Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Skeletal Muscle and Adipose Tissue
2.3. GLIM Criteria
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of the Patients
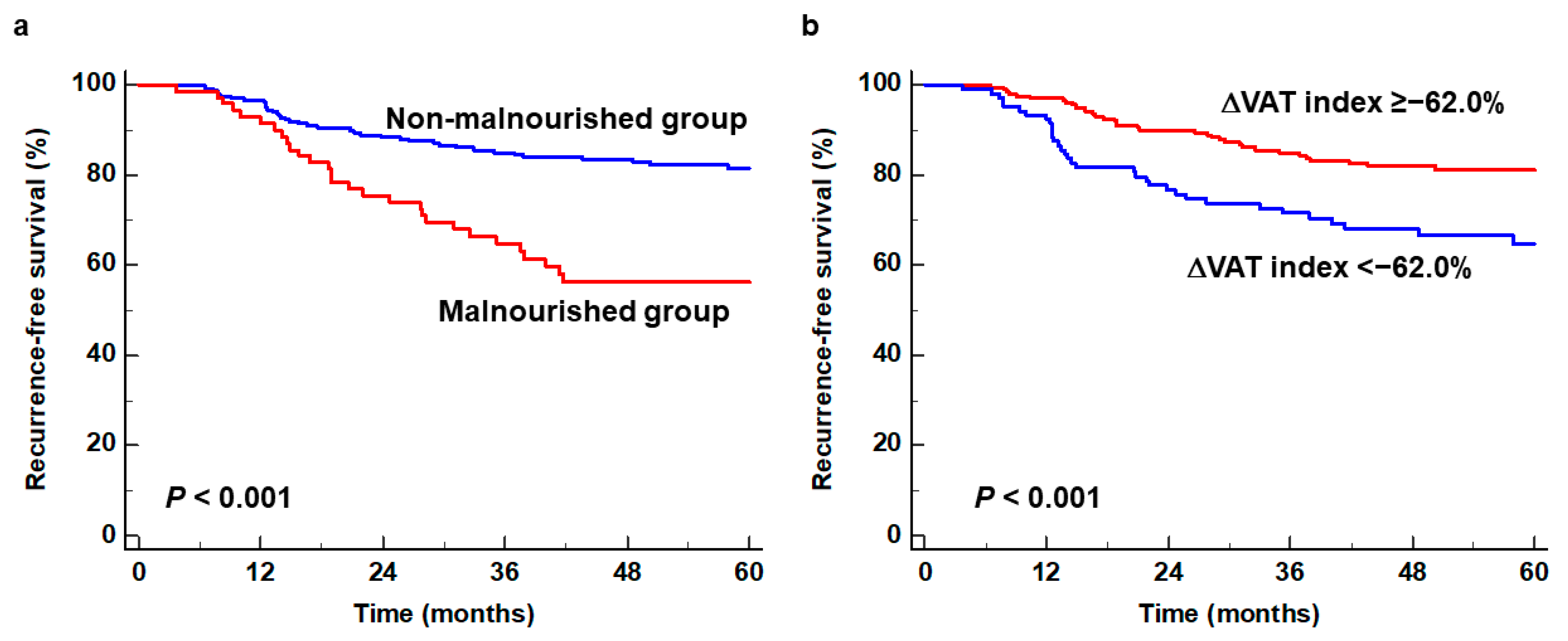
3.2. Survival Analysis for RFS
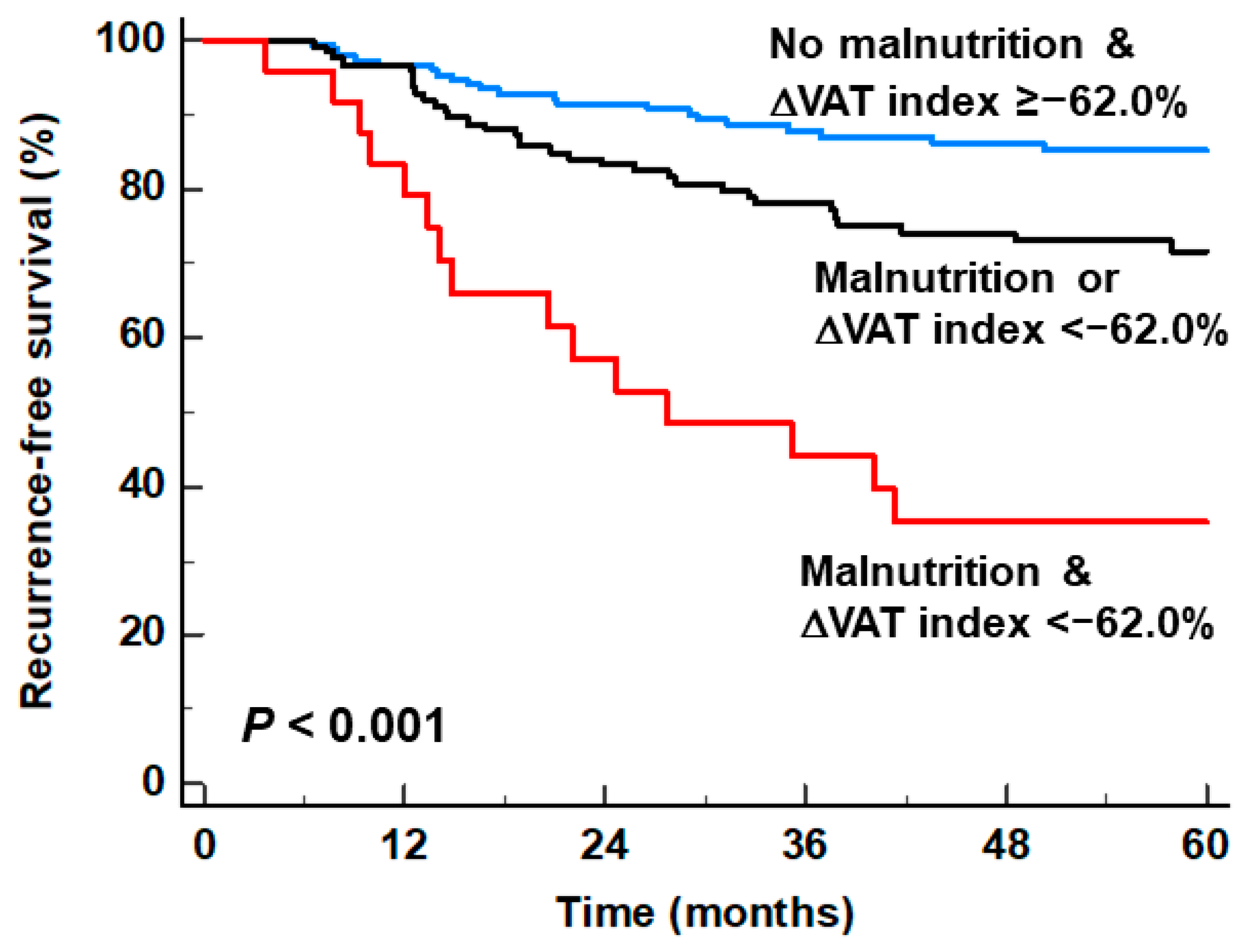
3.3. Stratification of RFS by Combining the GLIM Criteria and the ΔVAT Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Development Working Groups for the Korean Practice Guidelines for Gastric Cancer 2022 Task Force Team. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-Based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef]
- Desiderio, J.; Sagnotta, A.; Terrenato, I.; Garofoli, E.; Mosillo, C.; Trastulli, S.; Arteritano, F.; Tozzi, F.; D’Andrea, V.; Fong, Y.; et al. Long-term survival of patients with stage II and III gastric cancer who underwent gastrectomy with inadequate nodal assessment. World J. Gastrointest. Surg. 2021, 13, 1463–1483. [Google Scholar] [CrossRef]
- Iwasa, S.; Kudo, T.; Takahari, D.; Hara, H.; Kato, K.; Satoh, T. Practical guidance for the evaluation of disease progression and the decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. Int. J. Clin. Oncol. 2020, 25, 1223–1232. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.A.; Heo, S.J.; et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: Single-center study from the CLASSIC trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Zheng, H.L.; Lin, J.; Shen, L.L.; Yang, H.B.; Xu, B.B.; Xue, Z.; Wu, D.; Huang, J.B.; Lin, G.S.; Zheng, C.H.; et al. The GLIM criteria as an effective tool for survival prediction in gastric cancer patients. Eur. J. Surg. Oncol. 2023, 49, 964–973. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the Global Clinical Nutrition Community. JPEN J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T.; Fukunaga, T. Association of GLIM defined malnutrition according to preoperative chronic inflammation with long-term prognosis after gastrectomy in patients with advanced gastric cancer. J. Clin. Med. 2023, 12, 1579. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Su, P.; Yu, T.; Ma, Z.; Kang, W.; Liu, Y.; Jin, Z.; Yu, J. Visceral adipose tissue assessment enhances the prognostic value of GLIM criteria in patients with gastric cancer undergoing radical gastrectomy after neoadjuvant treatment. Nutrients 2022, 14, 5047. [Google Scholar] [CrossRef]
