A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Research
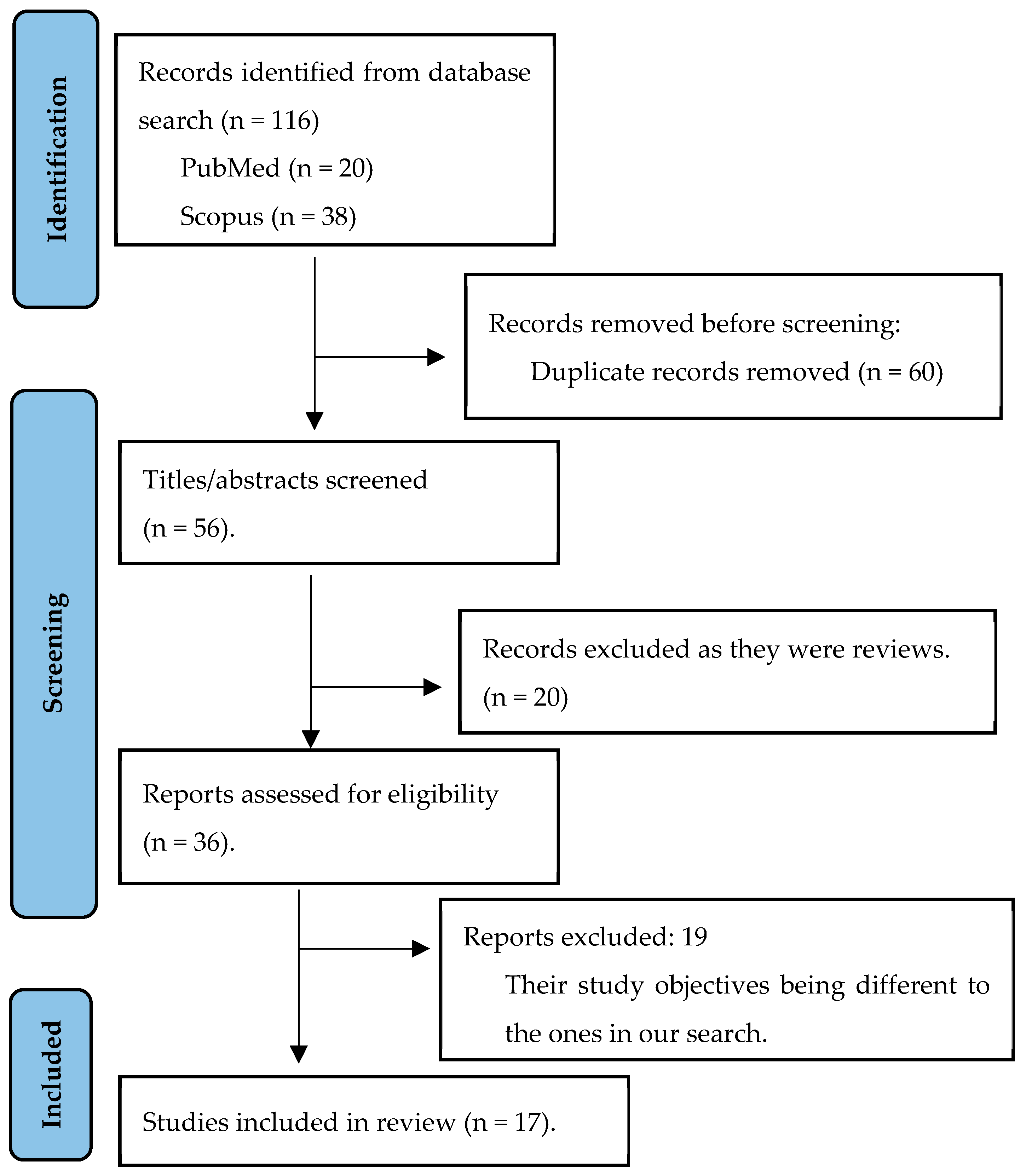
2.2. Study Eligibility, Selection and Data Extraction
3. Results
3.1. Summary of the Studies Included
Prevalence of Malnutrition in the Outpatient Cancer Population
3.2. Comparison with Different Screening Tools
3.3. Parameters Involved in the Development of Malnutrition
4. Discussion
4.1. Malnutrition Prevalence
4.2. Tumour Type
4.3. Age
4.4. Gender
4.5. Tumour Stage
4.6. Nutritional Status Assessment Factors in Nutritional Screening Tools
4.7. Choice of Nutritional Screening Tool
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Ulíbarri Pérez, J.I. Cribado Nutricional; control de la desnutrición clínica. Nutr. Hosp. 2014, 4, 797–811. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult Starvation and Disease-Related Malnutrition: A Proposal for Etiology-Based Diagnosis in the Clinical Practice Setting from the International Consensus Guideline Committee. J. Parenter. Enter. Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef]
- Virizuela, J.A.; Camblor-Álvarez, M.; Luengo-Pérez, L.M.; Grande, E.; Álvarez-Hernández, J.; Sendrós-Madroño, M.J.; Ji-ménez-Fonseca, P.; Cervera-Peris, M.; Ocón-Bretón, M.J. Nutritional Support and Parenteral Nutrition in Cancer Patients: An Expert Consensus Report. Clin. Transl. Oncol. 2018, 20, 619–629. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Valentini, L. Disease-Related Malnutrition and Sarcopenia as Determinants of Clinical Outcome. Visc. Med. 2019, 35, 282–291. [Google Scholar] [CrossRef] [PubMed]
- García de Lorenzo, A.; Álvarez Hernández, J.; Planas, M.; Burgos, R.; Araujo, K. Multidisciplinary consensus work-team on the approach to hospital malnutrition in Spain. Multidisciplinary consensus on the approach to hospital malnutrition in Spain. Nutr Hosp. 2011, 26, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated Screening Tools for the Assessment of Cachexia, Sarcopenia, and Malnutrition: A Systematic Review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arribas, L.; Hurtós, L.; Sendrós, M.J.; Peiró, I.; Salleras, N.; Fort, E.; Sánchez-Migallón, J.M. NUTRISCORE: A New Nutritional Screening Tool for Oncological Outpatients. Nutrition 2017, 33, 297–303. [Google Scholar] [CrossRef] [PubMed]
- De Van Der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on Validation of the Operational Criteria for the Diagnosis of Protein-Energy Malnutrition in Adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef]
- Gabrielson, D.K.; Scaffidi, D.; Leung, E.; Stoyanoff, L.; Robinson, J.; Nisenbaum, R.; Brezden-Masley, C.; Darling, P.B. Use of an Abridged Scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a Nutritional Screening Tool for Cancer Patients in an Outpatient Setting. Nutr. Cancer 2013, 65, 234–239. [Google Scholar] [CrossRef]
- Ottery, F.D. Definition of Standardized Nutritional Assessment and Interventional Pathways in Oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef]
