Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcomes and Measures
2.3. Composition and Infusion of Parenteral Nutrition
2.4. Central Venous Catheters and Their Related Complications
2.5. Statistical Analysis
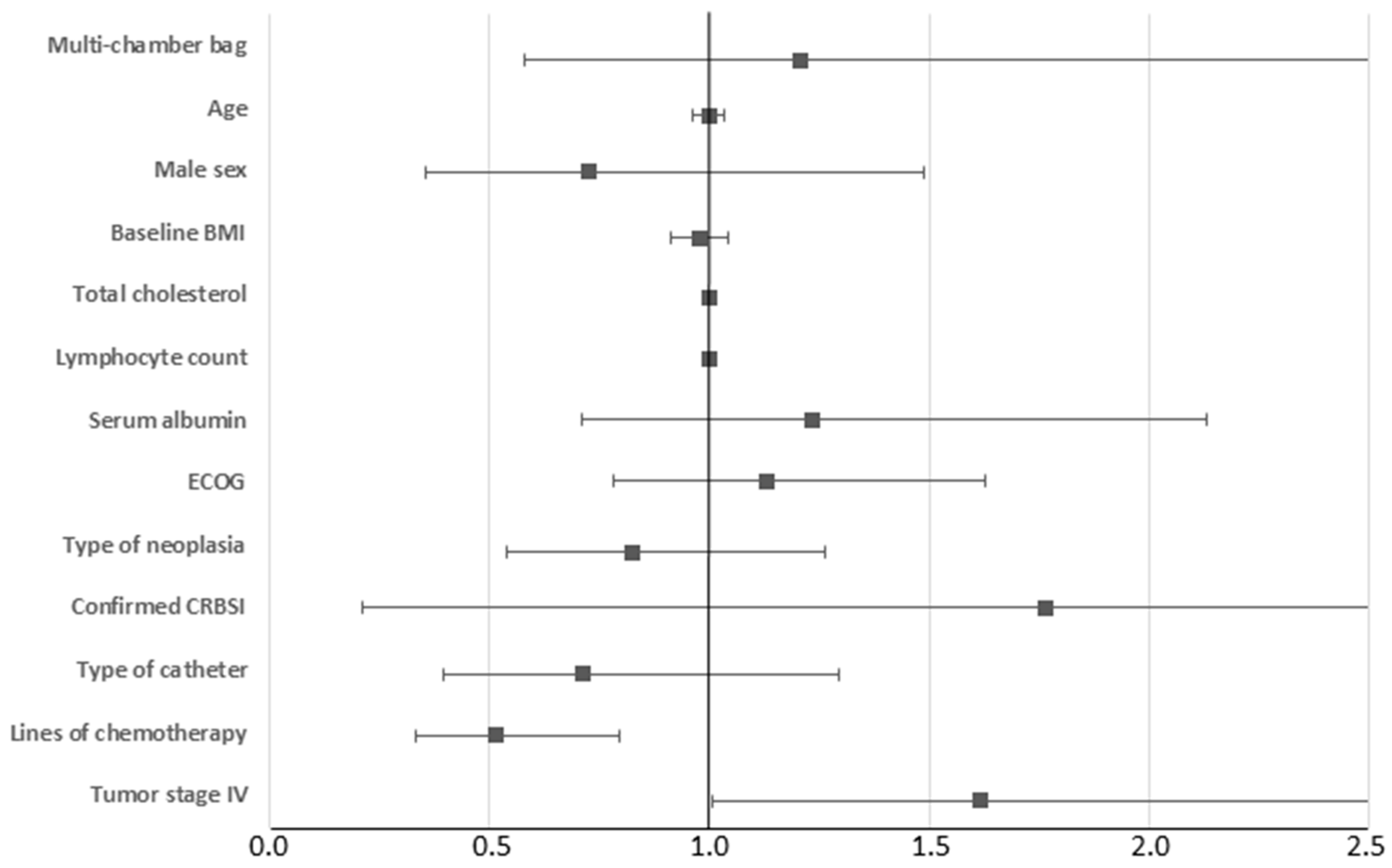
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, V.; Bielawska, B.; Jeejeebhoy, K.N.; Gramlich, L.M.; Raman, M.; Whittaker, J.S.; Armstrong, D.; Marliss, E.B.; Allard, J.P. Variations in practice patterns for adult cancer patients on home parenteral nutrition in Canada. Nutrition 2019, 65, 27–32. [Google Scholar] [CrossRef]
- Wanden-Berghe Lozano, C.; Cuerda Compes, C.; Maíz Jiménez, M.; Pereira Cunill, J.L.; Ramos Boluda, E.; Gómez Candela, C.; Virgili Casas, M.N.; Burgos Peláez, R.; de Luis Román, D.A.; Penacho Lázaro, M.; et al. Home and Ambulatory Artificial Nutrition (NADYA) Group Report. Home parenteral nutrition in Spain, 2018. Nutr. Hosp. 2020, 37, 403–407. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin. Nutr. 2019, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.J.; Huang, C.W.; Yeh, Y.S.; Tsai, H.L.; Su, W.C.; Chang, T.K.; Sun, L.C.; Shih, Y.L.; Yu, F.J.; Wu, D.C.; et al. Supplemental home parenteral nutrition improved nutrition status with comparable quality of life in malnourished unresectable/metastatic gastric cancer receiving salvage chemotherapy. Support. Care Cancer 2021, 29, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Cotogni, P.; Monge, T.; Passera, R.; Brossa, L.; De Francesco, A. Clinical characteristics and predictive factors of survival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study. Cancer Med. 2020, 9, 4686–4698. [Google Scholar] [CrossRef]
- Aria Guerra, E.; Cortes-Salgado, A.; Mateo-Lobo, R.; Nattero, L.; Riveiro, J.; Vega-Pinero, B.; Valbuena, B.; Carabana, F.; Carrero, C.; Grande, E.; et al. Role of Parenteral Nutrition in Oncologic Patients with Intestinal Occlusion and Peritoneal Carcinomatosis. Nutr. Hosp. 2015, 32, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Cotogni, P.; Ossola, M.; Passera, R.; Monge, T.; Fadda, M.; De Francesco, A.; Bozzetti, F. Home parenteral nutrition versus artificial hydration in malnourished patients with cancer in palliative care: A prospective, cohort survival study. BMJ Support. Palliat. Care 2022, 12, 114–120. [Google Scholar] [CrossRef]
- Theilla, M.; Cohen, J.; Kagan, I.; Attal-Singer, J.; Lev, S.; Singer, P. Home parenteral nutrition for advanced cancer patients: Contributes to survival? Nutrition 2018, 54, 197–200. [Google Scholar] [CrossRef]
- Webb, N.; Fricke, J.; Hancock, E.; Trueman, D.; Ghosh, S.; Winstone, J.; Miners, A.; Shepelev, J.; Valle, J.W. The clinical and cost-effectiveness of supplemental parenteral nutrition in oncology. ESMO Open 2020, 5, e000709. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Li, L.; Cui, J. Cost-Effectiveness Analyses of Home Parenteral Nutrition for Incurable Gastrointestinal Cancer Patients. Front. Oncol. 2022, 12, 858712. [Google Scholar] [CrossRef]