- Xu, L.B.; Mei, T.T.; Cai, Y.Q.; Chen, W.J.; Zheng, S.X.; Wang, L.; Chen, X.D.; Huang, Y.S. Correlation between components of malnutrition diagnosed by Global Leadership Initiative on Malnutrition criteria and the clinical outcomes in gastric cancer patients: A propensity score matching analysis. Front. Oncol. 2022, 12, 851091. [Google Scholar] [CrossRef]
- Feng, W.; Huang, M.; Zhao, X.; Chen, S.; Wang, C.; Chang, J.; Guo, W.; Chen, Z.; Zhu, H.; Zhu, X. Severe loss of visceral fat and skeletal muscle after chemotherapy predicts poor prognosis in metastatic gastric cancer patients without gastrectomy. J. Cancer 2020, 11, 3310–3317. [Google Scholar] [CrossRef]
- Park, Y.S.; Park, D.J.; Lee, Y.; Park, K.B.; Min, S.H.; Ahn, S.H.; Kim, H.H. Prognostic roles of perioperative body mass index and weight loss in the long-term survival of gastric cancer patients. Cancer Epidemiol. Biomark. Prev. 2018, 27, 955–962. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Lee, S.; Kim, H.H.; Kim, Y.J.; Lee, J.G.; Jeong, D.W.; Kim, Y.J. Long-term changes in gut hormones, appetite and food intake 1 year after subtotal gastrectomy with normal body weight. Eur. J. Clin. Nutr. 2010, 64, 826–831. [Google Scholar] [CrossRef]
- Kwon, Y.; Ha, J.; Kim, D.; Hwang, J.; Park, S.H.; Kwon, J.W.; Park, S. The association between weight change after gastric cancer surgery and type 2 diabetes risk: A nationwide cohort study. J. Cachexia Sarcopenia Muscle 2023, 14, 826–834. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Lee, S.; Kang, D.H.; Ahn, T.S.; Kim, S.S.; Yun, J.H.; Kim, H.J.; Seo, S.H.; Kim, T.W.; Kong, H.J.; Baek, M.J. The impact of pre-chemotherapy body composition and immunonutritional markers on chemotherapy adherence in stage III colorectal cancer patients. J. Clin. Med. 2023, 12, 1423. [Google Scholar] [CrossRef]