- Gascón-Ruiz, M.; Casas-Deza, D.; Torres-Ramón, I.; Zapata-García, M.; Alonso, N.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quílez, E.; Isla, D.; et al. Comparation of Different Malnutrition Screening Tools According to GLIM Criteria in Cancer Outpatients. Eur. J. Clin. Nutr. 2022, 76, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Gascón-Ruiz, M.; Casas-Deza, D.; Marti-Pi, M.; Torres-Ramón, I.; Zapata-García, M.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quilez, E.; Isla, D.; et al. Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients. Biomedicines 2022, 10, 2201. [Google Scholar] [CrossRef]
- Jendretzki, J.; Henniger, D.; Schiffmann, L.; Wolz, C.; Kollikowski, A.; Meining, A.; Einsele, H.; Winkler, M.; Löffler, C. Every Fifth Patient Suffered a High Nutritional Risk—Results of a Prospective Patient Survey in an Oncological Outpatient Center. Front. Nutr. 2022, 9, 1033265. [Google Scholar] [CrossRef]
- Opanga, Y.; Kaduka, L.; Bukania, Z.; Mutisya, R.; Korir, A.; Thuita, V.; Mwangi, M.; Muniu, E.; Mbakaya, C. Nutritional Status of Cancer Outpatients Using Scored Patient Generated Subjective Global Assessment in Two Cancer Treatment Centers, Nairobi, Kenya. BMC Nutr. 2017, 3, 63. [Google Scholar] [CrossRef]
- Helfenstein, S.F.; Uster, A.; Rühlin, M.; Pless, M.; Ballmer, P.E.; Imoberdorf, R. Are Four Simple Questions Able to Predict Weight Loss in Outpatients with Metastatic Cancer? A Prospective Cohort Study Assessing the Simplified Nutritional Appe-tite Questionnaire. Nutr. Cancer 2016, 68, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, J.; Madubhashini, P.; Miller, M. Agreement between the Malnutrition Universal Screening Tool and the Patient-Generated Subjective Global Assessment for Cancer Outpatients Receiving Chemotherapy: A Cross-Sectional Study. Nutr. Cancer 2018, 70, 1275–1282. [Google Scholar] [CrossRef]
- Auma, C.M.N.; Mweu, M.M.; Opiyo, R.O. Performance of Malnutrition Universal Screening Tool and Patient-Generated Global Subjective Assessment in Screening for Cancer-Related Malnutrition in Nairobi, Kenya. F1000Research 2022, 11, 755. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Amerio, M.L.; Biffi, R.; Caccialanza, R.; Capuano, G.; Correja, I.; Cozzaglio, L.; Di Leo, A.; et al. The Nutritional Risk in Oncology: A Study of 1453 Cancer Outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef]
- Hauner, H.; Kocsis, A.; Jaeckel, B.; Martignoni, M.; Hauner, D.; Holzapfel, C. Häufigkeit eines Risikos für Mangelernährung bei Patienten in onkologischen Schwerpunktpraxen—Eine Querschnittserhebung. DMW Dtsch. Med. Wochenschr. 2020, 145, e1–e9. [Google Scholar] [CrossRef]
- Kaduka, L.U.; Bukania, Z.N.; Opanga, Y.; Mutisya, R.; Korir, A.; Thuita, V.; Nyongesa, C.; Mwangi, M.; Mbakaya, C.F.L.; Muniu, E. Malnutrition and Cachexia among Cancer Out-Patients in Nairobi, Kenya. J. Nutr. Sci. 2017, 6, e63. [Google Scholar] [CrossRef] [PubMed]
- Sobrini, P.; Sánchez-Castellano, C.; Cruz-Jentoft, A.J. MNA-SF as a Screening Tool for Malnutrition Diagnosed with the Glim Criteria in Older Persons with Cancer. Eur. Geriatr. Med. 2021, 12, 653–656. [Google Scholar] [CrossRef]
- De Groot, L.M.; Lee, G.; Ackerie, A.; Van Der Meij, B.S. Malnutrition Screening and Assessment in the Cancer Care Ambula-tory Setting: Mortality Predictability and Validity of the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and the GLIM Criteria. Nutrients 2020, 12, 2287. [Google Scholar] [CrossRef]
