Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer
Abstract
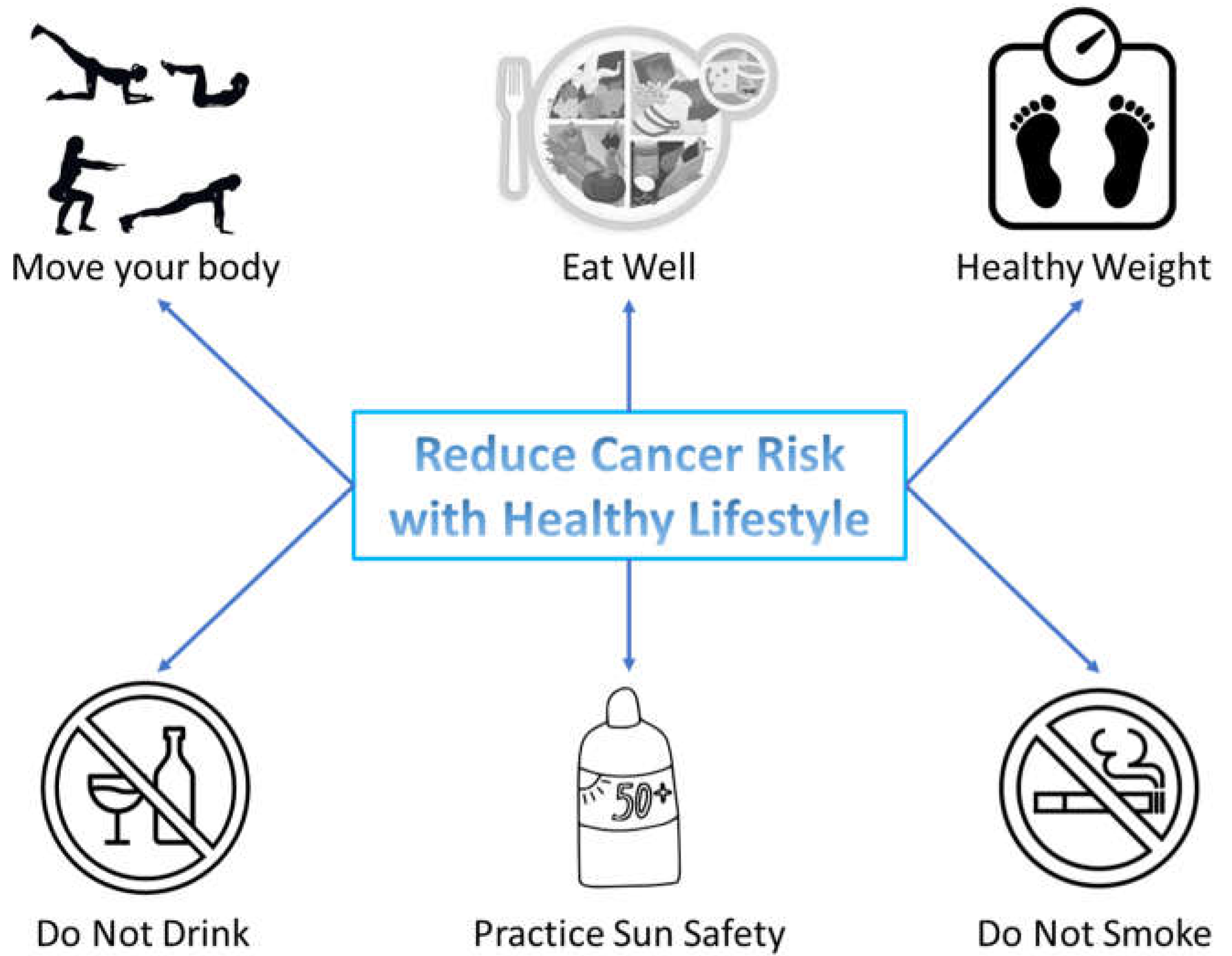
1. Introduction
1.1. Healthy Lifestyle Factors
1.2. Life’s Essential 8 and Cancer
2. Healthy Lifestyle and Cancer
2.1. Overweight and Obesity
2.2. Diet
2.3. Vitamin D
2.4. Physical Activity
2.5. Alcohol Consumption
2.6. Smoking
3. Healthy Lifestyle and Breast Cancer
3.1. Overweight and Obesity
3.2. Diet
3.3. Vitamin D
3.4. Physical Activity
3.5. Alcohol Consumption
3.6. Smoking
4. Lifestyle and Colorectal Cancer
4.1. Overweight and Obesity
4.2. Diet
4.3. Vitamin D
4.4. Physical Activity
4.5. Alcohol Consumption
4.6. Smoking
5. Lifestyle and Prostate Cancer
5.1. Overweight and Obesity
5.2. Diet
5.3. Vitamin D
5.4. Physical Activity
5.5. Alcohol Consumption
5.6. Smoking
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Zhang, Y.-G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.C.; Baltar, V.T.; Marchioni, D.M. Breast cancer and dietary patterns: A systematic review. Nutr. Rev. 2014, 72, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Morton, V.; Norat, T.; Moreira, A.; Potts, J.F.; Reeves, T.; Bakolis, I. Dietary patterns derived from principal component analysis (PCA) and risk of colorectal cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2019, 73, 366–386. [Google Scholar] [CrossRef]
- Bostan, N.; Beser, A. Factors affecting the healthy lifestyle behaviors of nurses/Hemsirelerin saglikli yasam bicimi davranislarini etkileyen faktorler. J. Educ. Res. Nurs. 2017, 14, 38–45. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Sert, H. Healthy lifestyle behaviors of university students and related factors. Acta Paul. De Enferm. 2021, 34, eAPE02942. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: http://dietandcancerreport.org (accessed on 25 October 2023).