- Pochettino, F.; Visconti, G.; Godoy, D.; Rivarola, P.; Crivelli, A.; Puga, M.; González, H.F.; Fernández, A. Association between Karnofsky performance status and outcomes in cancer patients on home parenteral nutrition. Clin. Nutr. ESPEN 2023, 54, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Caccialanza, R.; Cotogni, P.; Finocchiaro, C.; Pironi, L.; Santarpia, L.; Zanetti, M. SINPE Position Paper on the use of home parenteral nutrition in cancer patients. Nutrition 2022, 95, 111578. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Muhlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Cotogni, P.; Mussa, B.; Degiorgis, C.; De Francesco, A.; Pittiruti, M. Comparative Complication Rates of 854 Central Venous Access Devices for Home Parenteral Nutrition in Cancer Patients: A Prospective Study of Over 169,000 Catheter-Days. JPEN J. Parenter. Enter. Nutr. 2021, 45, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Buonomo, A.; Pagano, M.C.; Alfonsi, L.; Foggia, M.; Mottola, M.; Marinosci, G.Z.; Contaldo, F.; Pasanisi, F. Central venous catheter related bloodstream infections in adult patients on home parenteral nutrition: Prevalence, predictive factors, therapeutic outcome. Clin. Nutr. 2016, 35, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Pichitchaipitak, O.; Ckumdee, S.; Apivanich, S.; Chotiprasitsakul, D.; Shantavasinkul, P.C. Predictive factors of catheter-related bloodstream infection in patients receiving home parenteral nutrition. Nutrition 2018, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.; Clarivet, B.; Lotthe, A.; Bommart, S.; Parer, S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: A prospective cohort study. Antimicrob. Resist. Infect. Control 2017, 6, 18. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Carrero, C.; Guerra, E.; Valbuena, B.; Arrieta, F.; Calanas, A.; Zamarron, I.; Balsa, J.A.; Vazquez, C. Role of peripherally inserted central catheters in home parenteral nutrition: A 5-year prospective study. JPEN J. Parenter. Enter. Nutr. 2013, 37, 544–549. [Google Scholar] [CrossRef]
- Santacruz, E.; Mateo-Lobo, R.; Riveiro, J.; Nattero, L.; Vega-Piñero, B.; Lomba, G.; Sabido, R.; Carabaña, F.; Arrieta, F.J.; Botella-Carretero, J.I. Infectious complications in home parenteral nutrition: A long-term study with peripherally inserted central catheters, tunneled catheters, and ports. Nutrition 2019, 58, 89–93. [Google Scholar] [CrossRef]
- Cheon, S.; Oh, S.H.; Kim, J.T.; Choi, H.G.; Park, H.; Chung, J.E. Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients. Nutrients 2023, 15, 2531. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.X.; Liu, X.Y.; Li, H.L.; Mei, D. Comparison of effectiveness, safety, and costs of standardized and customized parenteral nutrition support among gastric cancer patients after gastrectomy: A retrospective cohort study. Asia Pac. J. Clin. Nutr. 2018, 27, 818–822. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Boleo-Tome, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br. J. Nutr. 2012, 108, 343–348. [Google Scholar] [CrossRef]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Cleveland Clinic Sample Size Calculator. Available online: https://riskcalc.org/samplesize/ (accessed on 12 September 2022).