- Ahn, H.; Song, G.J.; Jang, S.H.; Son, M.W.; Lee, H.J.; Lee, M.S.; Lee, J.H.; Oh, M.H.; Jeong, G.C.; Yun, J.H.; et al. Predicting the recurrence of gastric cancer using the textural features of perigastric adipose tissue on [(18)F]FDG PET/CT. Int. J. Mol. Sci. 2022, 23, 11985. [Google Scholar] [CrossRef]
- Huo, Z.; Chong, F.; Yin, L.; Li, N.; Liu, J.; Zhang, M.; Guo, J.; Fan, Y.; Zhang, L.; Lin, X.; et al. Investigation on Nutrition Status and Clinical Outcomes of Common Cancers Group. Comparison of the performance of the GLIM criteria, PG-SGA and mPG-SGA in diagnosing malnutrition and predicting survival among lung cancer patients: A multicenter study. Clin. Nutr. 2023, 42, 1048–1058. [Google Scholar] [CrossRef]
- Zhou, C.J.; Lin, Y.; Liu, J.Y.; Wang, Z.L.; Chen, X.Y.; Zheng, C.G. Malnutrition and visceral obesity predicted adverse short-term and long-term outcomes in patients undergoing proctectomy for rectal cancer. BMC Cancer 2023, 23, 576. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T. Effect of malnutrition as defined by the Global Leadership Initiative on Malnutrition criteria on compliance of adjuvant chemotherapy and relapse-free survival for advanced gastric cancer. Nutrition 2023, 109, 111958. [Google Scholar] [CrossRef]
- Soeters, P.; Bozzetti, F.; Cynober, L.; Forbes, A.; Shenkin, A.; Sobotka, L. Defining malnutrition: A plea to rethink. Clin. Nutr. 2017, 36, 896–901. [Google Scholar] [CrossRef]
- Lee, J.W.; Ban, M.J.; Park, J.H.; Lee, S.M. Visceral adipose tissue volume and CT-attenuation as prognostic factors in patients with head and neck cancer. Head Neck 2019, 41, 1605–1614. [Google Scholar] [CrossRef]
- Bonet, C.; Crous-Bou, M.; Tsilidis, K.K.; Gunter, M.J.; Kaaks, R.; Schulze, M.B.; Fortner, R.T.; Antoniussen, C.S.; Dahm, C.C.; Mellemkjær, L.; et al. The association between body fatness and mortality among breast cancer survivors: Results from a prospective cohort study. Eur. J. Epidemiol. 2023, 38, 545–557. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Hatano, E.; Okajima, H.; et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann. Surg. Oncol. 2017, 24, 3732–3740. [Google Scholar] [CrossRef]