- Zhu, M.; Wang, T.; Huang, Y.; Zhao, X.; Ding, Y.; Zhu, M.; Ji, M.; Wang, C.; Dai, J.; Yin, R. Genetic risk for overall cancer and the benefit of adherence to a healthy lifestyle. Cancer Res. 2021, 81, 4618–4627. [Google Scholar] [CrossRef]
- Jacob, L.; Freyn, M.; Kalder, M.; Dinas, K.; Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: A retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 2018, 9, 17420. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, F.D.; Ahmed, H.G.; Alshammari, D.; Alharbi, A.M.; Alsaedi, A.S.; Elasbaly, A. Population insight of the relationship between lifestyle and cancer: A population-based survey. AIMS Public Health 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Schmalwieser, A.W. Possibilities to estimate the personal UV radiation exposure from ambient UV radiation measurements. Photochem. Photobiol. Sci. 2020, 19, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; Yazar, S.; Young, A.R.; Norval, M.; de Gruijl, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.F.; Lu, K.Q. Vitamin D as a therapeutic option for sunburn: Clinical and biologic implications. DNA Cell Biol. 2017, 36, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral vitamin D rapidly attenuates inflammation from sunburn: An interventional study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef]
- Alfredsson, L.; Armstrong, B.K.; Butterfield, D.A.; Chowdhury, R.; de Gruijl, F.R.; Feelisch, M.; Garland, C.F.; Hart, P.H.; Hoel, D.G.; Jacobsen, R.; et al. Insufficient sun exposure has become a real public health problem. Int. J. Environ. Res. Public Health 2020, 17, 5014. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC–Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef]
- Hawkes, A.L.; Chambers, S.K.; Pakenham, K.I.; Patrao, T.A.; Baade, P.D.; Lynch, B.M.; Aitken, J.F.; Meng, X.; Courneya, K.S. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: A randomized controlled trial. J. Clin. Oncol. 2013, 31, 2313–2321. [Google Scholar] [CrossRef]
- Spring, B.; Moller, A.C.; Coons, M.J. Multiple health behaviours: Overview and implications. J. Public Health 2012, 34 (Suppl. S1), i3–i10. [Google Scholar] [CrossRef] [PubMed]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to multiple health behaviours in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef]
- Dalla Via, J.; Daly, R.; Fraser, S. The effect of exercise on bone mineral density in adult cancer survivors: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Bridgewater, J.; Steptoe, A.; Wardle, J. Lifestyle and quality of life in colorectal cancer survivors. Qual. Life Res. 2011, 20, 1237–1245. [Google Scholar] [CrossRef]
- Veronese, N.; Li, Y.; Manson, J.E.; Willett, W.C.; Fontana, L.; Hu, F.B. Combined associations of body weight and lifestyle factors with all cause and cause specific mortality in men and women: Prospective cohort study. BMJ 2016, 355, i5855. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kaluza, J.; Wolk, A. Combined impact of healthy lifestyle factors on lifespan: Two prospective cohorts. J. Intern. Med. 2017, 282, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Dinh, V.; Hosalli, R.; Aggarwal, B.A.; German, C.A.; Mullachery, P. Life’s Essential 8 and Mortality Risk: Associations of an Enhanced Cardiovascular Health Construct with All-Cause, Cardiovascular, and Cancer Mortality in US Adults From the 2011–2018 National Health and Nutrition Examination Survey. Circulation 2023, 147 (Suppl. S1), A62. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, W.; He, Y.; Da, M. The association between Life’s Essential 8 and all-cause, cancer and non-cancer mortality in US Cancer Survivors: A retrospective cohort study of NHANES. Prev. Med. 2024, 179, 107853. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, Z.; Zhang, Y.; Zhang, Q.; Chen, Z.; Tse, G.; Li, G.; Liu, T.; Wu, S. Association of Life’s Essential 8 with incident atherosclerotic cardiovascular disease in cancer patients: The Kailuan prospective cohort study. Eur. J. Prev. Cardiol. 2023, 30, e78–e80. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, Y.; Yu, Y.; Wang, Y.; Chen, C.; Tan, X.; Lu, Y.; Wang, N. Life’s Essential 8 and risk of non-communicable chronic diseases: Outcome-wide analyses. Chin. Med. J. 2023, 10, 1097. [Google Scholar] [CrossRef]
- Yi, J.; Wang, L.; Guo, X.; Ren, X. Association of Life’s Essential 8 with all-cause and cardiovascular mortality among US adults: A prospective cohort study from the NHANES 2005–2014. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, H.; Li, X.; Heianza, Y.; Manson, J.E.; Franco, O.H.; Qi, L. Life’s Essential 8 and Life Expectancy Free of Cardiovascular Disease, Diabetes, Cancer, and Dementia in Adults. Circulation 2023, 147 (Suppl. S1), A63. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Bandrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA A Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Sauer, A.G.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA A Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Islami, F.; Sauer, A.G.; Gapstur, S.M.; Jemal, A. Proportion of cancer cases attributable to excess body weight by US state, 2011–2015. JAMA Oncol. 2019, 5, 384–392. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Lazarus, E.; Bays, H.E. Cancer and obesity: An obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes. Pillars 2022, 3, 100026. [Google Scholar] [CrossRef]
- Célind, J.; Bygdell, M.; Martikainen, J.; Ohlsson, C.; Kindblom, J.M. Childhood overweight and risk of obesity-related adult cancer in men. Cancer Commun. 2022, 42, 576. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Almendros, I.; Martinez-Garcia, M.A.; Farré, R.; Gozal, D. Obesity, sleep apnea, and cancer. Int. J. Obes. 2020, 44, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, N.; Avgerinos, K.I.; Mantzoros, C.S.; Dalamaga, M. Classic and novel adipocytokines at the intersection of obesity and cancer: Diagnostic and therapeutic strategies. Curr. Obes. Rep. 2018, 7, 260–275. [Google Scholar] [CrossRef]
- Harris, B.H.L.; Macaulay, V.M.; Harris, D.A.; Klenerman, P.; Karpe, F.; Lord, S.R.; Harris, A.L.; Buffa, F.M. Obesity: A perfect storm for carcinogenesis. Cancer Metastasis Rev. 2022, 41, 491–515. [Google Scholar] [CrossRef]
- Levin, H.S.; Temkin, N.R.; Barber, J.; Nelson, L.D.; Robertson, C.; Brennan, J.; Stein, M.B.; Yue, J.K.; Giacino, J.T.; McCrea, M.A.; et al. Association of obesity with survival outcomes in patients with cancer: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Johansson, M.; Carreras-Torres, R.; Scelo, G.; Purdue, M.P.; Mariosa, D.; Muller, D.C.; Timpson, N.J.; Haycock, P.C.; Brown, K.M.; Wang, Z.; et al. The influence of obesity-related factors in the etiology of renal cell carcinoma—A mendelian randomization study. PLoS Med. 2019, 16, e1002724. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet Diabetes Endocrinol. 2014, 383, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Castelló, A.; Benavente, Y.; Marcos-Gragera, R.; Amiano, P.; Gracia-Lavedan, E.; Costas, L.; Robles, C.; Gonzalez-Barca, E.; de la Banda, E.; et al. Adherence to the Western, Prudent, and Mediterranean dietary patterns and chronic lymphocytic leukemia in the MCC-Spain study. Haematologica 2018, 103, 1881. [Google Scholar] [CrossRef] [PubMed]
