Organosulfur Compounds in Colorectal Cancer Prevention and Progression
Highlights
- A wide variety of organosulfur compounds from numerous plant sources in the Allium genus and Brassicaceae family have been demonstrated to have potent anti-cancer properties.
- Sulforaphane, phenethyl isothiocyanate, 3,3′-diindolylmethane, allicin, and diallyl disulfide have been extensively studied in multiple cell lines and animal models but lack studies in humans.
- All of them have demonstrated potent activities that could be useful in colorectal cancer prevention and treatment, such as repression of multidrug resistance proteins, triggering of apoptosis, inhibition of angiogenesis and metastasis mechanisms, and inhibition of cancer stem cells.
Abstract
1. Introduction
2. Organosulfur Compounds Derived from Cruciferous Vegetables
2.1. Cruciferous Vegetable Consumption
2.2. Cruciferous Vegetable Extracts
2.3. Glucosinolates
2.3.1. Sulforaphane
2.3.2. Sulforaphene
2.3.3. Allyl Isothiocyanate (AITC)
2.3.4. Phenethyl Isothiocyanate (PEITC)
2.3.5. Benzyl Isothiocyanate (BITC)
2.3.6. Iberin
2.4. Indoles
2.4.1. Indole-3-Carbinol
2.4.2. 3,3′-Diindolylmethane
3. Organosulfur Compounds Derived from Allium Species
3.1. Allium Vegetable Consumption
3.2. Allium Vegetable Extracts
3.2.1. Aged Garlic Extract
3.2.2. Allium roseum L. var. Grandiflorum Briq. Essential Oil
3.3. Bioactive Molecules
3.3.1. Allicin
3.3.2. Alliin/S-Allyl-L-Cysteine Sulfoxide (SACS)
3.3.3. Diallyl Sulfide (DAS)
3.3.4. Diallyl Disulfide (DADS)
3.3.5. Diallyl Trisulfide (DATS)
3.3.6. Diallyl Tetrasulfide
3.3.7. Methylsulfonylmethane (MSM) ((Dimethyl Sulfone (DMSO2))
3.3.8. (Z)-Ajoene
3.3.9. S-Allylmercaptocysteine (SAMC)
4. Conclusions
Funding
Conflicts of Interest
References
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; IARC: Lyon, France, 2020.
- Haque, T.R.; Bradshaw, P.T.; Crockett, S.D. Risk Factors for Serrated Polyps of the Colorectum. Dig. Dis. Sci. 2014, 59, 2874–2889. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Ward, H.A.; Jenab, M.; Rothwell, J.A.; Boutron-Ruault, M.-C.; Carbonnel, F.; Kvaskoff, M.; Kaaks, R.; Kühn, T.; Boeing, H.; et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1323–1331.e6. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Brown, L.S.; Fung, T.T. Dietary Patterns and Colorectal Cancer Risk: A Review of 17 Years of Evidence (2000–2016). Curr. Colorectal. Cancer Rep. 2017, 13, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; García, L.; Monte, J.; Villar, C.J.; Lombó, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes. 2018, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Merchea, A. Dysplasia and Cancer in Inflammatory Bowel Disease. Surg. Clin. North Am. 2017, 97, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and Cancer: Advances and New Agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A Genetic Model for Colorectal Tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Zoratto, F.; Rossi, L.; Verrico, M.; Papa, A.; Basso, E.; Zullo, A.; Tomao, L.; Romiti, A.; Lo Russo, G.; Tomao, S. Focus on Genetic and Epigenetic Events of Colorectal Cancer Pathogenesis: Implications for Molecular Diagnosis. Tumour Biol. 2014, 35, 6195–6206. [Google Scholar] [CrossRef]
- Bettington, M.; Walker, N.; Clouston, A.; Brown, I.; Leggett, B.; Whitehall, V. The Serrated Pathway to Colorectal Carcinoma: Current Concepts and Challenges. Histopathology 2013, 62, 367–386. [Google Scholar] [CrossRef]
- Haque, T.; Greene, K.G.; Crockett, S.D. Serrated Neoplasia of the Colon: What Do We Really Know? Curr. Gastroenterol. Rep. 2014, 16, 380. [Google Scholar] [CrossRef]
- Binefa, G. Colorectal Cancer: From Prevention to Personalized Medicine. World J. Gastroenterol. 2014, 20, 6786. [Google Scholar] [CrossRef]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of Histone-Deacetylase Activity by Short-Chain Fatty Acids and Some Polyphenol Metabolites Formed in the Colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 242711. [Google Scholar] [CrossRef]
- Fernández, J.; Moreno, F.J.; Olano, A.; Clemente, A.; Villar, C.J.; Lombó, F. A Galacto-Oligosaccharides Preparation Derived From Lactulose Protects Against Colorectal Cancer Development in an Animal Model. Front. Microbiol. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Miguélez, E.M.; Villar, C.J.; Clemente, A.; Lombó, F. Healthy Effects of Prebiotics and Their Metabolites against Intestinal Diseases and Colorectal Cancer. AIMS Microbiol. 2015, 1, 48–71. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon Microbiota Fermentation of Dietary Prebiotics towards Short-Chain Fatty Acids and Their Roles as Anti-Inflammatory and Antitumour Agents: A Review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Fernández, J.; Gallego-Lobillo, P.; Villar, C.J.; Lombó, F.; Moreno, F.J.; Villamiel, M. Behaviour of Citrus Pectin and Modified Citrus Pectin in an Azoxymethane/Dextran Sodium Sulfate (AOM/DSS)-Induced Rat Colorectal Carcinogenesis Model. Int. J. Biol. Macromol. 2021, 167, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Ledesma, E.; Monte, J.; Millán, E.; Costa, P.; de la Fuente, V.G.; García, M.T.F.; Martínez-Camblor, P.; Villar, C.J.; Lombó, F. Traditional Processed Meat Products Re-Designed Towards Inulin-Rich Functional Foods Reduce Polyps in Two Colorectal Cancer Animal Models. Sci. Rep. 2019, 9, 14783. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lai, C.; Wu, J.; Ho, C. Molecular Mechanisms for Chemoprevention of Colorectal Cancer by Natural Dietary Compounds. Mol. Nutr. Food Res. 2011, 55, 32–45. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 2192. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Jang, K.-J.; Yang, Y.M. Methylsulfonylmethane Induces Cell Cycle Arrest and Apoptosis, and Suppresses the Stemness Potential of HT-29 Cells. Anticancer Res. 2020, 40, 5191–5200. [Google Scholar] [CrossRef]
- Wargovich, M.J. Diallylsulfide and Allylmethylsulfide Are Uniquely Effective among Organosulfur Compounds in Inhibiting CYP2E1 Protein in Animal Models. J. Nutr. 2006, 136, 832S–834S. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, M.; Huang, D. Dietary Organosulfur-Containing Compounds and Their Health-Promotion Mechanisms. Annu. Rev. Food Sci. Technol. 2022, 13, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Di Gioia, F.; Ntatsi, G. Vegetable Organosulfur Compounds and Their Health Promoting Effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-S.; Kim, J.S. Understanding of MYB Transcription Factors Involved in Glucosinolate Biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef]
- Mithen, R.F.; Dekker, M.; Verkerk, R.; Rabot, S.; Johnson, I.T. The Nutritional Significance, Biosynthesis and Bioavailability of Glucosinolates in Human Foods. J. Sci. Food Agric. 2000, 80, 967–984. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl Isothiocyanate as a Cancer Chemopreventive Phytochemical. Mol. Nutr. Food Res. 2010, 54, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Ge, P.; Zhang, B.; Wen, L.; Gu, C.; Zhou, X. Sulforaphene: Formation, Stability, Separation, Purification, Determination and Biological Activities. Sep. Purif. Rev. 2022, 51, 330–339. [Google Scholar] [CrossRef]
- Al Janobi, A.A.; Mithen, R.F.; Gasper, A.V.; Shaw, P.N.; Middleton, R.J.; Ortori, C.A.; Barrett, D.A. Quantitative Measurement of Sulforaphane, Iberin and Their Mercapturic Acid Pathway Metabolites in Human Plasma and Urine Using Liquid Chromatography-Tandem Electrospray Ionisation Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 844, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, H.; Wang, L.; Yu, B. Biosynthesis of the High-Value Plant Secondary Product Benzyl Isothiocyanate via Functional Expression of Multiple Heterologous Enzymes in Escherichia Coli. ACS Synth. Biol. 2016, 5, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Dave, R.A. Pharmacokinetics and Pharmacodynamics of Phenethyl Isothiocyanate: Implications in Breast Cancer Prevention. AAPS J. 2014, 16, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Revelou, P.-K.; Pappas, C.; Constantinou-Kokotou, V. High Resolution Mass Spectrometry Studies of Sulforaphane and Indole-3-Carbinol in Broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef]
- Ciska, E.; Verkerk, R.; Honke, J. Effect of Boiling on the Content of Ascorbigen, Indole-3-Carbinol, Indole-3-Acetonitrile, and 3,3’-Diindolylmethane in Fermented Cabbage. J. Agric. Food Chem. 2009, 57, 2334–2338. [Google Scholar] [CrossRef]
- Cruciferous Vegetable Consumption and Multiple Health Outcomes: An Umbrella Review of 41 Systematic Reviews and Meta-Analyses of 303 Observational Studies—Food & Function; RSC Publishing: Tokyo, Japan, 2022.