- Pacquelet, B.; Morello, R.; Pelage, J.P.; Eid, Y.; Lebreton, G.; Alves, A.; Fohlen, A. Abdominal adipose tissue quantification and distribution with CT: Prognostic value for surgical and oncological outcome in patients with rectal cancer. Eur. Radiol. 2022, 32, 6258–6269. [Google Scholar] [CrossRef]
- Basile, D.; Ba rtoletti, M.; Polano, M.; Bortot, L.; Gerratana, L.; Di Nardo, P.; Borghi, M.; Fanotto, V.; Pelizzari, G.; Lisanti, C.; et al. Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clin. Nutr. 2021, 40, 286–294. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T.; Kokura, Y.; Momosaki, R. Preoperative high visceral fat increases severe complications but improves long-term prognosis after gastrectomy for patients with advanced gastric cancer: A propensity score matching analysis. Nutrients 2022, 14, 4236. [Google Scholar] [CrossRef]
- Kamiya, H.; Komatsu, S.; Nishibeppu, K.; Ohashi, T.; Konishi, H.; Shiozaki, A.; Kubota, T.; Fujiwara, H.; Otsuji, E. Obesity paradox as a new insight from postoperative complications in gastric cancer. Sci. Rep. 2023, 13, 10116. [Google Scholar] [CrossRef]
- Park, K.B.; Kwon, O.K.; Yu, W. Midterm body composition changes after open distal gastrectomy for early gastric cancer. Ann. Surg. Treat. Res. 2018, 95, 192–200. [Google Scholar] [CrossRef]
- Miki, M.; Lee, L.; Hisano, T.; Sugimoto, R.; Furukawa, M. Loss of adipose tissue or skeletal muscle during first-line gemcitabine/nab-paclitaxel therapy is associated with worse survival after second-line therapy of advanced pancreatic cancer. Asia Pac. J. Clin. Oncol. 2022, 18, e297–e305. [Google Scholar] [CrossRef]
- Ahn, H.; Song, G.J.; Lee, M.S.; Lee, J.H.; Jang, S.H.; Oh, M.H.; Yun, J.H.; Lee, S.M.; Lee, J.W. Relationship of FDG uptake of the reticuloendothelial system with tumor immune microenvironment and prognosis in patients with gastric cancer. Life 2023, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Horimai, C.; Katsukawa, F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: A meta-analysis. Acta Diabetol. 2003, 40 (Suppl. S1), S302–S304. [Google Scholar] [CrossRef] [PubMed]