- Carretero, I.V.; Ravasco, P. PP078-MON: Must in Cancer Patients Undergoing Chemotherapy: Valid? Clin. Nutr. 2014, 33, S159. [Google Scholar] [CrossRef]
- Tobberup, R.; Thoresen, L. Accuracy of Screening Tools in Predicting Malnutrition in Cancer Outpatients According to the Glim Criteria. Clin. Nutr. ESPEN 2020, 40, 565–566. [Google Scholar] [CrossRef]
- Anzévui, A.; Frateur, L.; Bertrand, B.; Gihousse, D.; Joly, E.; Hamoir, M.; Méan, A. Sun applicability of the Malnutrition Screening Tool for Cancer patients (MSTC) in adult outpatients, suffering from any tumour types, in Belgium. Clin. Nutr. Suppl. 2012, 7, 107–108. [Google Scholar] [CrossRef]
- Kuzma, S.; Mahon, E.; Ramage, J.; Camilleri, B.; Todd, A. Malnutrition prevalence in an outpatient cancer care setting. Asia Pac. J. Clin. Oncol. 2014, 10, 126–209. [Google Scholar] [CrossRef]
- Segura, A.; Pardo, J.; Jara, C.; Zugazabeitia, L.; Carulla, J.; De Las Peñas, R.; García-Cabrera, E.; Luz Azuara, M.; Casadó, J.; Gómez-Candela, C. An Epidemiological Evaluation of the Prevalence of Malnutrition in Spanish Patients with Locally Ad-vanced or Metastatic Cancer. Clin. Nutr. 2005, 24, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martínez, L.; Castro-Eguiluz, D.; Copca-Mendoza, E.T.; Pérez-Camargo, D.A.; Reyes-Torres, C.A.; Ávila, E.A.; López-Córdova, G.; Fuentes-Hernández, M.R.; Cetina-Pérez, L.; Milke-García, M.D.P. Nutritional Assessment Tools for the Identification of Malnutrition and Nutritional Risk Associated with Cancer Treatment. Rev. Investig. Clin. 2018, 70, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle 2018, 9, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Puizina Mladinic, E.; Usljebrka, M.; Kadic, S.; Lesin, A.; Vilovic, M.; Lupi-Ferandin, S.; Ercegovic, S.; Kumric, M.; et al. Nutritional Management of Patients with Head and Neck Cancer—A Comprehensive Review. Nutrients 2023, 15, 1864. [Google Scholar] [CrossRef]
- Sonneborn-Papakostopoulos, M.; Dubois, C.; Mathies, V.; Heß, M.; Erickson, N.; Ernst, T.; Huebner, J. Quality of life, symptoms and dietary habits in oncology outpatients with malnutrition: A cross-sectional study. Med. Oncol. 2021, 38, 20. [Google Scholar] [CrossRef]
- Argiles, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa ESilva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Zhang, X.; Edwards, B.J. Malnutrition in Older Adults with Cancer. Curr. Oncol. Rep. 2019, 21, 80. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Masoni, M.C.; Fini, M.; Pagani, S.; Giampietro, O.; Carpi, A. Malnutrition, anorexia and cachexia in cancer patients: A mini-review on pathogenesis and treatment. Biomed. Pharmacother. 2013, 67, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic Effect of Weight Loss Prior Tochemotherapy in Cancer Patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyan, N. Cost and benefits of nutrition support in cancer. Oncology 1995, 9, 79–84. [Google Scholar]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between Markers of Malnutrition and Clinical Outcomes in Older Adults with Cancer: Systematic Review, Narrative Synthesis and Meta-Analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.; Luo, M.; Matheson, E. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