- Fang, W.; Qu, X.; Shi, J.; Li, H.; Guo, X.; Wu, X.; Liu, Y.; Li, Z. Cruciferous Vegetables and Colorectal Cancer Risk: A Hospital-Based Matched Case–Control Study in Northeast China. Eur. J. Clin. Nutr. 2019, 73, 450–457. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous Vegetables Intake and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef]
- Mori, N.; Sawada, N.; Shimazu, T.; Yamaji, T.; Goto, A.; Takachi, R.; Ishihara, J.; Iwasaki, M.; Inoue, M.; Tsugane, S. Cruciferous Vegetable Intake and Colorectal Cancer Risk: Japan Public Health Center-Based Prospective Study. Eur. J. Cancer Prev. 2019, 28, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Siersema, P.D.; Overvad, K.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.-C.; Bastide, N.; Fagherazzi, G.; Katzke, V.; Kühn, T.; et al. Subtypes of Fruit and Vegetables, Variety in Consumption and Risk of Colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 137, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Abernathy, B.E.; Trudo, S.P.; Gallaher, D.D. Colon Cancer Risk of a Westernized Diet Is Reduced in Mice by Feeding Cruciferous or Apiaceous Vegetables at a Lower Dose of Carcinogen but Not a Higher Dose. J. Cancer Prev. 2020, 25, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Trudo, S.P.; Gallaher, D.D. Apiaceous and Cruciferous Vegetables Fed During the Post-Initiation Stage Reduce Colon Cancer Risk Markers in Rats. J. Nutr. 2019, 149, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Fuller, Z.; Collins, A.R.; Ratcliffe, B. Comparison of the Effect of Raw and Blanched-Frozen Broccoli on DNA Damage in Colonocytes. Cell Biochem. Funct. 2015, 33, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A.; Suzuki, H.; Mutoh, M.; Kamoshida, T.; Kakinoki, N.; Yoshida, S.; Hirose, M.; Ebihara, T.; Hyodo, I. Chemoprevention against Colon Cancer by Dietary Intake of Sulforaphane. Funct. Foods Health Dis. 2019, 9, 392–411. [Google Scholar] [CrossRef]
- Baenas, N.; Silván, J.M.; Medina, S.; de Pascual-Teresa, S.; García-Viguera, C.; Moreno, D.A. Metabolism and Antiproliferative Effects of Sulforaphane and Broccoli Sprouts in Human Intestinal (Caco-2) and Hepatic (HepG2) Cells. Phytochem. Rev. 2015, 14, 1035–1044. [Google Scholar] [CrossRef]
- Pereira, L.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.; Albuquerque, C.; Serra, A. Targeting Colorectal Cancer Proliferation, Stemness and Metastatic Potential Using Brassicaceae Extracts Enriched in Isothiocyanates: A 3D Cell Model-Based Study. Nutrients 2017, 9, 368. [Google Scholar] [CrossRef]
- Kassie, F.; Uhl, M.; Rabot, S.; Grasl-Kraupp, B.; Verkerk, R.; Kundi, M.; Chabicovsky, M.; Schulte-Hermann, R.; Knasmüller, S. Chemoprevention of 2-Amino-3-Methylimidazo[4, 5-f]Quinoline (IQ)-Induced Colonic and Hepatic Preneoplastic Lesions in the F344 Rat by Cruciferous Vegetables Administered Simultaneously with the Carcinogen. Carcinogenesis 2003, 24, 255–261. [Google Scholar] [CrossRef]
- Uhl, M.; Kassie, F.; Rabot, S.; Grasl-Kraupp, B.; Chakraborty, A.; Laky, B.; Kundi, M.; Knasmüller, S. Effect of Common Brassica Vegetables (Brussels Sprouts and Red Cabbage) on the Development of Preneoplastic Lesions Induced by 2-Amino-3-Methylimidazo[4,5-f]Quinoline (IQ) in Liver and Colon of Fischer 344 Rats. J. Chromatogr. B 2004, 802, 225–230. [Google Scholar] [CrossRef]
- Rajendran, P.; Dashwood, W.-M.; Li, L.; Kang, Y.; Kim, E.; Johnson, G.; Fischer, K.A.; Löhr, C.V.; Williams, D.E.; Ho, E.; et al. Nrf2 Status Affects Tumor Growth, HDAC3 Gene Promoter Associations, and the Response to Sulforaphane in the Colon. Clin. Epigenetics 2015, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Byun, D.-S.; Popova, N.; Murray, L.B.; L’Italien, K.; Sowa, Y.; Arango, D.; Velcich, A.; Augenlicht, L.H.; Mariadason, J.M. Histone Deacetylase 3 (HDAC3) and Other Class I HDACs Regulate Colon Cell Maturation and P21 Expression and Are Deregulated in Human Colon Cancer*. J. Biol. Chem. 2006, 281, 13548–13558. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, H.; Huang, K.; Sun, X.; Li, M. Sulforaphane Inhibits Proliferation and Apoptosis of Colorectal Cancer Cells by Down-Regulating the Cyclooxygenase-2/Protein Kinase B/Glycogen Synthase Kinase-3 Beta Signaling Pathway. Indian. J. Pharm. Sci. 2022, 84, 219–223. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, M.; Sun, N.-X.; Zhu, C.; Lin, Y.-M.; Li, C.; Liu, F.; Zhu, W.-W. Sulforaphane Suppresses Carcinogenesis of Colorectal Cancer through the ERK/Nrf2 UDP Glucuronosyltransferase 1A Metabolic Axis Activation. Oncol. Rep. 2020, 43, 1067–1080. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Wang, M.; Sun, N.-X.; Qing, Y.; Yin, T.-F.; Li, C.; Wu, D. Sulforaphane induced Epigenetic Regulation of Nrf2 Expression by DNA Methyltransferase in Human Caco 2 Cells. Oncol. Lett. 2019, 18, 2639–2647. [Google Scholar] [CrossRef]