- Fliss-Isakov, N.; Kariv, R.; Webb, M.; Ivancovsky, D.; Margalit, D.; Zelber-Sagi, S. Mediterranean dietary components are inversely associated with advanced colorectal polyps: A case-control study. World J. Gastroenterol. WJG 2018, 24, 2617. [Google Scholar] [CrossRef] [PubMed]
- Farràs Mañé, M.; Almanza-Aguilera, E.; Hernáez, Á.; Agustí, N.; Julve, J.; Castañer, O. Beneficial effects of olive oil and Mediterranean diet on cancer physio-pathology and incidence. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The mediterranean diet: From an environment-driven food culture to an emerging medical prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef]
- Muffone, A.R.M.; de Oliveira Lübke, P.D.; Rabito, E.I. Mediterranean diet and infertility: A systematic review with meta-analysis of cohort studies. Nutr. Rev. 2023, 81, 775–789. [Google Scholar] [CrossRef]
- Goñi, I.; Hernández-Galiot, A. Intake of nutrient and non-nutrient dietary antioxidants. contribution of macromolecular antioxidant polyphenols in an elderly Mediterranean population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef]
- De Matteis, C.; Crudele, L.; Gadaleta, R.M.; Di Buduo, E.; Novielli, F.; Petruzzelli, S.; Cariello, M.; Moschetta, A. Low Adherence to Mediterranean Diet Characterizes Metabolic Patients with Gastrointestinal Cancer. Nutrients 2024, 16, 630. [Google Scholar] [CrossRef]
- Agaj, A.; Peršurić, Ž.; Pavelić, S.K. Mediterranean Food Industry By-Products as a Novel Source of Phytochemicals with a Promising Role in Cancer Prevention. Molecules 2022, 27, 8655. [Google Scholar] [CrossRef] [PubMed]
- Kuan, A.S.; Green, J.; Kitahara, C.M.; De González, A.B.; Key, T.; Reeves, G.K.; Floud, S.; Balkwill, A.; Bradbury, K.; Liao, L.M.; et al. Diet and risk of glioma: Combined analysis of 3 large prospective studies in the UK and USA. Neuro-Oncol. 2019, 21, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Farvid, M.S.; Poustchi, H.; Murphy, G.; Etemadi, A.; Hekmatdoost, A.; Kamangar, F.; Sheikh, M.; Pourshams, A.; Sepanlou, S.G.; et al. The application of six dietary scores to a Middle Eastern population: A comparative analysis of mortality in a prospective study. Eur. J. Epidemiol. 2019, 34, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Yuan, C.; Sato, K.; Hollis, B.W.; Zhang, S.; Niedzwiecki, D.; Ou, F.-S.; Chang, I.-W.; O’Neil, B.H.; Innocenti, F.; Lenz, H.-J.; et al. Plasma 25-hydroxyvitamin D levels and survival in patients with advanced or metastatic colorectal cancer: Findings from CALGB/SWOG 80405 (Alliance). Clin. Cancer Res. 2019, 25, 7497–7505. [Google Scholar] [CrossRef]
- Talib, W.H.; Jum’ah, D.A.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front. Nutr. 2023, 10, 1281879. [Google Scholar] [CrossRef] [PubMed]
- Yonaga, H.; Okada, S.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect modification of vitamin D supplementation by histopathological characteristics on survival of patients with digestive tract cancer: Post hoc analysis of the AMATERASU randomized clinical trial. Nutrients 2019, 11, 2547. [Google Scholar] [CrossRef]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of high-dose vs. standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The SUNSHINE randomized clinical trial. JAMA 2019, 321, 1370–1379. [Google Scholar] [CrossRef]
- Urashima, M.; Ohdaira, H.; Akutsu, T.; Okada, S.; Yoshida, M.; Kitajima, M.; Suzuki, Y. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: The AMATERASU randomized clinical trial. JAMA 2019, 321, 1361–1369. [Google Scholar] [CrossRef]
- Machado, M.R.M.; Almeida-Filho, B.d.S.; Vespoli, H.D.L.; Schmitt, E.B.; Nahas-Neto, J.; Nahas, E.A. Low pretreatment serum concentration of vitamin D at breast cancer diagnosis in postmenopausal women. Menopause 2019, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The role of vitamin D in breast cancer risk and progression. Endocr.-Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, H.; Zhao, Y.; Hu, C.; Atanda, A.; Qin, X.; Jia, P.; Jiang, Y.; Qi, Z. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: A systematic review and dose-response meta-analysis. BMJ Open 2019, 9, e030513. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Mandle, H.B.; Zhu, W.; Hughes, D.J.; Siddiq, A.; Ferrari, P.; Romieu, I.; Riboli, E.; Bueno-De-Mesquita, B.; van Duijnhoven, F.J.; et al. Vitamin D-related genes, blood vitamin D levels and colorectal cancer risk in western european populations. Nutrients 2019, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Nettey, O.S.; Gogana, P.; Sheikh, U.; Macias, V.; Kajdacsy-Balla, A.; Sharifi, R.; Kittles, R.A.; Murphy, A.B. Physiologic serum 1, 25 dihydroxyvitamin D is inversely associated with prostatic Ki67 staining in a diverse sample of radical prostatectomy patients. Cancer Causes Control 2019, 30, 207–214. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Steck, S.E.; Arab, L.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Johnson, C.S.; Mohler, J.L.; Smith, G.J.; Su, L.J.; et al. Association among plasma 1, 25(OH)2D, ratio of 1, 25(OH)2D to 25(OH)D, and prostate cancer aggressiveness. Prostate 2019, 79, 1117–1124. [Google Scholar] [CrossRef]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Sun, R.; Xie, Z. Vitamin D receptor regulates proliferation and differentiation of thyroid carcinoma via the E-cadherin-β-catenin complex. J. Mol. Endocrinol. 2022, 68, 137–151. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A narrative review of the evidence for variations in serum 25-hydroxyvitamin D concentration thresholds for optimal health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Rihal, V.; Khan, H.; Kaur, A.; Singh, T.G. Vitamin D as therapeutic modulator in cerebrovascular diseases: A mechanistic perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 7772–7794. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic health, mitochondrial fitness, physical activity, and cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical activity in cancer prevention and survival: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.N.; Clare, P.J.; Katzmarzyk, P.T.; Cruz, B.d.P.; Lee, I.M.; Stamatakis, E. Vigorous physical activity, incident heart disease, and cancer: How little is enough? Eur. Heart J. 2022, 43, 4801–4814. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors after colon cancer diagnosis: The CALGB 89803/Alliance trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, C.; Chorin, F.; Beltran, E.E.M.; Neuzillet, C.; Cardot-Ruffino, V. Physical activity and mortality in cancer survivors: A systematic review and meta-analysis. JNCI Cancer Spectr. 2020, 4, 5402. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef]