| Autor (Year) | n | Design | Objective Intervention | Intervention | Time | Conclusions |
|---|---|---|---|---|---|---|
| Gascón et al. (2022) [18] | 165 | Cross-sectional | Purchase of different screening tools following GLIM criteria. | Head and neck, upper digestive tract, and colorectal tumours. | 8 months | Nutriscore and MNA-SF showed better performance in patients with high-risk tumours and older people. MUST showed high specificity and sensitivity in the detection of malnutrition. |
| Gascón et al. (2022) [19] | 165 | Longitudinal | To assess the ability of GLIM criteria to predict mortality in outpatients. | Head and neck, upper digestive tract, and colorectal tumours. | 6 months | GLIM criteria identify malnutrition in more than half of oncology outpatients and are useful in predicting clinical outcome: increased treatment toxicity, increased mortality, and tumour progression. |
| Jendretzki et al. (2022) [20] | 311 | Cross-sectional | To identify nutritional risk in groups of patients in an outpatient center. | 8 months | Establishes the MUST tool as a valid screening method. Unintentional weight loss is highlighted as a relevant parameter, especially in overweight patients. | |
| Opanga et al. (2017) [21] | 471 | Cross-sectional | To assess the nutritional status of patients in Kenya on active treatment using PG-SGA. | Outpatients. | PG-SGA is recommended for its efficacy in detecting malnutrition. The prevalence of malnutrition varies according to tumour type, sex, and tumour stage, being higher in advanced stages. | |
| Helfenstein et al. (2016) [22] | 118 | Longitudinal | To assess the validity of the SNAQ tool in detecting impending weight loss. | Patients with metastatic cancer. | 3 months | SNAQ is not a suitable tool for predicting nutritional status evolution. |
| Hettiarachchi et al. (2018) [23] | 100 | Cross-sectional | To determine the prevalence of malnutrition and compare different screening methods with the PG-SGA tool. | Breast, ovarian, prostate, lung, gastrointestinal, and leukaemia tumours. | Prevalence of malnutrition in 45% of patients. The use of MUST was validated, presenting a sensitivity of 89.7% and a specificity of 98.2%, with a positive predictive value of 92.9% and a negative predictive value of 89.7%. Its implementation in clinical practice is recommended as it is simple and does not require training, unlike PG-SGA. BMI was disregarded because of its limited ability to detect malnutrition, misclassifying 30 patients. However, %PP demonstrated adequate specificity and positive predictive value. | |
| Auma et al. (2022) [24] | 188 | Cross-sectional multicentred | To evaluate the efficacy of MUST and PG-SGA in detecting nutritional risk to improve early identification and management of malnutrition. | Patients with head and neck, respiratory, and gastrointestinal cancers. | MUST and PG-SGA are suitable for the detection of nutritional risk. The results revealed a sensitivity of 83.1% and a specificity of 85.7% for MUST. However, it underestimated nutritional risk in patients with oedema or ascites. The PG-SGA demonstrated a sensitivity of 92.4% and a specificity of 72.5%, being highly sensitive, but its accuracy is influenced by the experience of the observer, which may affect its reliability in nutritional assessment. | |