- Martin, S.L.; Kala, R.; Tollefsbol, T.O. Mechanisms for the Inhibition of Colon Cancer Cells by Sulforaphane through Epigenetic Modulation of MicroRNA-21 and Human Telomerase Reverse Transcriptase (HTERT) Down-Regulation. Curr. Cancer Drug Targets 2018, 18, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, A.; Mitra, J.; Johnson, G.S.; Li, L.; Dashwood, W.M.; Hegde, M.L.; Yue, C.; Dashwood, R.H.; Rajendran, P. Heterocyclic Analogs of Sulforaphane Trigger DNA Damage and Impede DNA Repair in Colon Cancer Cells: Interplay of HATs and HDACs. Mol. Nutr. Food Res. 2018, 62, 1800228. [Google Scholar] [CrossRef] [PubMed]
- Tafakh, M.S.; Saidijam, M.; Ranjbarnejad, T.; Malih, S.; Mirzamohammadi, S.; Najafi, R. Sulforaphane, a Chemopreventive Compound, Inhibits Cyclooxygenase-2 and Microsomal Prostaglandin E Synthase-1 Expression in Human HT-29 Colon Cancer Cells. Cells Tissues Organs 2018, 206, 46–53. [Google Scholar] [CrossRef]
- Liu, K.-C.; Shih, T.-Y.; Kuo, C.-L.; Ma, Y.-S.; Yang, J.-L.; Wu, P.-P.; Huang, Y.-P.; Lai, K.-C.; Chung, J.-G. Sulforaphane Induces Cell Death Through G2/M Phase Arrest and Triggers Apoptosis in HCT 116 Human Colon Cancer Cells. Am. J. Chin. Med. 2016, 44, 1289–1310. [Google Scholar] [CrossRef]
- Chung, Y.-K.; Chi-Hung Or, R.; Lu, C.-H.; Ouyang, W.-T.; Yang, S.-Y.; Chang, C.-C. Sulforaphane Down-Regulates SKP2 to Stabilize P27KIP1 for Inducing Antiproliferation in Human Colon Adenocarcinoma Cells. J. Biosci. Bioeng. 2015, 119, 35–42. [Google Scholar] [CrossRef]
- Rajendran, P.; Kidane, A.I.; Yu, T.-W.; Dashwood, W.-M.; Bisson, W.H.; Löhr, C.V.; Ho, E.; Williams, D.E.; Dashwood, R.H. HDAC Turnover, CtIP Acetylation and Dysregulated DNA Damage Signaling in Colon Cancer Cells Treated with Sulforaphane and Related Dietary Isothiocyanates. Epigenetics 2013, 8, 612–623. [Google Scholar] [CrossRef]
- Chen, M.-J.; Tang, W.-Y.; Hsu, C.-W.; Tsai, Y.-T.; Wu, J.-F.; Lin, C.-W.; Cheng, Y.-M.; Hsu, Y.-C. Apoptosis Induction in Primary Human Colorectal Cancer Cell Lines and Retarded Tumor Growth in SCID Mice by Sulforaphane. Evid. -Based Complement. Altern. Med. 2011, 2012, e415231. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; Wang, S.; Sun, D.-F.; Chen, J.; Li, Y.-Q.; Han, W.; Yang, X.-Y.; Gao, H.-Q. Effects of Phytochemicals Sulforaphane on Uridine Diphosphate-Glucuronosyltransferase Expression as Well as Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Chin. J. Physiol. 2012, 55, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Horinaka, M.; Sakai, T. Sulforaphane Enhances Apoptosis Induced by Lactobacillus Pentosus Strain S PT84 via the TNFα Pathway in Human Colon Cancer Cells. Oncol. Lett. 2019, 18, 4253–4261. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. Broccoli and Human Health: Immunomodulatory Effect of Sulforaphane in a Model of Colon Cancer. Int. J. Food Sci. Nutr. 2018, 69, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sung, B.; Kang, Y.J.; Hwang, S.Y.; Kim, M.J.; Yoon, J.-H.; Im, E.; Kim, N.D. Sulforaphane Inhibits Hypoxia-Induced HIF-1α and VEGF Expression and Migration of Human Colon Cancer Cells. Int. J. Oncol. 2015, 47, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Pogorzelska, A.; Wiktorska, K. Synergistic Interaction between 5-FU and an Analog of Sulforaphane—2-Oxohexyl Isothiocyanate—In an In Vitro Colon Cancer Model. Molecules 2021, 26, 3019. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lv, R.-B.; Liu, Y.; Hao, Q.; Liu, S.-J.; Zheng, Y.-Y.; Li, C.; Zhu, C.; Wang, M. Salinomycin and Sulforaphane Exerted Synergistic Antiproliferative and Proapoptotic Effects on Colorectal Cancer Cells by Inhibiting the PI3K/Akt Signaling Pathway in Vitro and in Vivo. Onco Targets Ther. 2020, 13, 4957–4969. [Google Scholar] [CrossRef] [PubMed]
- Erzinger, M.M.; Bovet, C.; Hecht, K.M.; Senger, S.; Winiker, P.; Sobotzki, N.; Cristea, S.; Beerenwinkel, N.; Shay, J.W.; Marra, G.; et al. Sulforaphane Preconditioning Sensitizes Human Colon Cancer Cells towards the Bioreductive Anticancer Prodrug PR-104A. PLoS ONE 2016, 11, e0150219. [Google Scholar] [CrossRef]
- Čižauskaitė, A.; Šimčikas, D.; Schultze, D.; Kallifatidis, G.; Bruns, H.; Čekauskas, A.; Herr, I.; Baušys, A.; Strupas, K.; Schemmer, P. Sulforaphane Has an Additive Anticancer Effect to FOLFOX in Highly Metastatic Human Colon Carcinoma Cells. Oncol. Rep. 2022, 48, 205. [Google Scholar] [CrossRef]
- Hu, R. Cancer Chemoprevention of Intestinal Polyposis in ApcMin/+ Mice by Sulforaphane, a Natural Product Derived from Cruciferous Vegetable. Carcinogenesis 2006, 27, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene Suppresses Growth of Colon Cancer-Derived Tumors via Induction of Glutathione Depletion and Microtubule Depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Du, F.; Wang, J.; Zhao, Y.; Liu, J.; Cai, D.; Zhang, X.; Wang, Y.; Zhang, S. Examination of the Differences between Sulforaphane and Sulforaphene in Colon Cancer: A Study Based on Next generation Sequencing. Oncol. Lett. 2021, 22, 690. [Google Scholar] [CrossRef]