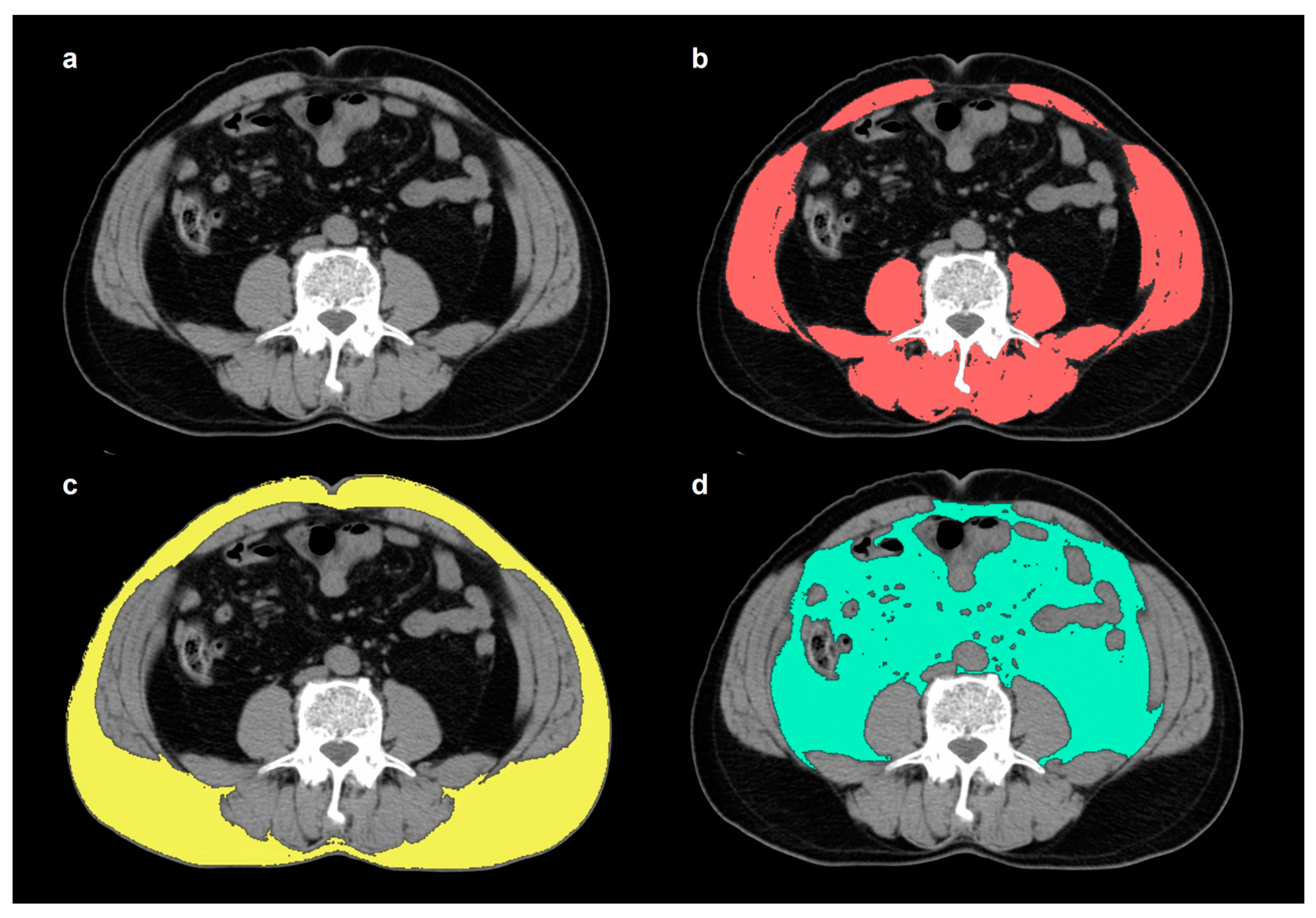
| Characteristics | Total (n = 302) | Patients with Events (n = 75) | Patients with No Events (n = 227) | p-Value |
|---|---|---|---|---|
| Age (years) | 60 (31–91) | 60 (31–80) | 60 (32–91) | 0.409 |
| Sex | 0.437 | |||
| Men | 219 (72.5%) | 57 (76.0%) | 162 (71.4%) | |
| Women | 83 (27.5%) | 18 (24.0%) | 65 (28.6%) | |
| Body mass index (kg/m2) | 23.6 (16.0–34.6) | 23.8 (16.2–33.3) | 23.6 (16.0–34.6) | 0.842 |
| Obesity | 0.365 | |||
| Underweight | 35 (11.6%) | 12 (16.0%) | 23 (10.1%) | |
| Normal | 187 (61.9%) | 43 (57.3%) | 144 (63.4%) | |
| Obese | 80 (26.5%) | 20 (26.7%) | 60 (26.4%) | |
| Tumor location | 0.227 | |||
| Upper | 68 (22.5%) | 22 (29.3%) | 46 (20.3%) | |
| Middle | 34 (11.3%) | 9 (12.0%) | 25 (11.0%) | |
| Lower | 200 (66.2%) | 44 (58.7%) | 156 (68.7%) | |
| Histopathological classification | 0.114 | |||
| Papillary/tubular type | 107 (35.4%) | 18 (24.0%) | 89 (39.2%) | |
| Poorly cohesive type | 172 (57.0%) | 51 (68.0%) | 121 (53.3%) | |
| Mucinous | 14 (4.6%) | 4 (5.3%) | 10 (4.4%) | |
| Others | 9 (3.0%) | 2 (2.7%) | 7 (3.1%) | |
| Lauren classification | 0.002 * | |||
| Intestinal type | 104 (34.4%) | 15 (20.0%) | 89 (39.2%) | |
| Diffuse/indeterminate type | 198 (65.6%) | 60 (80.0%) | 138 (60.8%) | |
| Surgery type | 0.006 * | |||
| Total gastrectomy | 80 (26.5%) | 30 (40.0%) | 50 (22.0%) | |
| Subtotal gastrectomy | 218 (72.2%) | 45 (60.0%) | 173 (76.2%) | |
| Proximal gastrectomy | 4 (1.3%) | 0 (0.0%) | 4 (1.8%) | |
| T stage | <0.001 * | |||
| T2 stage | 94 (31.1%) | 4 (5.3%) | 90 (39.6%) | |
| T3 stage | 135 (44.7%) | 36 (48.0%) | 99 (43.6%) | |
| T4 stage | 73 (24.2%) | 35 (46.7%) | 38 (16.7%) | |
| N stage | <0.001 * | |||
| N0 stage | 127 (42.1%) | 9 (12.0%) | 118 (52.0%) | |
| N1–3 stage | 175 (57.9%) | 66 (88.0%) | 109 (48.0%) | |
| TNM stage | <0.001 * | |||
| Stage I | 59 (19.5%) | 1 (1.3%) | 58 (25.6%) | |