- Anderson, B.O.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D.; et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Klein, W.M.; Jacobsen, P.B.; Helzlsouer, K.J. Alcohol and cancer risk: Clinical and research implications. JAMA 2020, 323, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose–response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018, 138, 345–355. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean diet and its benefits on health and mental health: A literature review. Clin. Pract. Epidemiol. Ment. Health: CP EMH 2020, 16 (Suppl. S1), 156–164. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.D.; McQueenie, R.; Nicholl, B.I.; Field, R.; Hanlon, P.; Gallacher, K.I.; Mair, F.S.; Lewsey, J. Association between patterns of alcohol consumption (beverage type, frequency and consumption with food) and risk of adverse health outcomes: A prospective cohort study. BMC Med. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An aspiring agent in promoting longevity and preventing chronic diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health: A critical review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Barbería-Latasa, M.; Gea, A.; Martínez-González, M.A. Alcohol, drinking pattern, and chronic disease. Nutrients 2022, 14, 1954. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Liao, L.M.; Reyes-Guzman, C.; Hartge, P.; Caporaso, N.; Freedman, N.D. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health–AARP Diet and Health Study. JAMA Intern. Med. 2017, 177, 87–95. [Google Scholar] [CrossRef]
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung cancer screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- McGee, E.E.; Jackson, S.S.; Petrick, J.L.; Van Dyke, A.L.; Adami, H.-O.; Albanes, D.; Andreotti, G.; Beane-Freeman, L.E.; de Gonzalez, A.B.; Buring, J.E.; et al. Smoking, alcohol, and biliary tract cancer risk: A pooling project of 26 prospective studies. JNCI J. Natl. Cancer Inst. 2019, 111, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Carter, P.; Kar, S.; Vithayathil, M.; Mason, A.M.; Michaëlsson, K.; Burgess, S. Smoking, alcohol consumption, and cancer: A mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 2020, 17, e1003178. [Google Scholar] [CrossRef] [PubMed]
- Krutz, M.; Acharya, P.; Chissoe, G.; Raj, V.; Driskill, L.; Krempl, G.; Zhao, D.; Mhawej, R.; Queimado, L. Tobacco cessation after head and neck cancer diagnosis is an independent predictor of treatment response and long-term survival. Oral Oncol. 2022, 134, 106072. [Google Scholar] [CrossRef]
- Wills, L.; Ables, J.L.; Braunscheidel, K.M.; Caligiuri, S.P.; Elayouby, K.S.; Fillinger, C.; Ishikawa, M.; Moen, J.K.; Kenny, P.J. Neurobiological mechanisms of nicotine reward and aversion. Pharmacol. Rev. 2022, 74, 271–310. [Google Scholar] [CrossRef]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C.; Ba, K.S.A. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA A Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef]
- Gritz, E.R.; Talluri, R.; Domgue, J.F.; Tami-Maury, I.; Shete, S. Smoking behaviors in survivors of smoking-related and non–smoking-related cancers. JAMA Netw. Open 2020, 3, e209072. [Google Scholar] [CrossRef]
- Sheikh, M.; Mukeriya, A.; Shangina, O.; Brennan, P.; Zaridze, D. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: A prospective cohort study. Ann. Intern. Med. 2021, 174, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F., Jr.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Narod, S.A.; Iqbal, J.; Miller, A.B. Why have breast cancer mortality rates declined? J. Cancer Policy 2015, 5, 8–17. [Google Scholar] [CrossRef]
- Britt, K.L.; Cuzick, J.; Phillips, K.-A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, M.; Niakan, B.; Keshtkar, A.; Rafiei, E.; Salamat, F. Subtypes of benign breast disease as a risk factor for breast cancer: A systematic review and meta-analysis protocol. Iran. J. Med. Sci. 2018, 43, 355. [Google Scholar]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Gard, C.C.; Tice, J.A.; Ziv, E.; Cummings, S.R.; Miglioretti, D.L.; Consortium, B.C.S. Risk factors that increase risk of estrogen receptor–positive and–negative breast cancer. J. Natl. Cancer Inst. 2017, 109, djw276. [Google Scholar] [CrossRef]
- Bravi, F.; Decarli, A.; Russo, A.G. Risk factors for breast cancer in a cohort of mammographic screening program: A nested case–control study within the FR iCaM study. Cancer Med. 2018, 7, 2145–2152. [Google Scholar] [CrossRef]
- Chen, M.-J.; Wu, W.Y.-Y.; Yen, A.M.-F.; Fann, J.C.-Y.; Chen, S.L.-S.; Chiu, S.Y.-H.; Chen, H.-H.; Chiou, S.-T. Body mass index and breast cancer: Analysis of a nation-wide population-based prospective cohort study on 1 393 985 Taiwanese women. Int. J. Obes. 2016, 40, 524–530. [Google Scholar] [CrossRef]
- Miller, E.; Wilson, C.; Chapman, J.; Flight, I.; Nguyen, A.-M.; Fletcher, C.; Ramsey, I. Connecting the dots between breast cancer, obesity and alcohol consumption in middle-aged women: Ecological and case control studies. BMC Public Health 2018, 18, 460. [Google Scholar] [CrossRef]
- van den Brandt, P.A.; Ziegler, R.G.; Wang, M.; Hou, T.; Li, R.; Adami, H.-O.; Agnoli, C.; Bernstein, L.; Buring, J.E.; Chen, Y. Body size and weight change over adulthood and risk of breast cancer by menopausal and hormone receptor status: A pooled analysis of 20 prospective cohort studies. Eur. J. Epidemiol. 2021, 36, 37–55. [Google Scholar] [CrossRef]
- Rosner, B.; Eliassen, A.H.; Toriola, A.T.; Chen, W.Y.; Hankinson, S.E.; Willett, W.C.; Berkey, C.S.; Colditz, G.A. Weight and weight changes in early adulthood and later breast cancer risk. Int. J. Cancer 2017, 140, 2003–2014. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Chaiwerawattana, A.; Ploysawang, P.; Nooklang, K.; Jamsri, P.; Somharnwong, S. Obesity, diet and physical inactivity and risk of breast cancer in Thai women. Asian Pac. J. Cancer Prev. 2013, 14, 7023–7027. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.J.; Han, W. A review of the epidemiology of breast cancer in Asia: Focus on risk factors. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.; Andergassen, U.; Hepp, P.; Schindlbeck, C.; Friedl, T.W.; Harbeck, N.; Kiechle, M.; Sommer, H.; Hauner, H.; Friese, K. Obesity as an independent risk factor for decreased survival in node-positive high-risk breast cancer. Breast Cancer Res. Treat. 2015, 151, 569–576. [Google Scholar] [CrossRef]
- Taha, Z.; Eltom, S.E. The role of diet and lifestyle in women with breast cancer: An update review of related research in the Middle East. BioResearch Open Access 2018, 7, 73–80. [Google Scholar] [CrossRef]
- Jia, T.; Liu, Y.; Fan, Y.; Wang, L.; Jiang, E.J. Association of healthy diet and physical activity with breast cancer: Lifestyle interventions and oncology education. Front. Public Health 2022, 10, 558. [Google Scholar] [CrossRef]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary patterns and breast cancer risk: A systematic review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Hua, T.N. Impact of lifestyle behaviors on cancer risk and prevention. J. Lifestyle Med. 2021, 11, 1. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef]
