Chlorella Supplementation Reduces Blood Lactate Concentration and Increases O2 Pulse during Submaximal and Maximal Cycling in Young Healthy Adults
Abstract
1. Introduction
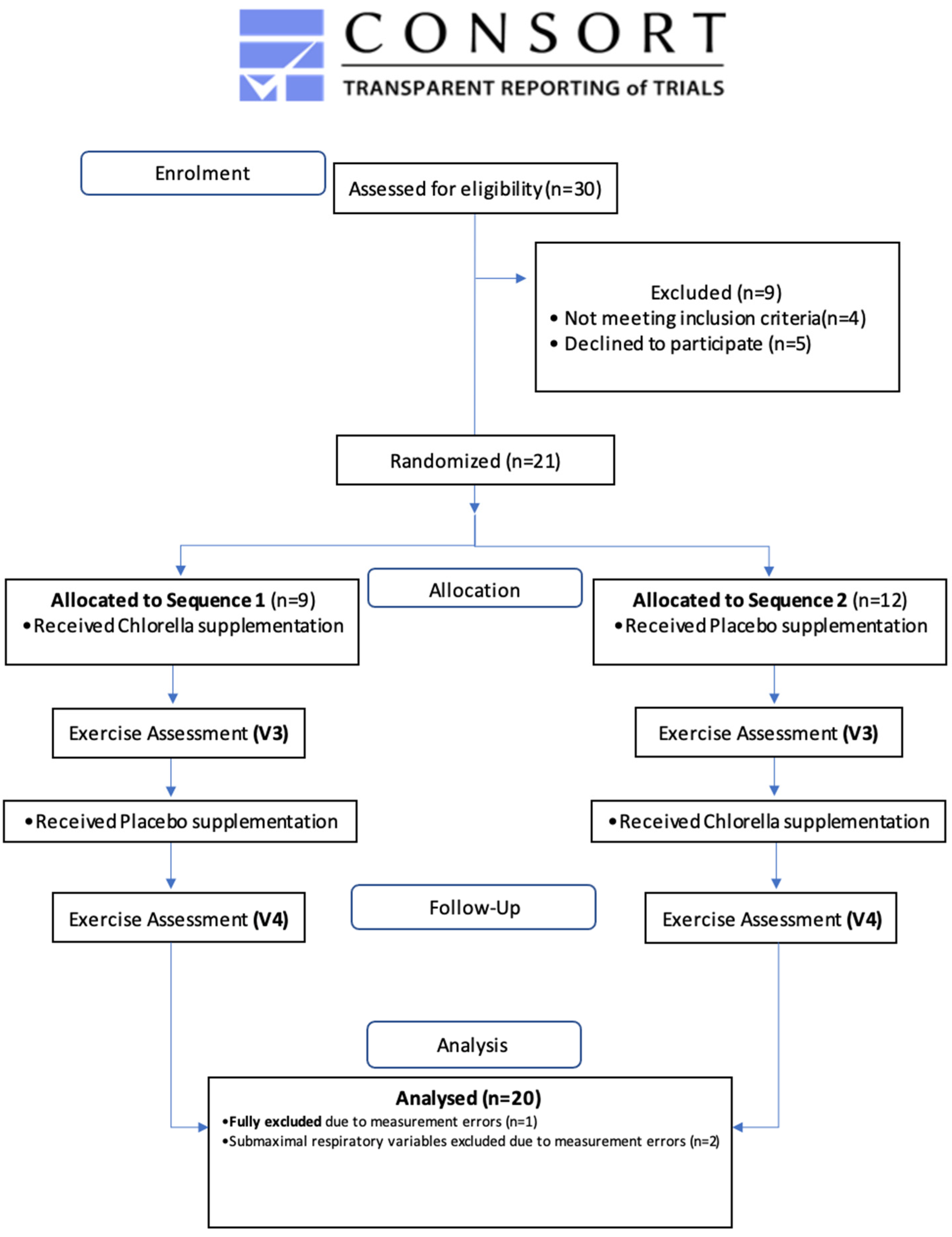