| Bozzetti et al. (2017) [25] | 1000 | Longitudinal | Longitudinal assessment of nutritional status using the NRS-2002 tool to screen for the presence of nutritional risk in outpatients. | Solid tumours of oesophagus, stomach, pancreas, small intestine, colon, lung, and head–neck at any stage. | 3 years | Gastrointestinal tumours, such as oesophageal, stomach, and pancreatic tumours, caused greater weight loss, with patients affected by oesophageal and pancreatic cancer being at higher nutritional risk. The severity of their weight loss was found to be related to anorexia. Both weight loss and decreased dietary intake, are relevant parameters in the assessment of nutritional status. In addition, weight loss was related to the type of tumour, the stage of tumours, and the ECOG scale. Although nutritional support is less effective in advanced stages, initiating it in early stages is essential for these patients |
| Bozzetti et al. (2012) [26] | 1453 | Longitudinal multicentred | To identify nutritional risk and determine patterns of nutritional risk scores in outpatients. | NRS-2002 in patients with solid tumours of the oesophagus, stomach, pancreas, small bowel, colon, lung and head–neck. | 4 years | NRS-2002 is an effective tool to detect malnutrition, showing a prevalence of 32%. Tumour location, performance on the ECOG scale, and symptomatology are factors that predict a high score on the NRS-2002. In addition, clinical variables such as weight loss, anorexia, ECOG, and gastrointestinal disorders are indicative of nutritional risk. |
| Hauner et al. (2020) [27] | 765 | Longitudinal multicentred | To assess the frequency of undernutrition in outpatients. | NRS-2002 MUST | 11 months | Both NRS-2002 and MUST are useful tools, although their efficacy may vary according to tumour type and MUST shows lower sensitivity and specificity than NRS-2002. The prevalence of malnutrition varies according to the type of tumour was 34.9% according to MUST, with a higher incidence in digestive tumours, while the NRS-2002 presented a prevalence of 29.1%, highlighting the relevance in haematopoietic tumours. These discrepancies in the data are due to differences in classification and scoring criteria. |
| Kaduka et al. (2017) [28] | 512 | Cross-sectional multicentred | To study the risk of malnutrition and factors associated with malnutrition and cachexia to inform cancer management in Kenya. | Breast, cervical and digestive tract cancer at various stages, especially advanced stages. | 1 month | Malnutrition affects 33.1% of patients, revealing that tumour location and treatment affect the prevalence of cancer-related malnutrition. The MUST screening tool is proposed for early identification. |