- Lo, J.J.; Park, Y.-M.; Sinha, R.; Sandler, D.P. Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int. J. Cancer 2020, 146, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C. Dietary modification and breast cancer mortality: Long-term follow-up of the Women’s Health Initiative randomized trial. J. Clin. Oncol. 2020, 38, 1419. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M. Diet and risk of breast cancer. Contemp. Oncol. Współczesna Onkol. 2016, 20, 13–19. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential role of natural antioxidant products in oncological diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef]
- Zhang, D.; Nichols, H.B.; Troester, M.; Cai, J.; Bensen, J.T.; Sandler, D.P. Tea consumption and breast cancer risk in a cohort of women with family history of breast cancer. Int. J. Cancer 2020, 147, 876–886. [Google Scholar] [CrossRef]
- Ratnani, S.; Malik, S. Therapeutic properties of green tea: A review. J. Multidiscip. Appl. Nat. Sci. 2022, 2, 90–102. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; Romanos-Nanclares, A.; Navarro, A.M.; Gea, A.; Cervantes, S.; Martínez-González, M.Á.; Toledo, E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020, 59, 3461–3471. [Google Scholar] [CrossRef]
- Farvid, M.S.; Spence, N.D.; Rosner, B.A.; Willett, W.C.; Eliassen, A.H.; Holmes, M.D. Post-diagnostic coffee and tea consumption and breast cancer survival. Br. J. Cancer 2021, 124, 1873–1881. [Google Scholar] [CrossRef]
- DeBose, M.M.; Cormier, P.; Lewis, K.; Harris, A.L. Breast Cancer Risk, Coffee Consumption, and Postdiagnosis Survival. Nurs. Women’s Health 2023, 27, 378–384. [Google Scholar] [CrossRef]
- Christakos, S.; Li, S.; De La Cruz, J.; Bikle, D.D. New developments in our understanding of vitamin D metabolism, action and treatment. Metabolism 2019, 98, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol./Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef]
- Atoum, M.; Alzoughool, F. Vitamin D and breast cancer: Latest evidence and future steps. Breast Cancer Basic Clin. Res. 2017, 11, 1178223417749816. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D signaling in inflammation and cancer: Molecular mechanisms and therapeutic implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.H.; Jeon, J.Y.; Ryu, J.; Kim, S.; Kim, J.Y.; Park, H.S.; Kim, S.I.; Park, B.-W. Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among Korean women: A case–control study. Breast Cancer Res. Treat. 2015, 152, 147–154. [Google Scholar] [CrossRef]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and risk of breast cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019, 21, 84. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations≥ 60 vs< 20 ng/mL (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Shamsi, U.; Khan, S.; Azam, I.; Khan, A.H.; Maqbool, A.; Hanif, M.; Gill, T.; Iqbal, R.; Callen, D. A multicenter case control study of association of vitamin D with breast cancer among women in Karachi, Pakistan. PLoS ONE 2020, 15, e0225402. [Google Scholar] [CrossRef]
- Niedermaier, T.; Gredner, T.; Kuznia, S.; Schöttker, B.; Mons, U.; Lakerveld, J.; Ahrens, W.; Brenner, H.; On behalf of the PEN-Consortium. Vitamin D food fortification in European countries: The underused potential to prevent cancer deaths. Eur. J. Epidemiol. 2022, 37, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Q.; Zhang, Y.; Xie, Q.; Tan, X. Physical activity and risk of breast cancer: A meta-analysis of 38 cohort studies in 45 study reports. Value Health 2019, 22, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Boraka, Ö.; Klintman, M.; Rosendahl, A.H. Physical activity and long-term risk of breast cancer, associations with time in life and body composition in the prospective Malmö diet and cancer study. Cancers 2022, 14, 1960. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef]
- Neilson, H.K.; Farris, M.S.; Stone, C.R.; Vaska, M.M.; Brenner, D.R.; Friedenreich, C.M. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: A systematic review and meta-analysis. Menopause 2017, 24, 322–344. [Google Scholar] [CrossRef]
- McTiernan, A.; Kooperberg, C.; White, E.; Wilcox, S.; Coates, R.; Adams-Campbell, L.L.; Woods, N.; Ockene, J. Recreational physical activity and the risk of breast cancer in postmenopausal women: The Women’s Health Initiative Cohort Study. JAMA Oncol. 2003, 290, 1331–1336. [Google Scholar] [CrossRef]
- Holick, C.N.; Newcomb, P.A.; Trentham-Dietz, A.; Titus-Ernstoff, L.; Bersch, A.J.; Stampfer, M.J.; Baron, J.A.; Egan, K.M.; Willett, W.C. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 379–386. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, K.-Y.; Goodman, M.T. Differences in incidence, mortality and survival of breast cancer by regions and countries in Asia and contributing factors. Asian Pac. J. Cancer Prev. 2015, 16, 2857–2870. [Google Scholar] [CrossRef]
- Lee, J. A meta-analysis of the association between physical activity and breast cancer mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Tobar, J.S.S.; Dardes, R.; Thuler, L.C.S. Alcohol consumption as a risk factor for breast cancer development: A case-control study in Brazil. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 703. [Google Scholar] [CrossRef] [PubMed]
- Erol, A.; Ho, A.M.C.; Winham, S.J.; Karpyak, V.M. Sex hormones in alcohol consumption: A systematic review of evidence. Addict. Biol. 2019, 24, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Zeinomar, N.; Knight, J.A.; Genkinger, J.M.; Phillips, K.-A.; Daly, M.B.; Milne, R.L.; Dite, G.S.; Kehm, R.D.; Liao, Y.; Southey, M.C. Alcohol consumption, cigarette smoking, and familial breast cancer risk: Findings from the Prospective Family Study Cohort (ProF-SC). Breast Cancer Res. 2019, 21, 128. [Google Scholar] [CrossRef]
- Fakhri, N.; Chad, M.A.; Lahkim, M.; Houari, A.; Dehbi, H.; Belmouden, A.; El Kadmiri, N. Risk factors for breast cancer in women: An update review. Med. Oncol. 2022, 39, 197. [Google Scholar] [CrossRef]
- Romieu, I.; Scoccianti, C.; Chajès, V.; De Batlle, J.; Biessy, C.; Dossus, L.; Baglietto, L.; Clavel-Chapelon, F.; Overvad, K.; Olsen, A. Alcohol intake and breast cancer in the E uropean prospective investigation into cancer and nutrition. Int. J. Cancer 2015, 137, 1921–1930. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, N.; Colditz, G.A. Links between alcohol consumption and breast cancer: A look at the evidence. Women’s Health 2015, 11, 65–77. [Google Scholar] [CrossRef]
- Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Wu, Y.; Zheng, Y.; Zhai, Z.; Hao, Q.; Song, D. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the Global Burden of Disease Study 2017. J. Hematol. Oncol. Rep. 2019, 12, 140. [Google Scholar] [CrossRef]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast cancer primary prevention and diet: An umbrella review. Int. J. Environ. Res. Public Health 2020, 17, 4731. [Google Scholar] [CrossRef]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global increase in breast cancer incidence: Risk factors and preventive measures. BioMed Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef]