2. Materials and Methods
2.1. Participants
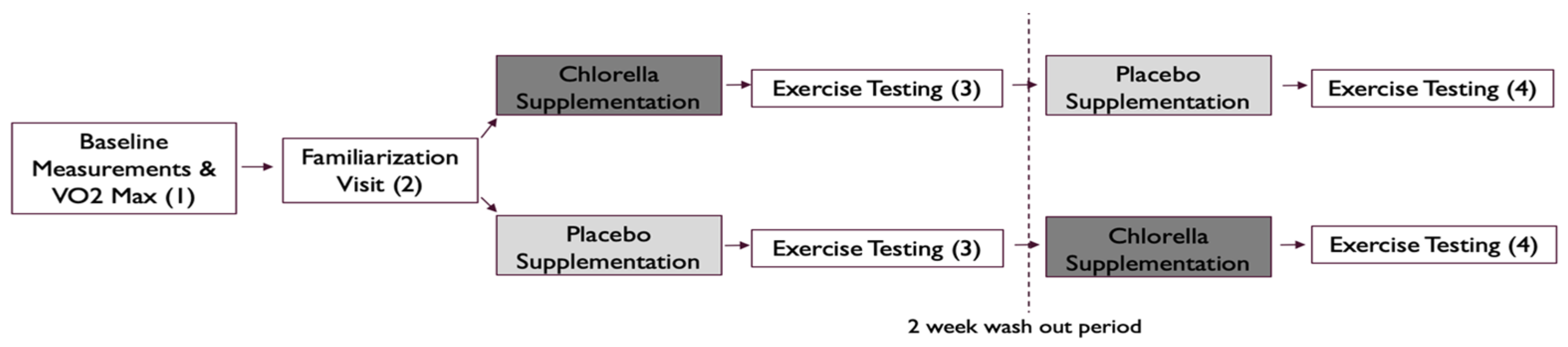
2.2. Study Design
2.3. Chlorella and Placebo Capsules
2.4. Familiarization Visit (V1)
2.5. Exercise Testing (V2, V3, V4)