| Sobrini et al. (2021) [29] | 40 | Longitudinal | To analyse the malnutrition detection validity of the MNA-SF screening tool compared with GLIM criteria. | Patients older than 70 years with a recent diagnosis of cancer and a score <14 on the G8 screening tool. | 6 months | MNA-SF is an effective tool to detect malnutrition in cancer patients with frail functional capacity, with 100% sensitivity and 50% specificity. Due to its high sensitivity, it can detect most cancer patients at nutritional risk. However, there is a disparity in the prevalence of malnutrition detected by the MNA, with 80%, compared with the GLIM criteria, which was 57.5%. |
| De Groot et al. (2020) [30] | 246 | Prospective cohorts | To assess the prevalence of malnutrition in outpatients and the validity of the PG-SGA tool and its reduced version compared with GLIM criteria. | Patients with gastrointestinal, lung, and head and neck cancers. | 2 weeks | PG-SGA and PG-SGA SF were neither sufficiently sensitive nor specific to detect malnutrition and showed low concordance with GLIM. |
| Carretero & Ravasco (2014) [31] | 460 | Cross-sectional | To validate the MUST screening tool. | Cancer patients on outpatient chemotherapy. | MUST is valid for detecting malnutrition in oncology outpatients and shows good concordance with PG-SGA. The importance of the weight loss parameter in the MUST tool stands out, with a sensitivity of 49% and a specificity of 79%. | |
| Tobberup & Thoresen (2020) [32] | 120 | Retrospective cross-sectional | To evaluate the accuracy of different screening tools in detecting malnutrition with reference to GLIM criteria. | Patients with unresectable lung cancer, using NRS-2002, MUST, SNAQ, PG-SGA SF, MST, MNA, and GLIM. | Concordance between NRS-2002 and MUST, with a higher specificity in MST and Nutriscore compared with NRS-2002 and MUST. While MNA-SF shows a higher rate of positive predictive value, its specificity is inadequate and its negative predictive value is low. In outpatients with lung cancer, PG-SGA stands out as the most effective tool to detect malnutrition. | |
| Anzévui et al. (2012) [33] | 95 | Cross-sectional | To analyse the MSTC tool in outpatients. | Outpatients with any type of tumour. | MSTC screening tool is characterised as fast (5 min), practical and consistent. It has limitations in factors such as oedema, amputation, voluntary weight loss, cortisone use and extreme BMI. | |
| Kuzma et al. (2014) [34] | 76 | Cross-sectional | To assess the need for nutritional screening in patients in the outpatient setting. | Breast, gynaecological, lung, and gastrointestinal. | In total, 52.6% of patients had MST 0, indicating no risk. Prevalence of malnutrition of 3.9% in outpatient centres. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Andrés, D.; Cabañas-Alite, L. A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients. Nutrients 2024, 16, 752. https://doi.org/10.3390/nu16050752
Gil-Andrés D, Cabañas-Alite L. A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients. Nutrients. 2024; 16(5):752. https://doi.org/10.3390/nu16050752
Chicago/Turabian StyleGil-Andrés, Delia, and Luis Cabañas-Alite. 2024. "A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients" Nutrients 16, no. 5: 752. https://doi.org/10.3390/nu16050752
APA StyleGil-Andrés, D., & Cabañas-Alite, L. (2024). A Narrative Review Comparing Nutritional Screening Tools in Outpatient Management of Cancer Patients. Nutrients, 16(5), 752. https://doi.org/10.3390/nu16050752