- Wada, K.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Husband’s smoking status and breast cancer risk in Japan: From the Takayama study. Cancer Sci. 2015, 106, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Margolis, K.L.; Wactawski-Wende, J.; Horn, K.; Messina, C.; Stefanick, M.L.; Tindle, H.A.; Tong, E.; Rohan, T.E. Association of active and passive smoking with risk of breast cancer among postmenopausal women: A prospective cohort study. BMJ 2011, 342, d1016. [Google Scholar] [CrossRef]
- French, D.P.; Howell, A.; Evans, D.G. Psychosocial issues of a population approach to high genetic risk identification: Behavioural, emotional and informed choice issues. Breast 2018, 37, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Schoemaker, M.J.; Wright, L.B.; Ashworth, A.; Swerdlow, A.J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 2017, 19, 118. [Google Scholar] [CrossRef]
- Tong, J.-h.; Li, Z.; Shi, J.; Li, H.-m.; Wang, Y.; Fu, L.-y.; Liu, Y.-p. Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking Chinese urban women: A hospital-based matched case control study. PLoS ONE 2014, 9, e97498. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- El Din, K.S.; Loree, J.M.; Sayre, E.C.; Gill, S.; Brown, C.J.; Dau, H.; De Vera, M.A. Trends in the epidemiology of young-onset colorectal cancer: A worldwide systematic. BMC Cancer 2020, 20, 288. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.K.; Mohiuddin, A.; Ming, L.C. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Xie, P.; Wu, S.; Kuo, Z.; Tian, H.; He, Q.; Li, Y.; Mi, N.; Hu, L.; Zhao, H.; Li, W. Association of modifiable lifestyle with colorectal cancer incidence and mortality according to metabolic status: Prospective cohort study. Front. Oncol. 2023, 13, 1162221. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Luo, D.; Wang, J.; Liu, J.; Luo, Y.; Zhou, W.; Zhuo, Z.; Guo, K.; Zeng, R. Association between cardiovascular risk factors and colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine 2021, 34, 100794. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Song, S.; Li, X.; Geng, C.; Wang, C. Excessive body fat at a young age increases the risk of colorectal cancer: A systematic review and meta-analysis. Nutr. Cancer 2021, 73, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3· 6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Rodriguez, R.M.; Segura-Sampedro, J.J.; Ochogavía-Seguí, A.; Romaguera, D.; Barceló-Coblijn, G. Insights behind the Relationship between Colorectal Cancer and Obesity: Is Visceral Adipose Tissue the Missing Link? Int. J. Mol. Sci. 2022, 23, 13128. [Google Scholar] [CrossRef]
- Tzenios, N. Obesity as a Risk Factor for Different Types of Cancer. EPRA Int. J. Res. Dev. 2023, 8, 97–100. [Google Scholar] [CrossRef]
- Mandic, M.; Safizadeh, F.; Niedermaier, T.; Hoffmeister, M.; Brenner, H. Association of Overweight, Obesity, and Recent Weight Loss With Colorectal Cancer Risk. JAMA Netw. Open 2023, 6, e239556. [Google Scholar] [CrossRef]
- Carr, P.; Alwers, E.; Bienert, S.; Weberpals, J.; Kloor, M.; Brenner, H.; Hoffmeister, M. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: A systematic review and meta-analyses. Ann. Oncol. 2018, 29, 825–834. [Google Scholar] [CrossRef]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef]
- Aykan, N.F. Red meat and colorectal cancer. Oncol. Rev. 2015, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Aran, V.; Victorino, A.P.; Thuler, L.C.; Ferreira, C.G. Colorectal cancer: Epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin. Color. Cancer 2016, 15, 195–203. [Google Scholar] [CrossRef]
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del Vecchio, V.; Caliendo, D.; Schiavone, V.; Fusco, R.; Granata, V.; Arra, C.; Cuomo, A. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): An overview on the current state of knowledge. Infect. Agents Cancer 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Yoon, Y.; Jo, C.; Jeong, J.Y.; Lee, K.T. Effect of dietary red meat on colorectal cancer risk—A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1812–1824. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ocalewski, J.; Jankowski, M.; Zegarski, W.; Migdalski, A.; Buczkowski, K. The Role of Health Behaviors in Quality of Life: A Longitudinal Study of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2023, 20, 5416. [Google Scholar] [CrossRef]
- Thélin, C.; Sikka, S. Epidemiology of colorectal Cancer—Incidence, lifetime risk factors statistics and temporal trends. Screen. Color. Cancer Colonoscopy 2015, 1, 61–77. [Google Scholar]
- Gandomani, H.S.; Aghajani, M.; Mohammadian-Hafshejani, A.; Tarazoj, A.A.; Pouyesh, V.; Salehiniya, H. Colorectal cancer in the world: Incidence, mortality and risk factors. Biomed. Res. Ther. 2017, 4, 1656–1675. [Google Scholar] [CrossRef]
- Hori, M.; Sawada, N.; Kito, K.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Vegetable and fruit intake and colorectal cancer risk by smoking status in adults: The Japan Public Health Center-based Prospective Study. Eur. J. Clin. Nutr. 2023, 77, 255–263. [Google Scholar] [CrossRef]
- Kumar, A.; Chinnathambi, S.; Kumar, M.; Pandian, G.N. Food intake and colorectal cancer. Nutr. Cancer 2023, 75, 1710–1742. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Garland, C.F.; Gorham, E.D. Dose-response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: A meta-analysis. J. Steroid Biochem. Mol. Biol. 2017, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lipsyc-Sharf, M.; Zong, X.; Wang, X.; Hur, J.; Song, M.; Wang, M.; Smith-Warner, S.A.; Fuchs, C.; Ogino, S.; et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterol. Rev./Przegląd Gastroenterol. 2021, 161, 1208–1217. [Google Scholar] [CrossRef]
- Huang, D.; Lei, S.; Wu, Y.; Weng, M.; Zhou, Y.; Xu, J.; Xia, D.; Xu, E.; Lai, M.; Zhang, H. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2525–2538. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D intake and the risk of colorectal cancer: An updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef]
- Koc, S.; Esin, M.N.; Ardic, A. Colorectal cancer prevention and risk counseling. In Colorectal Cancer-From Pathogenesis to Treatment; IntechOpen: London, UK, 2016. [Google Scholar]
- An, S.; Park, S. Association of physical activity and sedentary behavior with the risk of colorectal cancer. J. Korean Med. Sci. 2022, 37, e158. [Google Scholar] [CrossRef]
- Xie, F.; You, Y.; Huang, J.; Guan, C.; Chen, Z.; Fang, M.; Yao, F.; Han, J. Association between physical activity and digestive-system cancer: An updated systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 4–13. [Google Scholar] [CrossRef]
- Chang, W.Y.; Chiu, H.M. Beyond colonoscopy: Physical activity as a viable adjunct to prevent colorectal cancer. Dig. Endosc. 2023, 35, 33–46. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, J.; Wong, M.C.S. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2023, 62, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. Mol. Biol. 2016, 7, 105. [Google Scholar]
