Swapping White for High-Fibre Bread Increases Faecal Abundance of Short-Chain Fatty Acid-Producing Bacteria and Microbiome Diversity: A Randomized, Controlled, Decentralized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Recruitment and Screening
2.3. Study Design and Intervention
2.4. Twenty-Four-Hour Dietary Recall
2.5. Digestive Comfort
2.6. Faecal Sample Collection
2.7. Microbiome Analysis
2.7.1. Faecal DNA Extraction Method
2.7.2. Microbiome Analysis
2.8. Quantification of the Faecal BCoAT Gene Content
2.9. Statistical Analyses
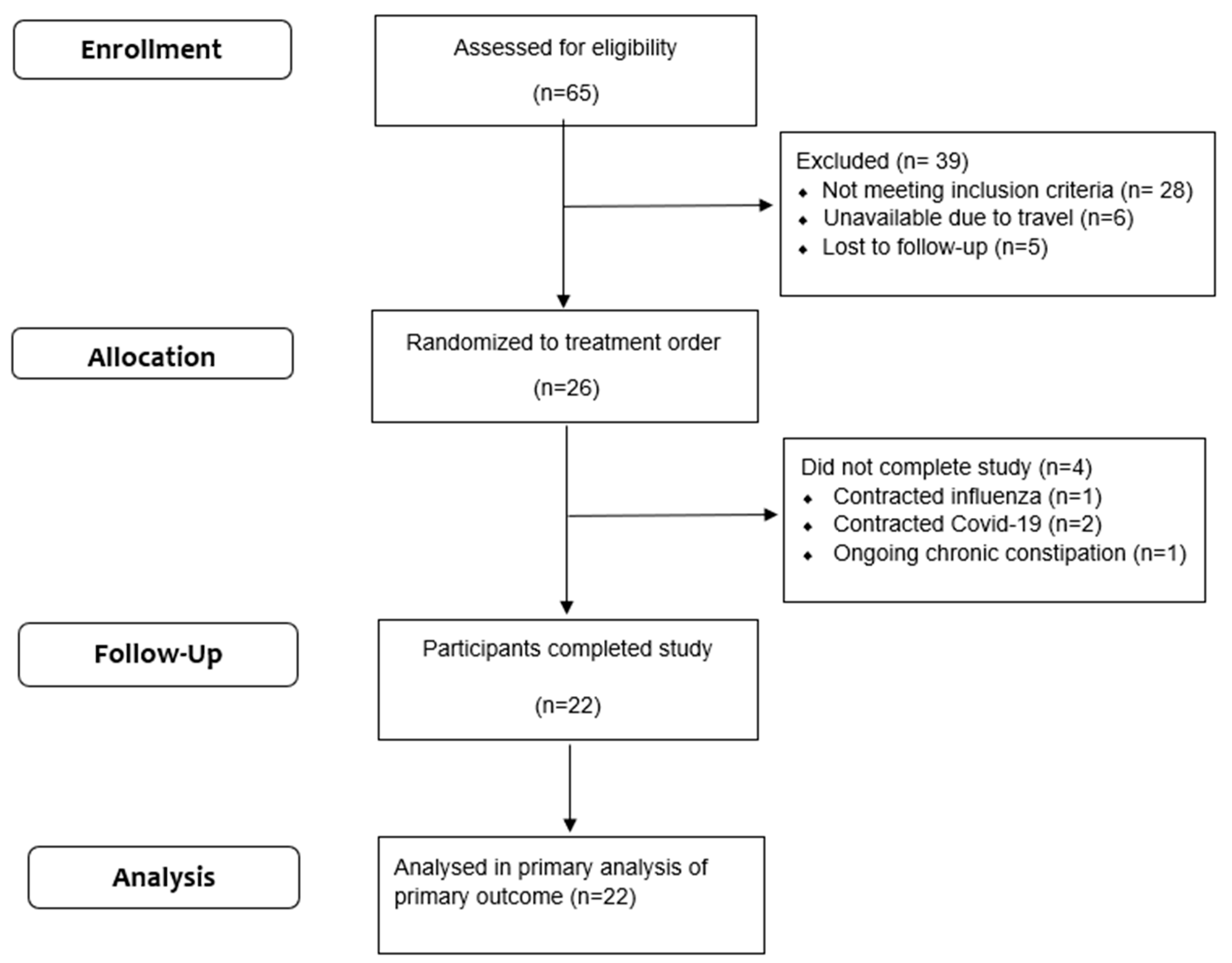
3. Results
3.1. Participants’ Characteristics
3.2. Dietary Intake and Compliance
3.3. Effect of High-Fibre Bread on Faecal Consistency