- Chiang, J.-H.; Tsai, F.-J.; Hsu, Y.-M.; Yin, M.-C.; Chiu, H.-Y.; Yang, J.-S. Sensitivity of Allyl Isothiocyanate to Induce Apoptosis via ER Stress and the Mitochondrial Pathway upon ROS Production in Colorectal Adenocarcinoma Cells. Oncol. Rep. 2020, 44, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-C.; Lu, C.-C.; Tang, Y.-J.; Chiang, J.-H.; Kuo, D.-H.; Chen, F.-A.; Chen, I.-L.; Yang, J.-S. Allyl Isothiocyanate Inhibits Cell Metastasis through Suppression of the MAPK Pathways in Epidermal Growth Factor stimulated HT29 Human Colorectal Adenocarcinoma Cells. Oncol. Rep. 2014, 31, 189–196. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, X.; Meng, Y.; Zhang, Q.; Zhu, J.; Chen, J.; Cao, W.; Wang, X.; Xie, C.; et al. Phenethyl Isothiocyanate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Phytother. Res. 2018, 32, 2447–2455. [Google Scholar] [CrossRef]
- Gupta, R.; Bhatt, L.K.; Momin, M. Potent Antitumor Activity of Laccaic Acid and Phenethyl Isothiocyanate Combination in Colorectal Cancer via Dual Inhibition of DNA Methyltransferase-1 and Histone Deacetylase-1. Toxicol. Appl. Pharmacol. 2019, 377, 114631. [Google Scholar] [CrossRef]
- Shin, J.M.; Lim, E.; Cho, Y.S.; Nho, C.W. Cancer-Preventive Effect of Phenethyl Isothiocyanate through Tumor Microenvironment Regulation in a Colorectal Cancer Stem Cell Xenograft Model. Phytomedicine 2021, 84, 153493. [Google Scholar] [CrossRef]
- Liu, X.; Takano, C.; Shimizu, T.; Yokobe, S.; Abe-Kanoh, N.; Zhu, B.; Nakamura, T.; Munemasa, S.; Murata, Y.; Nakamura, Y. Inhibition of Phosphatidylinositide 3-Kinase Ameliorates Antiproliferation by Benzyl Isothiocyanate in Human Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 491, 209–216. [Google Scholar] [CrossRef]
- Abe, N.; Hou, D.-X.; Munemasa, S.; Murata, Y.; Nakamura, Y. Nuclear Factor-KappaB Sensitizes to Benzyl Isothiocyanate-Induced Antiproliferation in P53-Deficient Colorectal Cancer Cells. Cell Death Dis. 2014, 5, e1534. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Chen, C.D.; Jimenez, A.; Steele, V.E.; Velasco, M.; Stephens, L.C.; Price, R.; Gray, K.; Kelloff, G.J. Aberrant Crypts as a Biomarker for Colon Cancer: Evaluation of Potential Chemopreventive Agents in the Rat. Cancer Epidemiol. Biomark. Prev. 1996, 5, 355–360. [Google Scholar]
- Barrera, L.N.; Johnson, I.T.; Bao, Y.; Cassidy, A.; Belshaw, N.J. Colorectal Cancer Cells Caco-2 and HCT116 Resist Epigenetic Effects of Isothiocyanates and Selenium in Vitro. Eur. J. Nutr. 2013, 52, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Sachlova, M.; Brezkova, V.; Hezova, R.; Kovarikova, A.; Bischofová, S.; Sevcikova, S.; Bienertova-Vasku, J.; Vasku, A.; Svoboda, M.; et al. Identification of MicroRNAs Regulated by Isothiocyanates and Association of Polymorphisms Inside Their Target Sites with Risk of Sporadic Colorectal Cancer. Nutr. Cancer 2013, 65, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, H.M.; Lee, C.M.; Park, S.-H.; Nam, M.J. Indole-3-Carbinol Inhibits the Proliferation of Colorectal Carcinoma LoVo Cells through Activation of the Apoptotic Signaling Pathway. Hum. Exp. Toxicol. 2021, 40, 2099–2112. [Google Scholar] [CrossRef]
- Megna, B.W.; Carney, P.R.; Nukaya, M.; Geiger, P.; Kennedy, G.D. Indole-3-Carbinol Induces Tumor Cell Death: Function Follows Form. J. Surg. Res. 2016, 204, 47–54. [Google Scholar] [CrossRef]
- Fadlalla, K.; Elgendy, R.; Gilbreath, E.; Pondugula, S.R.; Yehualaeshet, T.; Mansour, M.; Serbessa, T.; Manne, U.; Samuel, T. 3-(2-Bromoethyl)-Indole Inhibits the Growth of Cancer Cells and NF-ΚB Activation. Oncol. Rep. 2015, 34, 495–503. [Google Scholar] [CrossRef]
- de Moura, N.A.; Caetano, B.F.R.; de Moraes, L.N.; Carvalho, R.F.; Rodrigues, M.A.M.; Barbisan, L.F. Enhancement of Colon Carcinogenesis by the Combination of Indole-3 Carbinol and Synbiotics in Hemin-Fed Rats. Food Chem. Toxicol. 2018, 112, 11–18. [Google Scholar] [CrossRef]
- de Moura, N.A.; Caetano, B.F.R.; Bidinotto, L.T.; Rodrigues, M.A.M.; Barbisan, L.F. Synbiotic Supplementation Attenuates the Promoting Effect of Indole-3-Carbinol on Colon Tumorigenesis. Benef. Microbes 2021, 12, 493–501. [Google Scholar] [CrossRef]
- Wu, Y.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Kim, Y.S.; Wang, T.T.Y.; Smith, A.D. Indole-3-Carbinol Inhibits Citrobacter Rodentium Infection through Multiple Pathways Including Reduction of Bacterial Adhesion and Enhancement of Cytotoxic T Cell Activity. Nutrients 2020, 12, 917. [Google Scholar] [CrossRef]
- Tian, X.; Liu, K.; Zu, X.; Ma, F.; Li, Z.; Lee, M.; Chen, H.; Li, Y.; Zhao, Y.; Liu, F.; et al. 3,3’-Diindolylmethane Inhibits Patient-Derived Xenograft Colon Tumor Growth by Targeting COX1/2 and ERK1/2. Cancer Lett. 2019, 448, 20–30. [Google Scholar] [CrossRef]
- Zhang, X.; Sukamporn, P.; Zhang, S.; Min, K.-W.; Baek, S.J. 3,3’-Diindolylmethane Downregulates Cyclin D1 through Triggering Endoplasmic Reticulum Stress in Colorectal Cancer Cells. Oncol. Rep. 2017, 38, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Grafi-Cohen, M.; Napso, T.; Azzam, N.; Fares, F. The Indolic Diet-Derivative, 3,3′-Diindolylmethane, Induced Apoptosis in Human Colon Cancer Cells through Upregulation of NDRG1. BioMed. Res. Int. 2011, 2012, e256178. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Min, K.-W.; Zhang, X.; Baek, S.J. 3,3′-Diindolylmethane Induces Activating Transcription Factor 3 (ATF3) via ATF4 in Human Colorectal Cancer Cells. J. Nutr. Biochem. 2013, 24, 664–671. [Google Scholar] [CrossRef]