- Pedersen, A.; Johansen, C.; Grønbaek, M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut 2003, 52, 861–867. [Google Scholar] [CrossRef]
- Rossi, M.; Jahanzaib Anwar, M.; Usman, A.; Keshavarzian, A.; Bishehsari, F. Colorectal cancer and alcohol consumption—Populations to molecules. Cancers 2018, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Xiao, J.; Sun, J.; Yu, L.; Zhang, H.; Meng, X.; Yuan, S.; Timofeeva, M.; Law, P.J. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer 2022, 151, 83–94. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk factors associated with early-onset colorectal cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759. [Google Scholar] [CrossRef]
- Botteri, E.; Peveri, G.; Berstad, P.; Bagnardi, V.; Chen, S.L.; Sandanger, T.M.; Hoff, G.; Dahm, C.C.; Antoniussen, C.S.; Tjønneland, A. Changes in lifestyle and risk of colorectal cancer in the European prospective investigation into cancer and nutrition. Off. J. Am. Coll. Gastroenterol. ACG 2022, 10, 14309. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Gora, A.; Rudzki, S. Risk factors for the diagnosis of colorectal cancer. Cancer Control 2022, 29, 10732748211056692. [Google Scholar] [CrossRef]
- Florensa, D.; Mateo, J.; Solsona, F.; Galván, L.; Mesas, M.; Piñol, R.; Espinosa-Leal, L.; Godoy, P. Acetylsalicylic Acid Effect in Colorectal Cancer Taking into Account the Role of Tobacco, Alcohol and Excess Weight. Int. J. Environ. Res. Public Health 2023, 20, 4104. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Key, T.J.; Allen, N.E.; Fensom, G.K.; Bradbury, K.E.; Martin, R.M.; Travis, R.C. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br. J. Cancer 2017, 117, 1562–1571. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Plym, A.; Zhang, Y.; Stopsack, K.H.; Delcoigne, B.; Wiklund, F.; Haiman, C.; Kenfield, S.A.; Kibel, A.S.; Giovannucci, E.; Penney, K.L. A healthy lifestyle in men at increased genetic risk for prostate cancer. Eur. Urol. 2023, 83, 343–351. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Kalla, A.A.; Islam, M.M.; Sathekge, M.; Moorad, R. Prostate cancer: Epigenetic alterations, risk factors, and therapy. Prostate Cancer 2016, 2016, 5653862. [Google Scholar] [CrossRef]
- Hurwitz, L.M.; Dogbe, N.; Barry, K.H.; Koutros, S.; Berndt, S.I. Obesity and prostate cancer screening, incidence, and mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. JNCI J. Natl. Cancer Inst. 2023, 115, 1506–1514. [Google Scholar] [CrossRef]
- Möller, E.; Wilson, K.M.; Batista, J.L.; Mucci, L.A.; Bälter, K.; Giovannucci, E. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int. J. Cancer 2016, 138, 853–865. [Google Scholar] [CrossRef]
- Popovici, D.; Stanisav, C.; Pricop, M.; Dragomir, R.; Saftescu, S.; Ciurescu, D. Associations between Body Mass Index and Prostate Cancer: The Impact on Progression-Free Survival. Medicina 2023, 59, 289. [Google Scholar] [CrossRef]
- Vidal, A.C.; Oyekunle, T.; Howard, L.E.; De Hoedt, A.M.; Kane, C.J.; Terris, M.K.; Cooperberg, M.R.; Amling, C.L.; Klaassen, Z.; Freedland, S.J. Obesity, race, and long-term prostate cancer outcomes. Cancer Biol. Ther. 2020, 126, 3733–3741. [Google Scholar] [CrossRef]
- Taghizadeh, N.; Boezen, H.M.; Schouten, J.P.; Schröder, C.P.; Vries, E.E.d.; Vonk, J.M. BMI and lifetime changes in BMI and cancer mortality risk. PLoS ONE 2015, 10, e0125261. [Google Scholar] [CrossRef] [PubMed]
- Tzenios, N.; Tazanios, M.E.; Chahine, M. The impact of body mass index on prostate cancer: An updated systematic review and meta-analysis. Medicine 2022, 101, e30191. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. J. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef] [PubMed]
- Langlais, C.S.; Graff, R.E.; Van Blarigan, E.L.; Palmer, N.R.; Washington, S.L.; Chan, J.M.; Kenfield, S.A. Post-diagnostic dietary and lifestyle factors and prostate cancer recurrence, progression, and mortality. Curr. Oncol. Rep. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Minelli, L.; Bertarelli, G.; Bacci, S. A western dietary pattern increases prostate cancer risk: A systematic review and meta-analysis. Nutrients 2016, 8, 626. [Google Scholar] [CrossRef]
- Jalilpiran, Y.; Dianatinasab, M.; Zeighami, S.; Bahmanpour, S.; Ghiasvand, R.; Mohajeri, S.A.R.; Faghih, S. Western dietary pattern, but not mediterranean dietary pattern, increases the risk of prostate cancer. Nutr. Cancer 2018, 70, 851–859. [Google Scholar] [CrossRef]
- Capurso, C.; Vendemiale, G. The Mediterranean diet reduces the risk and mortality of the prostate cancer: A narrative review. Front. Nutr. 2017, 4, 38. [Google Scholar] [CrossRef]
- Leitão, C.; Matos, B.; Roque, F.; Herdeiro, M.T.; Fardilha, M. The impact of lifestyle on prostate cancer: A road to the discovery of new biomarkers. J. Clin. Med. 2022, 11, 2925. [Google Scholar] [CrossRef]
- Pelser, C.; Mondul, A.M.; Hollenbeck, A.R.; Park, Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Liss, M.A.; Al-Bayati, O.; Gelfond, J.; Goros, M.; Ullevig, S.; DiGiovanni, J.; Hamilton-Reeves, J.; O’Keefe, D.; Bacich, D.; Weaver, B. Higher baseline dietary fat and fatty acid intake is associated with increased risk of incident prostate cancer in the SABOR study. Prostate Cancer Prostatic Dis. 2019, 22, 244–251. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary factors and prostate cancer development, progression, and reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Henneberg, M. Prostate Cancer incidence is correlated to Total meat intake–a cross-National Ecologic Analysis of 172 countries. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2229. [Google Scholar] [CrossRef]
- Mazidi, M.; Ferns, G.A.; Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: Results from a multi-ethnic cohort. Public Health Nutr. 2020, 23, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, J.-F.; Gevariya, N.; Larose, J.; Robitaille, K.; Roy, J.; Oger, C.; Galano, J.-M.; Bergeron, A.; Durand, T.; Fradet, Y. Long chain omega-3 fatty acids and their oxidized metabolites are associated with reduced prostate tumor growth. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102215. [Google Scholar] [CrossRef] [PubMed]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.M.; De Souza, R.J.; Salehi-Abargouei, A. The effect of vitamin D supplementation on prostate cancer: A systematic review and meta-analysis of clinical trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef]
- Song, Z.-Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin d level and mortality in prostate cancer patients: A dose–response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef]
- Shephard, R.J. Physical activity and prostate cancer: An updated review. Sports Med. 2017, 47, 1055–1073. [Google Scholar] [CrossRef]
- Campos, C.; Sotomayor, P.; Jerez, D.; González, J.; Schmidt, C.B.; Schmidt, K.; Banzer, W.; Godoy, A.S. Exercise and prostate cancer: From basic science to clinical applications. Prostate 2018, 78, 639–645. [Google Scholar] [CrossRef]
