.Y.; Jung, Y.P.; Koozehchian, M.; et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl. Physiol. Nutr. Metab. 2017, 42, 216–227. [Google Scholar] [CrossRef]
- Coletta, A.M.; Sanchez, B.; O’Connor, A.; Dalton, R.; Springer, S.; Koozehchian, M.S.; Murano, P.S.; Woodman, C.R.; Rasmussen, C.; Kreider, R.B. Alignment of diet prescription to genotype does not promote greater weight loss success in women with obesity participating in an exercise and weight loss program. Obes. Sci. Pract. 2018, 4, 554–574. [Google Scholar] [CrossRef]
- Galbreath, M.; Campbell, B.; La Bounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.L.; Levers, K.; Galvan, E.; et al. Effects of Adherence to a Higher Protein Diet on Weight Loss, Markers of Health, and Functional Capacity in Older Women Participating in a Resistance-Based Exercise Program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wismann-Bunn, J.; Fogt, D.; Thomas, A.R.; Taylor, L.; Campbell, B.I.; Wilborn, C.D.; Harvey, T.; Roberts, M.D.; La Bounty, P.; et al. Changes in weight loss, body composition and cardiovascular disease risk after altering macronutrient distributions during a regular exercise program in obese women. Nutr. J. 2010, 9, 59. [Google Scholar] [CrossRef]
- Kreider, R.B.; Serra, M.; Beavers, K.M.; Moreillon, J.; Kresta, J.Y.; Byrd, M.; Oliver, J.M.; Gutierrez, J.; Hudson, G.; Deike, E.; et al. A structured diet and exercise program promotes favorable changes in weight loss, body composition, and weight maintenance. J. Am. Diet. Assoc. 2011, 111, 828–843. [Google Scholar] [CrossRef]
- Mardock, M.; Lockard, B.; Oliver, J.; Byrd, M.; Simbo, S.; Jagim, A.; Kresta, J.; Baetge, C.; Jung, P.; Koozehchian, M.; et al. Comparative effectiveness of two popular weight loss programs in women I: Body composition and resting energy expenditure. J. Int. Soc. Sports Nutr. 2011, 8, P4. [Google Scholar] [CrossRef]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Damasceno de Lima, R.; Fudoli Lins Vieira, R.; Rosetto Munoz, V.; Chaix, A.; Azevedo Macedo, A.P.; Calheiros Antunes, G.; Felonato, M.; Rosseto Braga, R.; Castelo Branco Ramos Nakandakari, S.; Calais Gaspar, R.; et al. Time-restricted feeding combined with resistance exercise prevents obesity and improves lipid metabolism in the liver of mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E513–E528. [Google Scholar] [CrossRef]
- Madrid, D.A.; Beavers, K.M.; Walkup, M.P.; Ambrosius, W.T.; Rejeski, W.J.; Marsh, A.P.; Weaver, A.A. Effect of exercise modality and weight loss on changes in muscle and bone quality in older adults with obesity. Exp. Gerontol. 2023, 174, 112126. [Google Scholar] [CrossRef]
- Jo, E.; Worts, P.R.; Elam, M.L.; Brown, A.F.; Khamoui, A.V.; Kim, D.H.; Yeh, M.C.; Ormsbee, M.J.; Prado, C.M.; Cain, A.; et al. Resistance training during a 12-week protein supplemented VLCD treatment enhances weight-loss outcomes in obese patients. Clin. Nutr. 2019, 38, 372–382. [Google Scholar] [CrossRef]
- Kleist, B.; Wahrburg, U.; Stehle, P.; Schomaker, R.; Greiwing, A.; Stoffel-Wagner, B.; Egert, S. Moderate Walking Enhances the Effects of an Energy-Restricted Diet on Fat Mass Loss and Serum Insulin in Overweight and Obese Adults in a 12-Week Randomized Controlled Trial. J. Nutr. 2017, 147, 1875–1884. [Google Scholar] [CrossRef]
- Serra, M.C.; Ryan, A.S. Bone Mineral Density Changes during Weight Regain following Weight Loss with and without Exercise. Nutrients 2021, 13, 848. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Das, S.K.; Redman, L.; Smith, S.R.; Saltzman, E.; Bales, C.; Rochon, J.; Pieper, C.; Huang, M.; et al. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J. Bone Miner. Res. 2016, 31, 40–51. [Google Scholar] [CrossRef]
- O’Bryan, S.J.; Giuliano, C.; Woessner, M.N.; Vogrin, S.; Smith, C.; Duque, G.; Levinger, I. Progressive Resistance Training for Concomitant Increases in Muscle Strength and Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 1939–1960. [Google Scholar] [CrossRef]
- Klein, G.L. Insulin and bone: Recent developments. World J. Diabetes 2014, 5, 14–16. [Google Scholar] [CrossRef]
- Thomas, S.J.; Morimoto, K.; Herndon, D.N.; Ferrando, A.A.; Wolfe, R.R.; Klein, G.L.; Wolf, S.E. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery 2002, 132, 341–347. [Google Scholar] [CrossRef]
- Avnet, S.; Perut, F.; Salerno, M.; Sciacca, L.; Baldini, N. Insulin receptor isoforms are differently expressed during human osteoblastogenesis. Differentiation 2012, 83, 242–248. [Google Scholar] [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef]
- Clemens, T.L.; Karsenty, G. The osteoblast: An insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 2011, 26, 677–680. [Google Scholar] [CrossRef]
- Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 2011, 54, 1291–1297. [Google Scholar] [CrossRef]