- Wang, D.; Neupane, P.; Ragnarsson, L.; Capon, R.J.; Lewis, R.J. Diindolylmethane Derivatives: New Selective Blockers for T-Type Calcium Channels. Membranes 2022, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, S.; Zhang, Y.; Yang, Z.; Wang, W.; Meng, M.; Feng, J.; Zhang, P.; Xiao, L.; Lee, M.-H.; et al. 3,3′-Diindolylmethane Enhances Fluorouracil Sensitivity via Inhibition of Pyrimidine Metabolism in Colorectal Cancer. Metabolites 2022, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ni, J.; Tang, Y.; Wang, X.; Tang, H.; Li, H.; Zhang, S.; Shen, X. Allicin Inhibits Mouse Colorectal Tumorigenesis through Suppressing the Activation of STAT3 Signaling Pathway. Nat. Prod. Res. 2019, 33, 2722–2725. [Google Scholar] [CrossRef]
- Țigu, A.B.; Toma, V.-A.; Moț, A.C.; Jurj, A.; Moldovan, C.S.; Fischer-Fodor, E.; Berindan-Neagoe, I.; Pârvu, M. The Synergistic Antitumor Effect of 5-Fluorouracil Combined with Allicin against Lung and Colorectal Carcinoma Cells. Molecules 2020, 25, 1947. [Google Scholar] [CrossRef]
- Huang, W.; Wu, S.; Xu, S.; Ma, Y.; Wang, R.; Jin, S.; Zhou, S. Allicin Enhances the Radiosensitivity of Colorectal Cancer Cells via Inhibition of NF-ΚB Signaling Pathway. J. Food Sci. 2020, 85, 1924–1931. [Google Scholar] [CrossRef]
- Roy, N.; Nazeem, P.A.; Babu, T.D.; Abida, P.S.; Narayanankutty, A.; Valsalan, R.; Valsala, P.A.; Raghavamenon, A.C. EGFR Gene Regulation in Colorectal Cancer Cells by Garlic Phytocompounds with Special Emphasis on S-Allyl-L-Cysteine Sulfoxide. Interdiscip. Sci. 2018, 10, 686–693. [Google Scholar] [CrossRef]
- Hatono, S.; Jimenez, A.; Wargovich, M.J. Chemopreventive Effect of S-Allylcysteine and Its Relationship to the Detoxification Enzyme Glutathione S-Transferase. Carcinogenesis 1996, 17, 1041–1044. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Jimenez, A.; McKee, K.; Steele, V.E.; Velasco, M.; Woods, J.; Price, R.; Gray, K.; Kelloff, G.J. Efficacy of Potential Chemopreventive Agents on Rat Colon Aberrant Crypt Formation and Progression. Carcinogenesis 2000, 21, 1149–1155. [Google Scholar] [CrossRef]
- Sumiyoshi, H.; Wargovich, M.J. Chemoprevention of 1,2-Dimethylhydrazine-Induced Colon Cancer in Mice by Naturally Occurring Organosulfur Compounds1. Cancer Res. 1990, 50, 5084–5087. [Google Scholar]
- Sriram, N.; Kalayarasan, S.; Ashokkumar, P.; Sureshkumar, A.; Sudhandiran, G. Diallyl Sulfide Induces Apoptosis in Colo 320 DM Human Colon Cancer Cells: Involvement of Caspase-3, NF-ΚB, and ERK-2. Mol. Cell Biochem. 2008, 311, 157–165. [Google Scholar] [CrossRef]
- Lai, K.-C.; Hsu, S.-C.; Kuo, C.-L.; Yang, J.-S.; Ma, C.-Y.; Lu, H.-F.; Tang, N.-Y.; Hsia, T.-C.; Ho, H.-C.; Chung, J.-G. Diallyl Sulfide, Diallyl Disulfide, and Diallyl Trisulfide Inhibit Migration and Invasion in Human Colon Cancer Colo 205 Cells through the Inhibition of Matrix Metalloproteinase-2, -7, and -9 Expressions. Env. Toxicol. 2013, 28, 479–488. [Google Scholar] [CrossRef]
- Lai, K.-C.; Kuo, C.-L.; Ho, H.-C.; Yang, J.-S.; Ma, C.-Y.; Lu, H.-F.; Huang, H.-Y.; Chueh, F.-S.; Yu, C.-C.; Chung, J.-G. Diallyl Sulfide, Diallyl Disulfide and Diallyl Trisulfide Affect Drug Resistant Gene Expression in Colo 205 Human Colon Cancer Cells in Vitro and in Vivo. Phytomedicine 2012, 19, 625–630. [Google Scholar] [CrossRef]
- Kang, J.-S.; Kim, T.-M.; Shim, T.-J.; Salim, E.I.; Han, B.-S.; Kim, D.-J. Modifying Effect of Diallyl Sulfide on Colon Carcinogenesis in C57BL/6J-Apc^Min/+ Mice. Asian Pac. J. Cancer Prev. 2012, 13, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lin, J.; Su, J.; Oyang, L.; Wang, H.; Tan, S.; Tang, Y.; Chen, X.; Liu, W.; Luo, X.; et al. Diallyl Disulfide Inhibits Colon Cancer Metastasis by Suppressing Rac1-Mediated Epithelial-Mesenchymal Transition. Onco Targets Ther. 2019, 12, 5713–5728. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, S.; Kim, D.Y.; You, S.; Park, D.; Oh, S.C.; Lee, D.-H. Diallyl Disulfide (DADS) Boosts TRAIL-Mediated Apoptosis in Colorectal Cancer Cells by Inhibiting Bcl-2. Food Chem. Toxicol. 2019, 125, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Li, W.; Gray, Z.; Matter, M.S.; Colburn, N.H.; Young, M.R.; Kim, Y.S. Diallyl Disulfide (DADS), a Constituent of Garlic, Inactivates NF-ΚB and Prevents Colitis-Induced Colorectal Cancer by Inhibiting GSK-3β. Cancer Prev. Res. 2016, 9, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Huang, C.-F.; Tseng, Y.-T.; Kuo, S.-Y. Diallyl Disulfide Induces Ca2+ Mobilization in Human Colon Cancer Cell Line SW480. Arch. Toxicol. 2012, 86, 231–238. [Google Scholar] [CrossRef]