- Crooks, B.; Harrison, S.; Millward, G.; Hall, K.; Taylor, M.; Farrer, K.; Abraham, A.; Teubner, A.; Lal, S. Catheter-related infection rates in patients receiving customized home parenteral nutrition compared with multichamber bags. JPEN J. Parenter. Enter. Nutr. 2022, 46, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Planas, M.; Puiggros, C.; Sanchez, J.R.; Cots, I.; Tutusaus, M.; Rodriguez, T.; Perez-Portabella, C.; Gomez, R. Use of ready-to-use (RTU) products in home-based parenteral nutrition. Nutr. Hosp. 2006, 21, 64–70. [Google Scholar] [PubMed]
- Banko, D.; Rosenthal, N.; Chung, J.; Lomax, C.; Washesky, P.F. Comparing the risk of bloodstream infections by type of parenteral nutrition preparation method: A large retrospective, observational study. Clin. Nutr. ESPEN 2019, 30, 100–106. [Google Scholar] [CrossRef]
- Mateo-Lobo, R.; Riveiro, J.; Vega-Pinero, B.; Botella-Carretero, J.I. Infectious Complications in Home Parenteral Nutrition: A Systematic Review and Meta-Analysis Comparing Peripherally-Inserted Central Catheters with Other Central Catheters. Nutrients 2019, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Arruda, A.; Zaloga, G.; Wischmeyer, P.; Turpin, R.; Liu, F.X.; Mercaldi, C. Is there a difference in bloodstream infections in critically ill patients associated with ready-to-use versus compounded parenteral nutrition? Clin. Nutr. 2012, 31, 728–734. [Google Scholar] [CrossRef]
- Hall, J.W. Safety, cost, and clinical considerations for the use of premixed parenteral nutrition. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2015, 30, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.E.; Berlana, D.; Ukleja, A.; Boullata, J. Clinical, Ergonomic, and Economic Outcomes With Multichamber Bags Compared With (Hospital) Pharmacy Compounded Bags and Multibottle Systems: A Systematic Literature Review. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, H.; Alsemari, M.; Mohamed, G.; Alshahrani, A.; Islami, M. The Rate of Discontinuing Ready-to-Use Multi-Chamber Bag Parenteral Nutrition Secondary to High Serum Electrolyte Levels. Hosp. Pharm. 2023, 58, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Keane, N.; Fragkos, K.C.; Patel, P.S.; Bertsch, F.; Mehta, S.J.; Di Caro, S.; Rahman, F. Performance Status, Prognostic Scoring, and Parenteral Nutrition Requirements Predict Survival in Patients with Advanced Cancer Receiving Home Parenteral Nutrition. Nutr. Cancer 2018, 70, 73–82. [Google Scholar] [CrossRef]
- Bozzetti, F.; Cotogni, P.; Lo Vullo, S.; Pironi, L.; Giardiello, D.; Mariani, L. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann. Oncol. 2015, 26, 2335–2340. [Google Scholar] [CrossRef]
- Pasanisi, F.; Orban, A.; Scalfi, L.; Alfonsi, L.; Santarpia, L.; Zurlo, E.; Celona, A.; Potenza, A.; Contaldo, F. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001, 17, 581–584. [Google Scholar] [CrossRef]

| Gastrointestinal neoplasia | 89 |
| Esophageal cancer * | 8 |
| Gastric cancer with PC or obstruction | 38 |
| Pancreatic cancer with PC or obstruction | 9 |
| Intestinal adenocarcinoma with PC | 3 |
| Neuroendocrine tumor with obstruction | 4 |