| Stage II | 126 (41.7%) | 16 (21.3%) | 110 (48.5%) | |
| Stage III | 117 (38.7%) | 58 (77.3%) | 59 (26.0%) | |
| Adjuvant chemotherapy | <0.001 * | |||
| Yes | 263 (87.1%) | 74 (98.7%) | 189 (83.3%) | |
| No | 39 (12.9%) | 1 (1.3%) | 38 (16.7%) | |
| GLIM criteria | <0.001 * | |||
| Malnutrition | 70 (23.2%) | 31 (41.3%) | 39 (17.2%) | |
| No malnutrition | 232 (76.8%) | 44 (58.7%) | 188 (82.8%) | |
| Initial SAT index (cm2/m2) | 45.5 (2.2–162.3) | 42.1 (2.2–142.6) | 45.8 (4.4–162.3) | 0.649 |
| Initial VAT index (cm2/m2) | 43.5 (0.9–147.8) | 38.0 (2.8–147.8) | 44.6 (0.9–140.9) | 0.217 |
| Follow-up SAT index (cm2/m2) | 30.7 (1.0–111.6) | 26.1 (1.6–111.6) | 32.4 (1.0–101.2) | 0.007 * |
| Follow-up VAT index (cm2/m2) | 19.5 (1.2–103.1) | 12.8 (1.2–103.1) | 21.4 (1.2–72.3) | 0.012 * |
| ΔSAT index (%) | −26.3 (−97.4–235.5) | −35.5 (−86.2–220.5) | −23.9 (−97.4–235.5) | 0.005 * |
| ΔVAT index (%) | −52.0 (−98.6–369.7) | −59.7 (−93.5–139.7) | −48.8 (−98.6–369.7) | 0.006 * |
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | ||
| Age (<50 years vs.) | 50–70 years | 0.795 | 0.924 (0.514–1.665) | ||
| >70 years | 0.876 | 1.057 (0.527–2.119) | |||
| Sex (women vs. men) | 0.432 | 1.237 (0.728–2.102) | |||
| Obesity (underweight vs.) | Normal | 0.151 | 0.626 (0.330–1.187) | ||
| Obese | 0.284 | 0.676 (0.330–1.383) | |||
| Tumor location (upper vs.) | Middle | 0.648 | 0.835 (0.384–1.813) | ||
| Lower | 0.106 | 0.655 (0.392–1.093) | |||
| Histopathological classification (papillary/tubular type vs.) | Poorly cohesive type | 0.027 * | 1.839 (1.074–3.149) | 0.727 | 0.879 (0.425–1.818) |
| Mucinous | 0.339 | 1.698 (0.574–5.020) | 0.812 | 0.871 (0.280–2.712) | |
| Others | 0.453 | 1.750 (0.406–7.548) | 0.664 | 0.717 (0.160–3.208) | |
| Lauren classification (Intestinal vs. diffuse/indeterminate) | 0.004 * | 2.320 (1.317–4.087) | 0.153 | 1.764 (0.810–3.838) | |
| T stage (T2 vs.) | T3 | <0.001 * | 6.850 (2.437–19.251) | 0.004 * | 4.859 (1.680–14.052) |
| T4 | <0.001 * | 16.942 (6.009–47.767) | <0.001 * | 9.917 (3.355–29.316) | |