- Su, J.; Zhou, Y.; Pan, Z.; Shi, L.; Yang, J.; Liao, A.; Liao, Q.; Su, Q. Downregulation of LIMK1–ADF/Cofilin by DADS Inhibits the Migration and Invasion of Colon Cancer. Sci. Rep. 2017, 7, 45624. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; AlSuhaymi, N.; Alsugoor, M.H.; et al. The Effect of Liposomal Diallyl Disulfide and Oxaliplatin on Proliferation of Colorectal Cancer Cells: In Vitro and In Silico Analysis. Pharmaceutics 2022, 14, 236. [Google Scholar] [CrossRef]
- Saraf, A.; Dubey, N.; Dubey, N.; Sharma, M. Enhancement of Cytotoxicty of Diallyl Disulfide toward Colon Cancer by Eudragit S100/PLGA Nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 64, 102580. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.-T.; Chen, Y.; Chen, J.-Q.; Zhu, J.-Y.; Meng, Y.; Wang, X.-Q.; Li, Y.; Geng, S.-S.; Xie, C.-F.; et al. Wnt/β-Catenin Signaling Mediates the Suppressive Effects of Diallyl Trisulfide on Colorectal Cancer Stem Cells. Cancer Chemother. Pharmacol. 2018, 81, 969–977. [Google Scholar] [CrossRef]
- Yu, C.-S.; Huang, A.-C.; Lai, K.-C.; Huang, Y.-P.; Lin, M.-W.; Yang, J.-S.; Chung, J.-G. Diallyl Trisulfide Induces Apoptosis in Human Primary Colorectal Cancer Cells. Oncol. Rep. 2012, 28, 949–954. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; LLeonart, M.E. Insights into New Mechanisms and Models of Cancer Stem Cell Multidrug Resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Wu, P.-P.; Liu, K.-C.; Huang, W.-W.; Chueh, F.-S.; Ko, Y.-C.; Chiu, T.-H.; Lin, J.-P.; Kuo, J.-H.; Yang, J.-S.; Chung, J.-G. Diallyl Trisulfide (DATS) Inhibits Mouse Colon Tumor in Mouse CT-26 Cells Allograft Model in Vivo. Phytomedicine 2011, 18, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Yagdi, E.E.; Mazumder, A.; Lee, J.-Y.; Gaigneaux, A.; Radogna, F.; Nasim, M.J.; Christov, C.; Jacob, C.; Kim, K.-W.; Dicato, M.; et al. Tubulin-Binding Anticancer Polysulfides Induce Cell Death via Mitotic Arrest and Autophagic Interference in Colorectal Cancer. Cancer Lett. 2017, 410, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jeong, J.H.; Kwon, S.W.; Lee, S.K.; Lee, H.J.; Ryu, J.-H. Z-Ajoene Inhibits Growth of Colon Cancer by Promotion of CK1α Dependent β-Catenin Phosphorylation. Molecules 2020, 25, 703. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; So, K.F.; Wong, N.K.; Xiao, J. Anti-Cancer Activities of S-Allylmercaptocysteine from Aged Garlic. Chin. J. Nat. Med. 2019, 17, 43–49. [Google Scholar] [CrossRef]
- Li, S.; Guang, Y.; Zhu, X.; Lin, C.; Sun, Y.; Zhao, Z. Combination of Rapamycin and Garlic-Derived S-Allylmercaptocysteine Induces Colon Cancer Cell Apoptosis and Suppresses Tumor Growth in Xenograft Nude Mice through Autophagy/P62/Nrf2 Pathway. Oncol. Rep. 2017, 38, 1637–1644. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.Y.; Zhang, Z.H.; Bian, H.L.; Lin, G. Garlic-Derived Compound S-Allylmercaptocysteine Inhibits Cell Growth and Induces Apoptosis via the JNK and P38 Pathways in Human Colorectal Carcinoma Cells. Oncol. Lett. 2014, 8, 2591–2596. [Google Scholar] [CrossRef]
- Liang, D.; Qin, Y.; Zhao, W.; Zhai, X.; Guo, Z.; Wang, R.; Tong, L.; Lin, L.; Chen, H.; Wong, Y.C.; et al. S-Allylmercaptocysteine Effectively Inhibits the Proliferation of Colorectal Cancer Cells under in Vitro and in Vivo Conditions. Cancer Lett. 2011, 310, 69–76. [Google Scholar] [CrossRef]
- Tong, D.; Qu, H.; Meng, X.; Jiang, Y.; Liu, D.; Ye, S.; Chen, H.; Jin, Y.; Fu, S.; Geng, J. S-Allylmercaptocysteine Promotes MAPK Inhibitor-Induced Apoptosis by Activating the TGF-β Signaling Pathway in Cancer Cells. Oncol. Rep. 2014, 32, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Laukens, D.; Brinkman, B.M.; Raes, J.; De Vos, M.; Vandenabeele, P. Heterogeneity of the Gut Microbiome in Mice: Guidelines for Optimizing Experimental Design. FEMS Microbiol. Rev. 2016, 40, 117–132. [Google Scholar] [CrossRef]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, Chemical, and Biological Properties of s-Allylcysteine, an Amino Acid Derived from Garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Saito, K. S-Alk(En)Ylcysteine Sulfoxides in the Genus Allium: Proposed Biosynthesis, Chemical Conversion, and Bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kumagai, H. Characteristics, Biosynthesis, Decomposition, Metabolism and Functions of the Garlic Odour Precursor, S-Allyl-L-Cysteine Sulfoxide. Exp. Ther. Med. 2020, 19, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.S.; Midde, N.M.; Miller, D.D.; Chauhan, S.; Kumar, A.; Kumar, S. Diallyl Sulfide: Potential Use in Novel Therapeutic Interventions in Alcohol, Drugs, and Disease Mediated Cellular Toxicity by Targeting Cytochrome P450 2E1. Curr. Drug Metab. 2015, 16, 486–503. [Google Scholar] [CrossRef]
- Puccinelli, M.T.; Stan, S.D. Dietary Bioactive Diallyl Trisulfide in Cancer Prevention and Treatment. Int. J. Mol. Sci. 2017, 18, 1645. [Google Scholar] [CrossRef] [PubMed]