- Ha, Y.J.; Choi, Y.S.; Oh, Y.R.; Kang, E.H.; Khang, G.; Park, Y.B.; Lee, Y.J. Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling. Mar. Drugs 2021, 19, 132. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Dias, S.; Strasser, B.; Hoffmann, G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: A systematic review and network meta-analysis. PLoS ONE 2013, 8, e82853. [Google Scholar] [CrossRef]
- van Baak, M.A.; Pramono, A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: Systematic review and meta-analyses. Obes. Rev. 2021, 22 (Suppl. 4), e13239. [Google Scholar] [CrossRef]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Minton, D.; Lee, D.C.; Sui, X.; Fayad, R.; Lavie, C.J.; Blair, S.N. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin. Proc. 2013, 88, 1420–1426. [Google Scholar] [CrossRef]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A(y) mice. Lipids Health Dis. 2012, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.G.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Willmott, A.G.B.; Beasley, L.; Boal, M.; Davies, R.; Martin, L.; Chichger, H.; Gautam, L.; Del Coso, J. The Impact of Decaffeinated Green Tea Extract on Fat Oxidation, Body Composition and Cardio-Metabolic Health in Overweight, Recreationally Active Individuals. Nutrients 2021, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- Gillen, Z.M.; Mustad, V.A.; Shoemaker, M.E.; McKay, B.D.; Leutzinger, T.J.; Lopez-Pedrosa, J.M.; Rueda, R.; Cramer, J.T. Impact of slow versus rapid digesting carbohydrates on substrate oxidation in pre-pubertal children: A randomized crossover trial. Clin. Nutr. 2021, 40, 3718–3728. [Google Scholar] [CrossRef]
- Gorski, T.; De Bock, K. Metabolic regulation of exercise-induced angiogenesis. Vasc. Biol. 2019, 1, H1–H8. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, W.C.; Chu, P.M.; Hsieh, P.L.; Hung, C.H.; Tsai, K.L. Fucoxanthin Protects against oxLDL-Induced Endothelial Damage via Activating the AMPK-Akt-CREB-PGC1α Pathway. Mol. Nutr. Food Res. 2019, 63, 1801353. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Kargl, C.K.; Ferguson, R.; Gavin, T.P.; Hellsten, Y. Exercise-induced skeletal muscle angiogenesis: Impact of age, sex, angiocrines and cellular mediators. Eur. J. Appl. Physiol. 2023, 123, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Youn, K.; Yoon, J.H.; Lee, B.; Kim, D.H.; Jun, M. The Role of Fucoxanthin as a Potent Nrf2 Activator via Akt/GSK-3β/Fyn Axis against Amyloid-β Peptide-Induced Oxidative Damage. Antioxidants 2023, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, U.; Jazwa, A.; Maleszewska, M.; Mendel, M.; Szade, K.; Kozakowska, M.; Grochot-Przeczek, A.; Viscardi, M.; Czauderna, S.; Bukowska-Strakova, K.; et al. Nrf2 regulates angiogenesis: Effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid. Redox Signal 2014, 20, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Senchenkova, E. Integrated Systems Physiology—From Cell to Function. In Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef] [PubMed]
- Magrans-Courtney, T.; Wilborn, C.; Rasmussen, C.; Ferreira, M.; Greenwood, L.; Campbell, B.; Kerksick, C.M.; Nassar, E.; Li, R.; Iosia, M.; et al. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J. Int. Soc. Sports Nutr. 2011, 8, 8. [Google Scholar] [CrossRef]
- Beppu, F.; Hosokawa, M.; Yim, M.J.; Shinoda, T.; Miyashita, K. Down-regulation of hepatic stearoyl-CoA desaturase-1 expression by fucoxanthin via leptin signaling in diabetic/obese KK-Ay mice. Lipids 2013, 48, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.V.; Zhe, E.L.C.; Chin, Y.H.; Lim, W.H.; Goh, R.S.J.; Lin, C.; Ng, C.H.; Kong, G.; Tay, P.W.L.; Devi, K.; et al. Barriers and Facilitators to Engagement with a Weight Management Intervention in Asian Patients with Overweight or Obesity: A Systematic Review. Endocr. Pract. 2023, 29, 398–407. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickerson, B.; Maury, J.; Jenkins, V.; Nottingham, K.; Xing, D.; Gonzalez, D.E.; Leonard, M.; Kendra, J.; Ko, J.; Yoo, C.; et al. Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients 2024, 16, 990. https://doi.org/10.3390/nu16070990
Dickerson B, Maury J, Jenkins V, Nottingham K, Xing D, Gonzalez DE, Leonard M, Kendra J, Ko J, Yoo C, et al. Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients. 2024; 16(7):990. https://doi.org/10.3390/nu16070990
Chicago/Turabian StyleDickerson, Broderick, Jonathan Maury, Victoria Jenkins, Kay Nottingham, Dante Xing, Drew E. Gonzalez, Megan Leonard, Jacob Kendra, Joungbo Ko, Choongsung Yoo, and et al. 2024. "Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women" Nutrients 16, no. 7: 990. https://doi.org/10.3390/nu16070990
APA StyleDickerson, B., Maury, J., Jenkins, V., Nottingham, K., Xing, D., Gonzalez, D. E., Leonard, M., Kendra, J., Ko, J., Yoo, C., Johnson, S., Pradelles, R., Purpura, M., Jäger, R., Sowinski, R., Rasmussen, C. J., & Kreider, R. B. (2024). Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients, 16(7), 990. https://doi.org/10.3390/nu16070990