| N stage (N0 vs. N1–3) | <0.001 * | 6.449 (3.212–12.946) | <0.001 * | 4.234 (2.071–8.654) | |
| Adjuvant chemotherapy (no vs. yes) | 0.016 * | 11.363 (1.579–81.774) | 0.577 | 1.777 (0.236–13.397) | |
| GLIM criteria (no malnutrition vs. malnutrition) | <0.001 * | 2.695 (1.701–4.271) | 0.008 * | 2.016 (1.198–3.395) | |
| Initial SAT index (≥43.6 vs. <43.6) | 0.113 | 1.445 (0.917–2.277) | |||
| Initial VAT index (≥39.5 vs. <39.5) | 0.012 * | 1.797 (1.139–2.836) | 0.753 | 1.102 (0.602–2.019) | |
| Follow-up SAT index (≥29.5 vs. <29.5) | 0.007 * | 1.888 (1.190–2.998) | 0.997 | 0.999 (0.538–1.857) | |
| Follow-up VAT index (≥11.1 vs. <11.1) | <0.001 * | 2.561 (1.625–4.036) | 0.534 | 0.804 (0.403–1.602) | |
| ΔSAT index (≥−41.6 vs. <−41.6) | 0.002 * | 2.082 (1.317–3.292) | 0.146 | 1.569 (0.855–2.881) | |
| ΔVAT index (≥−62.0 vs. <−62.0) | <0.001 * | 2.168 (1.378–3.411) | 0.008 * | 2.191 (1.230–3.904) | |
| Patient Subgroup | Number of Events (%) | p-Value | Hazard Ratio (95% CI) |
|---|---|---|---|
| No malnutrition & ΔVAT index ≥ −62.0% (n = 152) | 23 (15.1%) | - | 1.00 |
| Malnutrition or ΔVAT index < −62.0% (n = 126) | 36 (28.6%) | 0.006 | 2.082 (1.233–3.515) |
| Malnutrition & ΔVAT index < −62.0% (n = 24) | 16 (66.7%) | <0.001 | 6.791 (3.573–12.907) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.J.; Ahn, H.; Son, M.W.; Yun, J.H.; Lee, M.-S.; Lee, S.M. Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients. Nutrients 2024, 16, 728. https://doi.org/10.3390/nu16050728
Song GJ, Ahn H, Son MW, Yun JH, Lee M-S, Lee SM. Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients. Nutrients. 2024; 16(5):728. https://doi.org/10.3390/nu16050728
Chicago/Turabian StyleSong, Geum Jong, Hyein Ahn, Myoung Won Son, Jong Hyuk Yun, Moon-Soo Lee, and Sang Mi Lee. 2024. "Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients" Nutrients 16, no. 5: 728. https://doi.org/10.3390/nu16050728
APA StyleSong, G. J., Ahn, H., Son, M. W., Yun, J. H., Lee, M.-S., & Lee, S. M. (2024). Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients. Nutrients, 16(5), 728. https://doi.org/10.3390/nu16050728