- Matsutomo, T.; Kodera, Y. Development of an Analytic Method for Sulfur Compounds in Aged Garlic Extract with the Use of a Postcolumn High Performance Liquid Chromatography Method with Sulfur-Specific Detection. J. Nutr. 2016, 146, 450S–455S. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, J.; Fang, W.X.; Guo, X.Y.; Zhang, L.Y.; Liu, Y.P.; Li, Z. Allium Vegetables Are Associated with Reduced Risk of Colorectal Cancer: A Hospital-Based Matched Case-Control Study in China. Asia Pac. J. Clin. Oncol. 2019, 15, e132–e141. [Google Scholar] [CrossRef]
- Zhou, X.; Qian, H.; Zhang, D.; Zeng, L. Garlic Intake and the Risk of Colorectal Cancer: A Meta-Analysis. Medicine 2020, 99, e18575. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, L.; Qi, L.; Zhong, R.; Miao, X. Allium Vegetables and Garlic Supplements Do Not Reduce Risk of Colorectal Cancer, Based on Meta-Analysis of Prospective Studies. Clin. Gastroenterol. Hepatol. 2014, 12, 1991–2001.e4. [Google Scholar] [CrossRef]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of Herbal and Specialty Supplements with Lung and Colorectal Cancer Risk in the VITamins and Lifestyle Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1419–1428. [Google Scholar] [CrossRef]
- Cascajosa-Lira, A.; Andreo-Martínez, P.; Prieto, A.I.; Baños, A.; Guillamón, E.; Jos, A.; Cameán, A.M. In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review. Foods 2022, 11, 2620. [Google Scholar] [CrossRef] [PubMed]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude Garlic Extract Inhibits Cell Proliferation and Induces Cell Cycle Arrest and Apoptosis of Cancer Cells in Vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- Su, C.-C.; Chen, G.W.; Tan, T.-W.; Lin, J.-G.; Chung, J.-G. Crude Extract of Garlic Induced Caspase-3 Gene Expression Leading to Apoptosis in Human Colon Cancer Cells. Int. Inst. Anticancer Res. 2006, 20, 85–90. [Google Scholar]
- Ghazanfari, T.; Yaraee, R.; Rahmati, B.; Hakimzadeh, H.; Shams, J.; Jalali-Nadoushan, M.R. In Vitro Cytotoxic Effect of Garlic Extract on Malignant and Nonmalignant Cell Lines. Immunopharmacol. Immunotoxicol. 2011, 33, 603–608. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Bruno, S.; Badalamenti, N.; Quassinti, L.; Bramucci, M.; Beghelli, D.; Lupidi, G.; Dall’Acqua, S. Hairy Garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn Analysis of Secondary Metabolites and in Vitro Biological Properties. Molecules 2020, 25, 2837. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, M.J.; Park, H.-J.; Chung, W.-Y.; Kim, K.-R.; Park, K.-K. Chemopreventive and Anticancer Activities of Allium victorialis var. platyphyllum Extracts. J. Cancer Prev. 2014, 19, 179–186. [Google Scholar] [CrossRef]
- Perez-Ortiz, J.M.; Galan-Moya, E.M.; de la Cruz-Morcillo, M.A.; Rodriguez, J.F.; Gracia, I.; Garcia, M.T.; Redondo-Calvo, F.J. Cost Effective Use of a Thiosulfinate-Enriched Allium sativum Extract in Combination with Chemotherapy in Colon Cancer. Int. J. Mol. Sci. 2020, 21, 2766. [Google Scholar] [CrossRef] [PubMed]
- Miraghajani, M.; Rafie, N.; Hajianfar, H.; Larijani, B.; Azadbakht, L. Aged Garlic and Cancer: A Systematic Review. Int. J. Prev. Med. 2018, 9, 84. [Google Scholar] [PubMed]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged Black Garlic Extract Inhibits HT29 Colon Cancer Cell Growth via the PI3K/Akt Signaling Pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Miyamae, Y.; Yamane, K.; Nagao, Y.; Hamada, Y.; Kawaguchi, N.; Katsuki, T.; Hirata, K.; Sumi, S.-I.; Ishikawa, H. Significance of Garlic and Its Constituents in Cancer and Cardiovascular Disease. J. Nutr. 2006, 136, 716–725. [Google Scholar]
- Katsuki, T.; Hirata, K.; Ishikawa, H.; Matsuura, N.; Sumi, S.-I.; Itoh, H. Significance of Garlic and Its Constituents in Cancer and Cardiovascular Disease Aged Garlic Extract Has Chemopreventative Effects On 1,2-Dimethylhydrazine-Induced Colon Tumors in Rats. J. Nutr. 2006, 136, 847S–851S. [Google Scholar] [CrossRef] [PubMed]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged Garlic Extract Inhibits 1,2-Dimethylhydrazine-Induced Colon Tumor Development by Suppressing Cell Proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Significance of Garlic and Its Constituents in Cancer and Cardiovascular Disease Aged Garlic Extract Has Potential Suppressive Effect on Colorectal Adenomas in Humans. J. Nutr. 2006, 136, 821S–826S. [Google Scholar] [CrossRef]
- Touihri, I.; Boukhris, M.; Marrakchi, N.; Luis, J.; Hanchi, B.; Kallech-Ziri, O. Chemical Composition and Biological Activities of Allium roseum L. var. Grandiflorum Briq. Essential Oil. J. Oleo Sci. 2015, 64, ess15055. [Google Scholar] [CrossRef]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
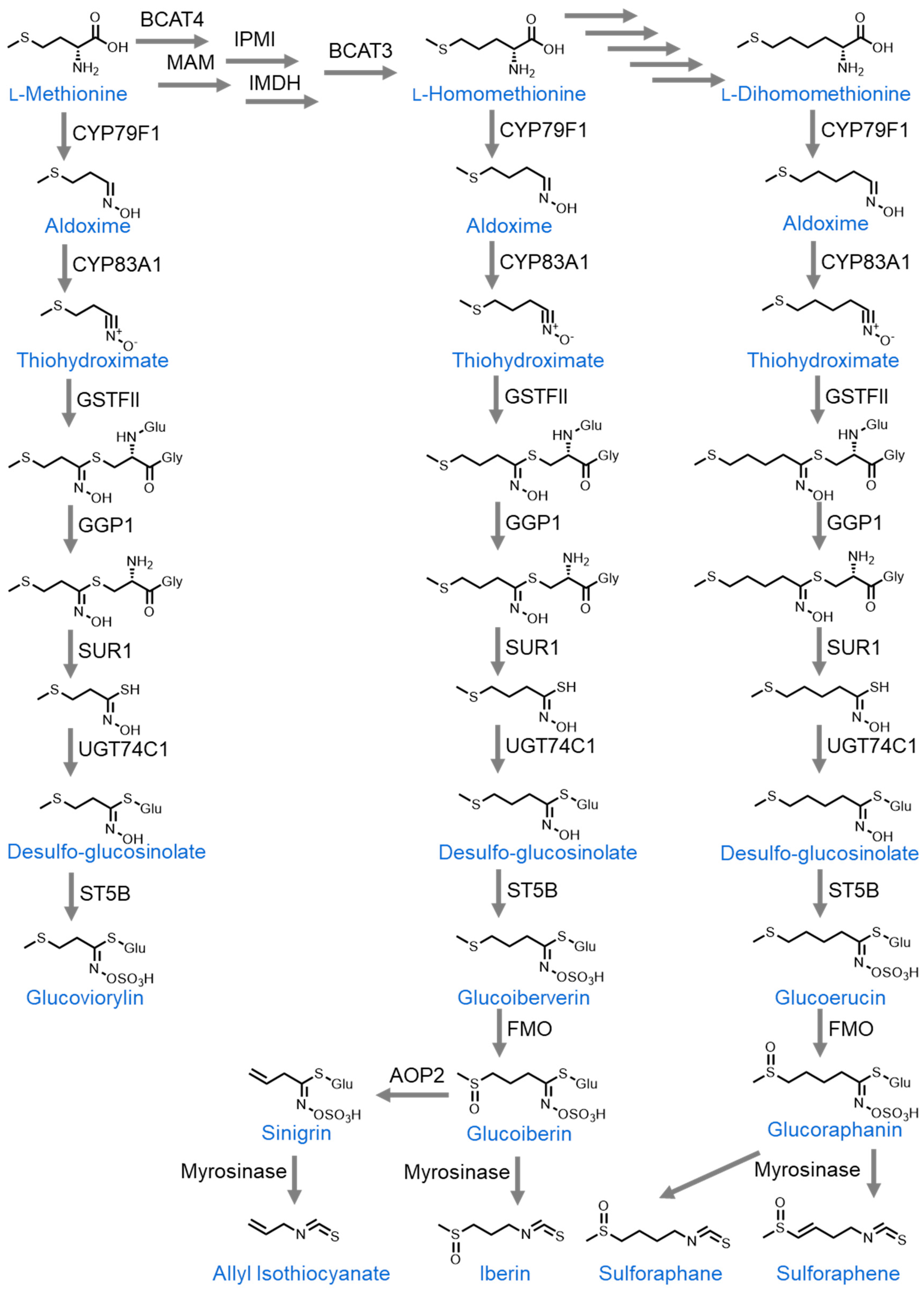

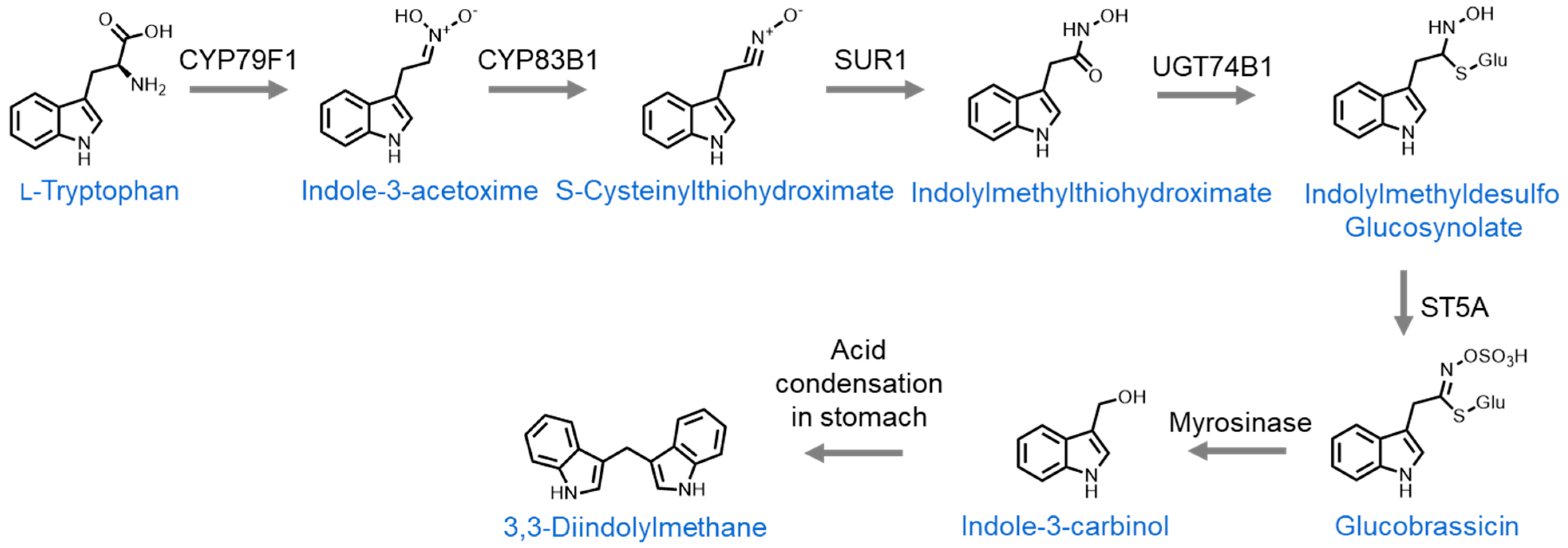

| Compound | Summary | Positive Dosing | Negative Dosing | Citations |
|---|---|---|---|---|
| Sulforaphane | Induced cell cycle arrest and apoptosis, and reduced angiogenesis and migration in vitro. Reduced xenograft size and tumor initiation in vivo. | 7–60 µM in cell culture, 0.08 µmoles via daily intraperitoneal injection, 300 ppm in diet (1.6 mg/day). | [47,48,49,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] | |