3.4. Effect of High-Fibre Bread on Gut Comfort
3.5. Effect of High-Fibre Bread on Faecal Microbial Diversity
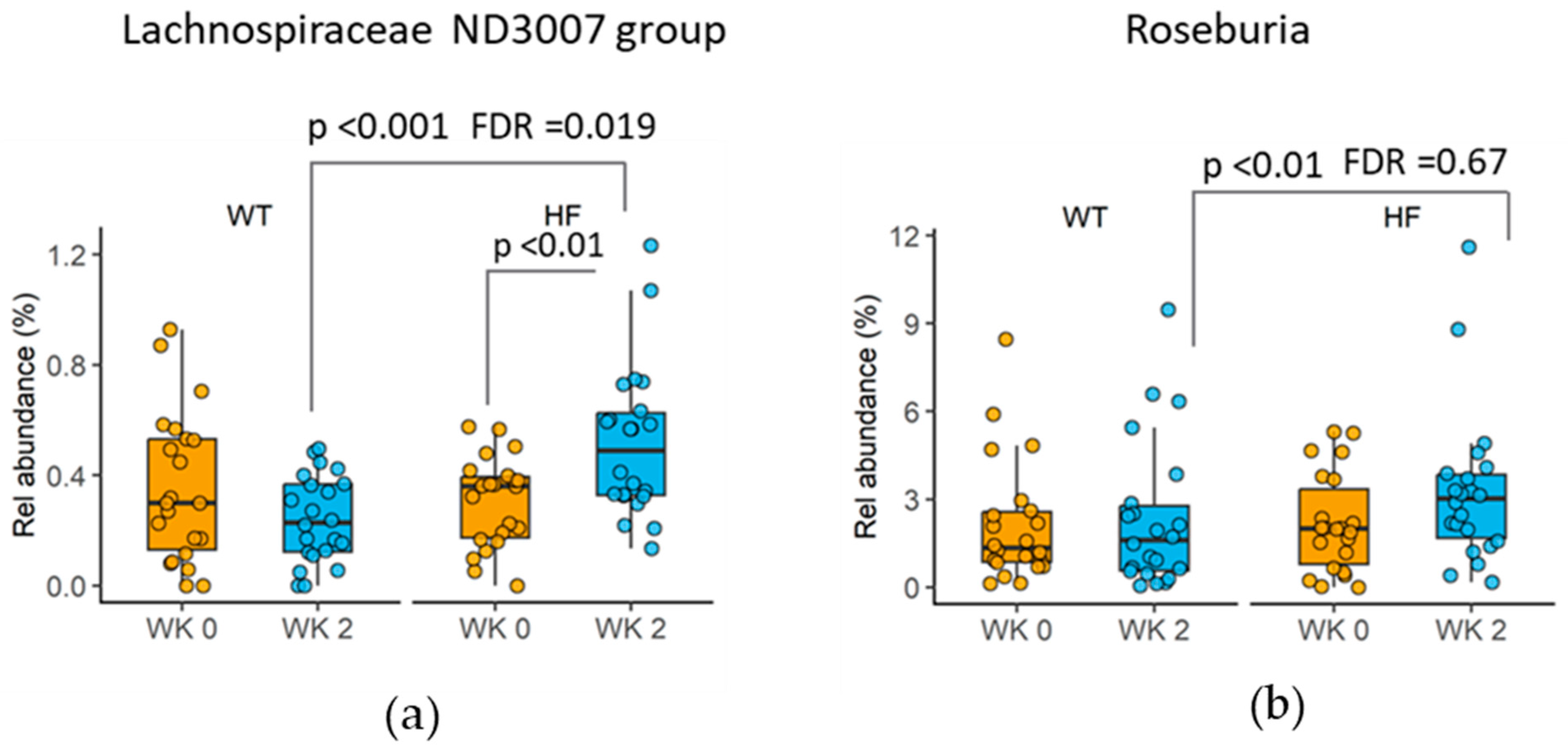
3.6. Changes in Taxa following High-Fibre Bread Consumption
3.7. Effect of High-Fibre Bread on BCoAT Gene Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary Fibre Intake in Australia. Paper II: Comparative Examination of Food Sources of Fibre among High and Low Fibre Consumers. Nutrients 2018, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Australian Government National Health and Medical Research Council; Manatu Hauora Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Available online: https://www.eatforhealth.gov.au/nutrient-reference-values/nutrients/dietary-fibre (accessed on 19 March 2024).