| Appendicular carcinoma | 1 |
| Colorectal cancer with PC | 25 |
| Rectal cancer with radiation enteritis | 1 |
| Gynecological neoplasia | 28 |
| Cervix carcinoma with PC | 1 |
| Breast cancer with PC | 5 |
| Ovarian cancer with PC | 19 |
| Fallopian tube carcinoma with PC | 2 |
| Endometrium cancer with PC | 1 |
| Others | 13 |
| Bladder cancer with PC or fistula | 4 |
| Bone sarcoma with PC | 1 |
| Lung cancer with GI involvement | 1 |
| Head and neck cancer * | 3 |
| GVHD after leukemia | 4 |
| Individual Compounded (n = 87) | Commercial Multichamber (n = 43) | |
|---|---|---|
| Age (y) | 58 ± 11 | 59 ± 13 |
| Female gender | 57 (66) | 30 (69) |
| Time on HPN (days) ‡ | 64 ± 113 | 64 ± 126 |
| Duration > 6 months | 70 (80) | 29 (68) |
| Bags infused per week (n) | 6.9 ± 0.6 | 7.0 ± 0.0 |
| Supplied energy (Kcal/kg/day) | 29.4 ± 7.9 | 28.7 ± 7.6 |
| Body weight (kg) | 53 ± 13 | 55 ± 14 |
| BMI (kg/m2) | 20.2 ± 4.4 | 21.1 ± 5.5 |
| Serum albumin (g/dL) | 2.7 ± 0.7 | 2.7 ± 0.5 |
| Total lymphocytes (n/103) | 1.2 ± 0.7 | 1.3 ± 0.8 |
| Total cholesterol (mg/dL) | 205 ± 205 | 163 ± 47 |
| CONUT index (score) | 7.2 ± 2.4 | 8.2 ± 2.8 |
| MUST index (score) | 1.9 ± 0.3 | 1.9 ± 0.3 |
| ECOG index (score) | 1.7 ± 0.9 | 1.7 ± 0.8 |
| Gastrointestinal neoplasia (n) | 62 (71) | 27 (63) |
| Stage IV (n) | 29 (48) | 14 (52) |
| Gynecologic neoplasia (n) | 18 (21) | 10 (21) |
| Stage IV (n) | 14 (78) | 7 (70) |
| Other type of neoplasia (n) | 7 (8) | 6 (14) |
| Stage IV (n) | 3 (43) | 3 (50) |
| Lines of chemotherapy before HPN (n) | 1.8 ± 1.8 | 1.3 ± 1.4 |
| Lines of chemotherapy after HPN (n) | 2.5 ± 2.0 | 1.9 ± 1.6 |
| PICC catheter (n) | 56 (65) | 33 (78) |
| Tunneled catheter (n) | 9 (10) | 2 (5) |
| Ports (n) | 22 (25) | 7 (17) |
| Multilumen catheter (n) | 12 (19) | 7 (21) |
| Suspected CRBSI (n) | 8 (9.2) | 2 (4.7) |
| Confirmed CRBSI (n) | 3 (3.4) | 1 (2.3) |
| Thrombosis (n) | 2 (2.3) | 0 (0) |
| Severe metabolic complications (n) † | 1 (1.2) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Argüeso, M.; Gómez-Bayona, E.; Ugalde, B.; Vega-Piñero, B.; Gil-Díaz, M.; Longo, F.; Pintor, R.; Botella-Carretero, J.I. Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study. Nutrients 2024, 16, 457. https://doi.org/10.3390/nu16030457
Fernández-Argüeso M, Gómez-Bayona E, Ugalde B, Vega-Piñero B, Gil-Díaz M, Longo F, Pintor R, Botella-Carretero JI. Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study. Nutrients. 2024; 16(3):457. https://doi.org/10.3390/nu16030457
Chicago/Turabian StyleFernández-Argüeso, María, Elena Gómez-Bayona, Beatriz Ugalde, Belén Vega-Piñero, Mayra Gil-Díaz, Federico Longo, Rosario Pintor, and José I. Botella-Carretero. 2024. "Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study" Nutrients 16, no. 3: 457. https://doi.org/10.3390/nu16030457
APA StyleFernández-Argüeso, M., Gómez-Bayona, E., Ugalde, B., Vega-Piñero, B., Gil-Díaz, M., Longo, F., Pintor, R., & Botella-Carretero, J. I. (2024). Ready-to-Use Multichamber Bags in Home Parenteral Nutrition for Patients with Advanced Cancer: A Single-Center Prospective Study. Nutrients, 16(3), 457. https://doi.org/10.3390/nu16030457