| Sulforaphene | Induced cell cycle arrest at the G2/M phase, upregulation of the JNK pathway, inhibition of microtubule polymerization, and increase in intracellular reactive oxygen species in vitro. Reduced xenograft size in vivo. | 5 µM in cell culture, 5 mg/kg of body weight daily intraperitoneal injection. | [74,75] | |
| Allyl isothiocyanate (AITC) | Induced cell cycle arrest and apoptosis, and reduced migration in vitro. | 10–20 µM in cell culture. | [76,77] | |
| Phenethyl isothiocyanate (PEITC) | Reduced viability, stemness, and spheroid formation in vitro. Reduced xenograft size, and tumor initiation in vivo. | 12–88 µM in vitro, 60 mg/kg of body weight daily intraperitoneal injection, and 20 mg/kg of body weight daily oral administration. | [78,79,80] | |
| Benzyl isothiocyanate (BITC) | Induced cell cycle arrest and apoptosis in vitro. Increased tumor initiation in vivo. | 10–20 µM in cell culture. | 0.5 g/kg of diet | [81,82,83] |
| Iberin | Reduced proliferation, methyl guanine methyl transferase methylation, and increased miRNA expression in vitro. | 8–10 µM in cell culture. | [84,85] | |
| Indole-3-carbinol | Reduced viability and proliferation, plus p53 upregulation in vitro. Both reduced and increased tumor initiation in vivo. | 500 µM to 1 mM in cell culture, 1 g/kg of diet. | 1 g/kg of diet. | [65,86,87,88,89,90,91] |
| 3,3′-Diindolylmethane | Reduced viability, cell cycle arrest, and COX1/2 and ERK1/2 protein inhibition in vitro. Reduced xenograft size in vivo. | 25–56 µM in cell culture, 40 mg/kg of body weight via intraperitoneal injection. | 40 mg/kg of body weight via oral administration. | [92,93,94,95,96,97] |
| Allicin | Reduced viability, proliferation, and migration in vitro. Reduced xenograft size and tumor initiation in vivo. | 25–50 µM for viability and 4 µg/mL for migration in cell culture. Intraperitoneal injection of 5 mg/kg of body weight and 48 mg/kg of diet. | [98,99,100,101] | |
| Alliin/S-Allyl-L-cysteine sulfoxide (SACS) | Reduced viability and EGFR (epithelial growth factor receptor) expression in vitro. Reduced tumor initiation in vivo. | 100 µg/mL in cell culture, 125 mg/kg of diet, 200 mg/kg of body weight administered orally. | [102,103,104,105] | |
| Diallyl sulfide (DAS) | Induced apoptosis and inhibited migration/metastasis in vitro. Non-significant reductions in xenograft size and tumor initiation in vivo. | 50 µM in cell culture. | Intraperitoneal injection of 6 mg/kg of body weight and 300 ppm in diet. | [106,107,108,109] |
| Diallyl disulfide (DADS) | Reduced viability, migration, and invasion, and increased apoptosis in vitro. Reduced metastasis, xenograft size (best in TRAIL overexpressing tumors), and tumor initiation in vivo. | 5–50 µM for viability and 25 µM for migration in cell culture. Intraperitoneal injection of 100 mg/kg of body weight and 42 ppm in diet. | [107,108,109,110,111,112,113,114,115,116] | |
| Diallyl trisulfide (DATS) | Induced apoptosis, reduced stem cell viability, and reduced migration/invasion in vitro. Reduced xenograft size and tumor initiation in vivo. | 30–40 µM for apoptosis, 40 µM for stem cells, 10 µM for migration. Intraperitoneal injection of 6 mg/kg of body weight and oral administration of 50 mg/kg of body weight. | [23,107,108,117,118,119,120] | |
| Diallyl tetrasulfide | Induced cell cycle arrest, apoptosis, and reduced spheroid formation in vitro. Modified molecule reduced xenograft size in vivo. | 10–50 µM in cell culture. An amount of 50 µM dibenzyl tetrasulfide in zebrafish. | [121] | |
| Methylsulfonylmethane (MSM) ((dimethyl sulfone (DMSO2)) | Reduced viability, stemness, and spheroid formation in vitro. | 100–250 mM in cell culture. | [24] | |
| (Z)-ajoene | Reduced viability and Wnt/β-catenin pathway inhibition in vitro. | 30 µM for 72 h of treatment. | [122] | |
| S-allylmercaptocysteine (SAMC) | Reduced proliferation, induced apoptosis, and reduced migration in vitro. Reduced xenograft size in combination treatment in vivo. | 200–450 µM for apoptosis, 400 µM for migration, and 300 mg/kg body weight/day administered orally in combination with rapamycin treatment. | [123,124,125,126,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAlpine, P.L.; Fernández, J.; Villar, C.J.; Lombó, F. Organosulfur Compounds in Colorectal Cancer Prevention and Progression. Nutrients 2024, 16, 802. https://doi.org/10.3390/nu16060802
McAlpine PL, Fernández J, Villar CJ, Lombó F. Organosulfur Compounds in Colorectal Cancer Prevention and Progression. Nutrients. 2024; 16(6):802. https://doi.org/10.3390/nu16060802
Chicago/Turabian StyleMcAlpine, Patrick L., Javier Fernández, Claudio J. Villar, and Felipe Lombó. 2024. "Organosulfur Compounds in Colorectal Cancer Prevention and Progression" Nutrients 16, no. 6: 802. https://doi.org/10.3390/nu16060802
APA StyleMcAlpine, P. L., Fernández, J., Villar, C. J., & Lombó, F. (2024). Organosulfur Compounds in Colorectal Cancer Prevention and Progression. Nutrients, 16(6), 802. https://doi.org/10.3390/nu16060802