- Sorial, E.; Si, S.; Fritschi, L.; Darcey, E.; Leavy, J.E.; Girschik, J.; Ambrosini, G.L.; Boyle, T. Lifetime recreational physical activity and the risk of prostate cancer. Cancer Causes Control 2019, 30, 617–625. [Google Scholar] [CrossRef]
- Benke, I.N.; Leitzmann, M.; Behrens, G.; Schmid, D. Physical activity in relation to risk of prostate cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1154–1179. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, F.J.; la Riva-Pérez, P.A.d.; Agudo-Martínez, A.; Sabatel-Hernández, G.; Calvo-Morón, M.C. Whole muscle 18F-choline uptake due to intense physical exercise. Int. Braz. J. Urol. 2019, 45, 851–852. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Wang, Q.; Neilson, H.K.; Kopciuk, K.A.; McGregor, S.E.; Courneya, K.S. Physical activity and survival after prostate cancer. Eur. Urol. 2016, 70, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Vaux-Bjerke, A.; Quam, J.B.; Piercy, K.L.; Troiano, R.P.; George, S.M.; Sprow, K.; Ballard, R.M.; Fulton, J.E.; Galuska, D.A. Physical activity guidelines for Americans. NADAR! SWIMMING MAGAZINE-Periódico Científico Em Esportes E Fit. Aquático-Natação Pólo Aquático Nado Sincronizado Saltos Ornamentais Travessias Aquáticas 2023, 3, 55–66. [Google Scholar]
- Macke, A.J.; Petrosyan, A. Alcohol and prostate cancer: Time to draw conclusions. Biomolecules 2022, 12, 375. [Google Scholar] [CrossRef]
- Rota, M.; Scotti, L.; Turati, F.; Tramacere, I.; Islami, F.; Bellocco, R.; Negri, E.; Corrao, G.; Boffetta, P.; La Vecchia, C. Alcohol consumption and prostate cancer risk: A meta-analysis of the dose–risk relation. Eur. J. Cancer Prev. 2012, 21, 350–359. [Google Scholar] [CrossRef]
- Zhao, J.; Stockwell, T.; Roemer, A.; Chikritzhs, T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta–analysis. BMC Cancer 2016, 16, 845. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Alcohol intake, drinking patterns, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1049–1058. [Google Scholar] [CrossRef]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C. Alcohol intake and risk of lethal prostate cancer in the health professionals follow-up study. J. Clin. Oncol. 2019, 37, 1499. [Google Scholar] [CrossRef]
- D’Ecclesiis, O.; Pastore, E.; Gandini, S.; Caini, S.; Marvaso, G.; Jereczek-Fossa, B.A.; Corrao, G.; Raimondi, S.; Bellerba, F.; Ciceri, S. Association between Alcohol Intake and Prostate Cancer Mortality and Survival. Nutrients 2023, 15, 925. [Google Scholar] [CrossRef]
- Huncharek, M.; Haddock, K.S.; Reid, R.; Kupelnick, B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am. J. Public Health 2010, 100, 693–701. [Google Scholar] [CrossRef]
- Islami, F.; Moreira, D.M.; Boffetta, P.; Freedland, S.J. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur. Urol. 2014, 66, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Travis, R.C.; Key, T.J.; Allen, N.E. Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: Implications for epidemiological research. Cancer Epidemiol. 2016, 45, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-M.; Li, Z.-M.; Shin, M.-H.; Kim, D.-H.; Lee, M.-S.; Ahn, Y.-O. Cigarette smoking and prostate cancer risk: Negative results of the Seoul Male Cancer Cohort Study. Asian Pac. J. Cancer Prev. 2013, 14, 4667–4669. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Mendoza, E.; Vázquez-Salas, R.A.; Barrientos-Gutierrez, T.; Reynales-Shigematsu, L.M.; Labra-Salgado, I.R.; Manzanilla-García, H.A.; Torres-Sánchez, L.E. Smoking and prostate cancer: A life course analysis. BMC Cancer 2018, 18, 160. [Google Scholar] [CrossRef]
- Foerster, B.; Pozo, C.; Abufaraj, M.; Mari, A.; Kimura, S.; D’Andrea, D.; John, H.; Shariat, S.F. Association of smoking status with recurrence, metastasis, and mortality among patients with localized prostate cancer undergoing prostatectomy or radiotherapy: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 953–961. [Google Scholar] [CrossRef]
- Wilson, K.M.; Markt, S.C.; Fang, F.; Nordenvall, C.; Rider, J.R.; Ye, W.; Adami, H.O.; Stattin, P.; Nyrén, O.; Mucci, L.A. Snus use, smoking and survival among prostate cancer patients. Int. J. Cancer 2016, 139, 2753–2759. [Google Scholar] [CrossRef]
- Jones, M.R.; Joshu, C.E.; Kanarek, N.; Navas-Acien, A.; Richardson, K.A.; Platz, E.A. Peer Reviewed: Cigarette Smoking and Prostate Cancer Mortality in Four US States, 1999–2010. Prev. Chronic Dis. 2016, 13, E51. [Google Scholar] [CrossRef][Green Version]
- Steinberger, E.; Kollmeier, M.; McBride, S.; Novak, C.; Pei, X.; Zelefsky, M.J. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015, 116, 596–603. [Google Scholar] [CrossRef]
- Rieken, M.; Shariat, S.F.; Kluth, L.A.; Fajkovic, H.; Rink, M.; Karakiewicz, P.I.; Seitz, C.; Briganti, A.; Rouprêt, M.; Loidl, W. Association of cigarette smoking and smoking cessation with biochemical recurrence of prostate cancer in patients treated with radical prostatectomy. Eur. Urol. 2015, 68, 949–956. [Google Scholar] [CrossRef]
- Riviere, P.; Kumar, A.; Luterstein, E.; Vitzthum, L.K.; Nalawade, V.; Sarkar, R.R.; Bryant, A.K.; Einck, J.P.; Mundt, A.J.; Murphy, J.D. Tobacco smoking and death from prostate cancer in US veterans. Prostate Cancer Prostatic Dis. 2020, 23, 252–259. [Google Scholar] [CrossRef] [PubMed]
| Recommendations | Aims | Advice |
|---|---|---|
| (1) Be a healthy weight. Body weight must be kept within a healthy limit, preventing weight increases over the years. |
|
|
| (2) Perform physical activity. Perform physical activity as part of daily life, move more, and stop less. |
|
|
| (3) Consume a diet rich in cereals, vegetables, fruit, and beans. Make these foods part of your daily diet. |
|
|
| (4) Restrict eating of “fast foods” and processed foods high in fat, starches, or sugars. Reducing these foods helps maintain a healthy weight. |
|
|
| (5) Reduce the use of red and processed meats.Consume red meat in small doses, such as beef, pork, and lamb. Eat small or eliminate processed meat. |
|
|
| (6) Reduce the use of sugary drinks.Drink mainly water and unsweetened beverages. |
|
|
| (7) Reduce or avoid alcohol use. For better cancer prevention, eliminate alcohol. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. https://doi.org/10.3390/nu16060800
Marino P, Mininni M, Deiana G, Marino G, Divella R, Bochicchio I, Giuliano A, Lapadula S, Lettini AR, Sanseverino F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients. 2024; 16(6):800. https://doi.org/10.3390/nu16060800
Chicago/Turabian StyleMarino, Pasquale, Mariangela Mininni, Giovanni Deiana, Graziella Marino, Rosa Divella, Ilaria Bochicchio, Alda Giuliano, Stefania Lapadula, Alessandro Rocco Lettini, and Francesca Sanseverino. 2024. "Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer" Nutrients 16, no. 6: 800. https://doi.org/10.3390/nu16060800
APA StyleMarino, P., Mininni, M., Deiana, G., Marino, G., Divella, R., Bochicchio, I., Giuliano, A., Lapadula, S., Lettini, A. R., & Sanseverino, F. (2024). Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients, 16(6), 800. https://doi.org/10.3390/nu16060800