- Grafenauer, S.; Curtain, F. An Audit of Australian Bread with a Focus on Loaf Breads and Whole Grain. Nutrients 2018, 10, 1106. [Google Scholar] [CrossRef]
- Bird, A.R.; Jackson, M.; King, R.A.; Davies, D.A.; Usher, S.; Topping, D.L. A novel high-amylose barley cultivar (Hordeum vulgare var. Himalaya 292) lowers plasma cholesterol and alters indices of large-bowel fermentation in pigs. Br. J. Nutr. 2004, 92, 607–615. [Google Scholar]
- Bird, A.R.; Vuaran, M.S.; King, R.A.; Noakes, M.; Keogh, J.; Morell, M.K.; Topping, D.L. Wholegrain foods made from a novel high-amylose barley variety (Himalaya 292) improve indices of bowel health in human subjects. Br. J. Nutr. 2008, 99, 1032–1040. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl. Environ. Microbiol. 2007, 73, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Clooney, A.G.; Carretero-Gomez, J.F.; Moran, C.; Sheehan, D.; Nolan, J.A.; Hill, C.; Gahan, C.G.M.; Joyce, S.A.; Shanahan, F.; et al. Determinants of Reduced Genetic Capacity for Butyrate Synthesis by the Gut Microbiome in Crohn’s Disease and Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Hurworth, M.; Giglia, R.; Trapp, G.; Strauss, P. Feasibility of a commercial smartphone application for dietary assessment in epidemiological research and comparison with 24-h dietary recalls. Nutr. J. 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, R.; Radd-Vagenas, S.; Singh, M.F.; Noble, Y.; Daniel, K.; Mavros, Y.; Sachdev, P.S.; Lautenschlager, N.; Cox, K.; Brodaty, H.; et al. Electronic food records among middle-aged and older people: A comparison of self-reported and dietitian-assisted information. Nutr. Diet. 2021, 78, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gondalia, S.V.; Wymond, B.; Benassi-Evans, B.; Berbezy, P.; Bird, A.R.; Belobrajdic, D.P. Substitution of Refined Conventional Wheat Flour with Wheat High in Resistant Starch Modulates the Intestinal Microbiota and Fecal Metabolites in Healthy Adults: A Randomized, Controlled Trial. J. Nutr. 2022, 152, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2 (vol 37, pg 852, 2019). Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.L.; Rideout, J.R.; Bolyen, E.; Li, H.L.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbiome Data. Msystems 2018, 3, e00219-18. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Shenoy, A.R. grafify: An R Package for Easy Graphs, ANOVAs and Post-Hoc Comparisons. Available online: https://grafify-vignettes.netlify.app/ (accessed on 22 October 2023).
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results–Foods and Nutrients. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/latest-release#data-downloads (accessed on 19 March 2024).
- Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ishida, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Short-Chain Fatty Acid-Producing Gut Microbiota Is Decreased in Parkinson’s Disease but Not in Rapid-Eye-Movement Sleep Behavior Disorder. Msystems 2020, 5, e00797-20. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, S.; Akagawa, Y.; Nakai, Y.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Chino, K.; Tamiya, T.; Hashiyada, M.; Akane, A.; et al. Fiber-Rich Barley Increases Butyric Acid-Producing Bacteria in the Human Gut Microbiota. Metabolites 2021, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Willis, H.J.; Slavin, J.L. The Influence of Diet Interventions Using Whole, Plant Food on the Gut Microbiome: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.I.H.; Morgan, X.C. Searching for a Consensus Among Inflammatory Bowel Disease Studies: A Systematic Meta-Analysis. Inflamm. Bowel Dis. 2023, 29, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Doetsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. Tuning Expectations to Reality: Don’t Expect Increased Gut Microbiota Diversity with Dietary Fiber. J. Nutr. 2023, 153, 3156–3163. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.P.; Cherbut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 2003, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 167, 1137. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Winter, J.M.; Christophersen, C.T.; Young, G.P.; Humphreys, K.J.; Hu, Y.; Gratz, S.W.; Miller, R.B.; Topping, D.L.; Bird, A.R.; et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: A randomised clinical trial. Br. J. Nutr. 2015, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High Molecular Weight Barley beta-Glucan Alters Gut Microbiota Toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- Wang, Y.; Leong, L.E.X.; Keating, R.L.; Kanno, T.; Abell, G.C.J.; Mobegi, F.M.; Choo, J.M.; Wesselingh, S.L.; Mason, A.J.; Burr, L.D.; et al. Opportunistic bacteria confer the ability to ferment prebiotic starch in the adult cystic fibrosis gut. Gut Microbes 2019, 10, 367–381. [Google Scholar] [CrossRef]



| White Bread 2 | High-Fibre Bread 3 | |||
|---|---|---|---|---|
| Per 100 g | Per 3 Slices (114 g) | Per 100 g | Per 3 Slices (150 g) | |
| Energy (kJ) | 1080 | 1231 | 1240 | 1860 |
| Protein (g) | 9.5 | 10.8 | 14.8 | 22.2 |
| Fat (total) (g) | 1.9 | 2.2 | 10.6 | 15.9 |
| Carbohydrate (g) | 54.0 | 61.6 | 24.7 | 37.1 |
| Starch | 50.5 | 57.6 | 22.0 | 33.0 |
| Sugars (g) | 3.5 | 4.0 | 2.7 | 4.1 |
| Total dietary fibre (g) | 2.7 | 3.1 | 15.3 | 23.0 |
| Resistant starch | 0.6 | 0.7 | 0.5 | 0.8 |
| Insoluble fibre (g) | 1.5 | 1.7 | 14.6 | 21.9 |
| Soluble fibre (g) | 1.3 | 1.5 | 2.1 | 3.2 |
| White Bread | High-Fibre Bread | ||||||
|---|---|---|---|---|---|---|---|
| WK 0 | WK 2 | ∆ | WK 0 | WK 2 | ∆ | p-Value | |
| Energy (kJ) | 8576 ± 2494 | 9309 ± 2418 | 732 ± 3100 | 8177 ± 2838 | 8333 ± 1909 | 157 ± 2626 | 0.601 |
| Carbohydrate (g) | 222.4 ± 76.9 | 249.2 ± 83.4 | 27 ± 108 | 196.3 ± 65.3 | 185.9 ± 46.4 | −10 ± 53 | 0.263 |
| Starch (g) | 128.3 ± 51.8 | 185.9 ± 67.6 | 57 ± 71 | 114.1 ± 37.2 | 104.1 ± 33.8 | −10 ± 33 | 0.008 |
| Sugars (g) | 93.3 ± 56.6 | 67.3 ± 25.9 | −26 ± 52 | 80.3 ± 43.9 | 73.9 ± 27.2 | −6 ± 35 | 0.189 |
| Total dietary fibre (g) | 21.9 ± 10.7 | 20.3 ± 8.1 | −2 ± 13 | 19.0 ± 7.2 | 40.1 ± 6.3 | 21 ± 9 | <0.001 |
| Grain (servings) | 5.5 ± 3.6 | 9.8 ± 4.5 | 4.3 ± 4.3 | 5.6 ± 1.9 | 6.1 ± 2.1 | 0.5 ± 2.8 | 0.026 |
| Refined grain (servings) | 4.4 ± 3.5 | 9.3 ± 4.0 | 4.9 ± 4.0 | 4.1 ± 2.0 | 4.1 ± 2.0 | 0 ± 0 | <0.001 |
| Wholegrain (servings) | 1.1 ± 1.3 | 0.5 ± 1.0 | −0.6 ± 1.6 | 1.5 ± 1.5 | 4.0 ± 0.5 | 2.5 ± 1.6 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wymond, B.; Tandon, H.; Belobrajdic, D.P. Swapping White for High-Fibre Bread Increases Faecal Abundance of Short-Chain Fatty Acid-Producing Bacteria and Microbiome Diversity: A Randomized, Controlled, Decentralized Trial. Nutrients 2024, 16, 989. https://doi.org/10.3390/nu16070989
Wang Y, Wymond B, Tandon H, Belobrajdic DP. Swapping White for High-Fibre Bread Increases Faecal Abundance of Short-Chain Fatty Acid-Producing Bacteria and Microbiome Diversity: A Randomized, Controlled, Decentralized Trial. Nutrients. 2024; 16(7):989. https://doi.org/10.3390/nu16070989
Chicago/Turabian StyleWang, Yanan, Brooke Wymond, Himanshu Tandon, and Damien P. Belobrajdic. 2024. "Swapping White for High-Fibre Bread Increases Faecal Abundance of Short-Chain Fatty Acid-Producing Bacteria and Microbiome Diversity: A Randomized, Controlled, Decentralized Trial" Nutrients 16, no. 7: 989. https://doi.org/10.3390/nu16070989
APA StyleWang, Y., Wymond, B., Tandon, H., & Belobrajdic, D. P. (2024). Swapping White for High-Fibre Bread Increases Faecal Abundance of Short-Chain Fatty Acid-Producing Bacteria and Microbiome Diversity: A Randomized, Controlled, Decentralized Trial. Nutrients, 16(7), 989. https://doi.org/10.3390/nu16070989