2.6. Statistical Analysis
3. Results
3.1. Submaximal Testing
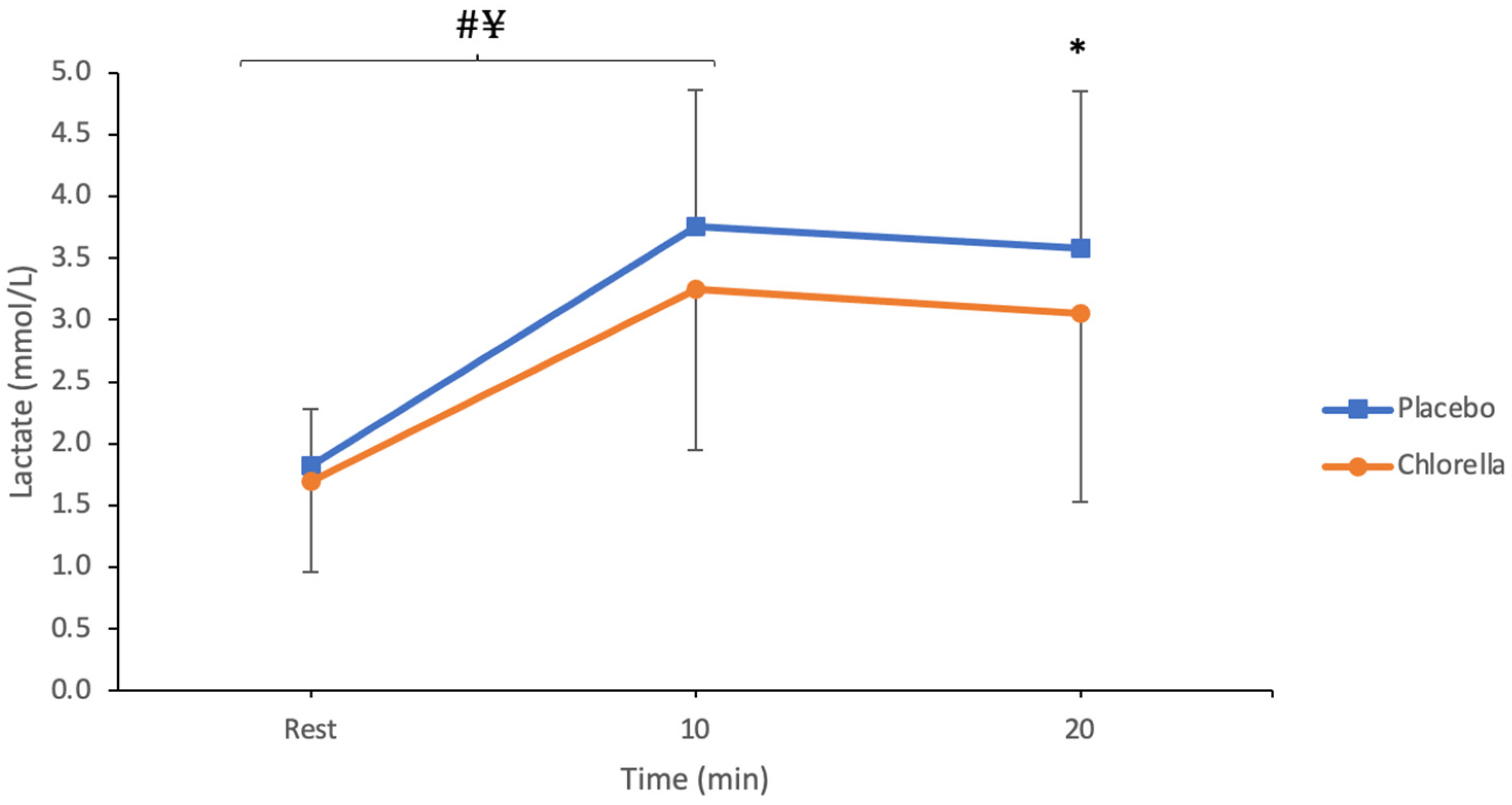
3.1.1. Lactate
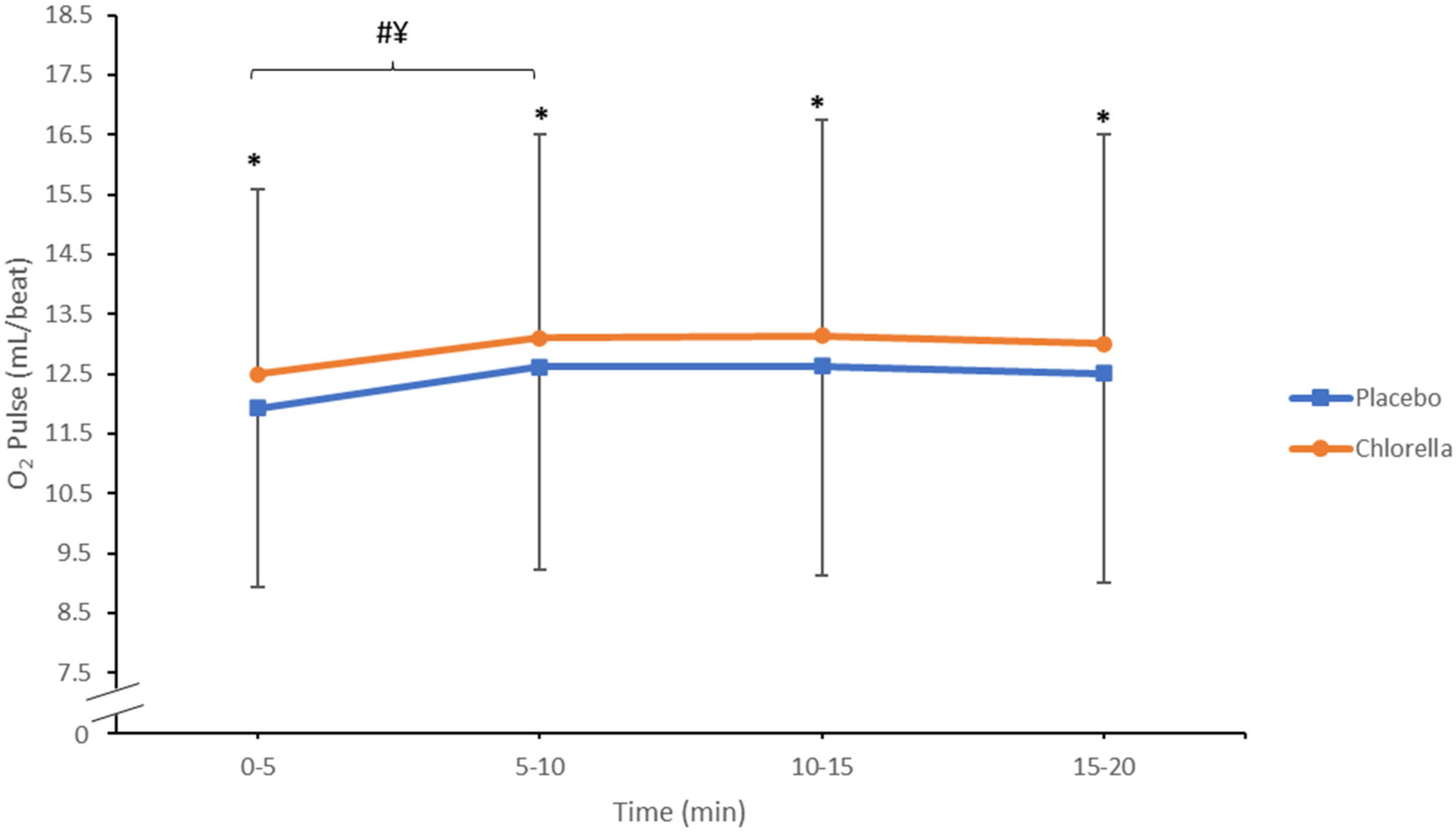
3.1.2. O2 Pulse
3.1.3. Heart Rate
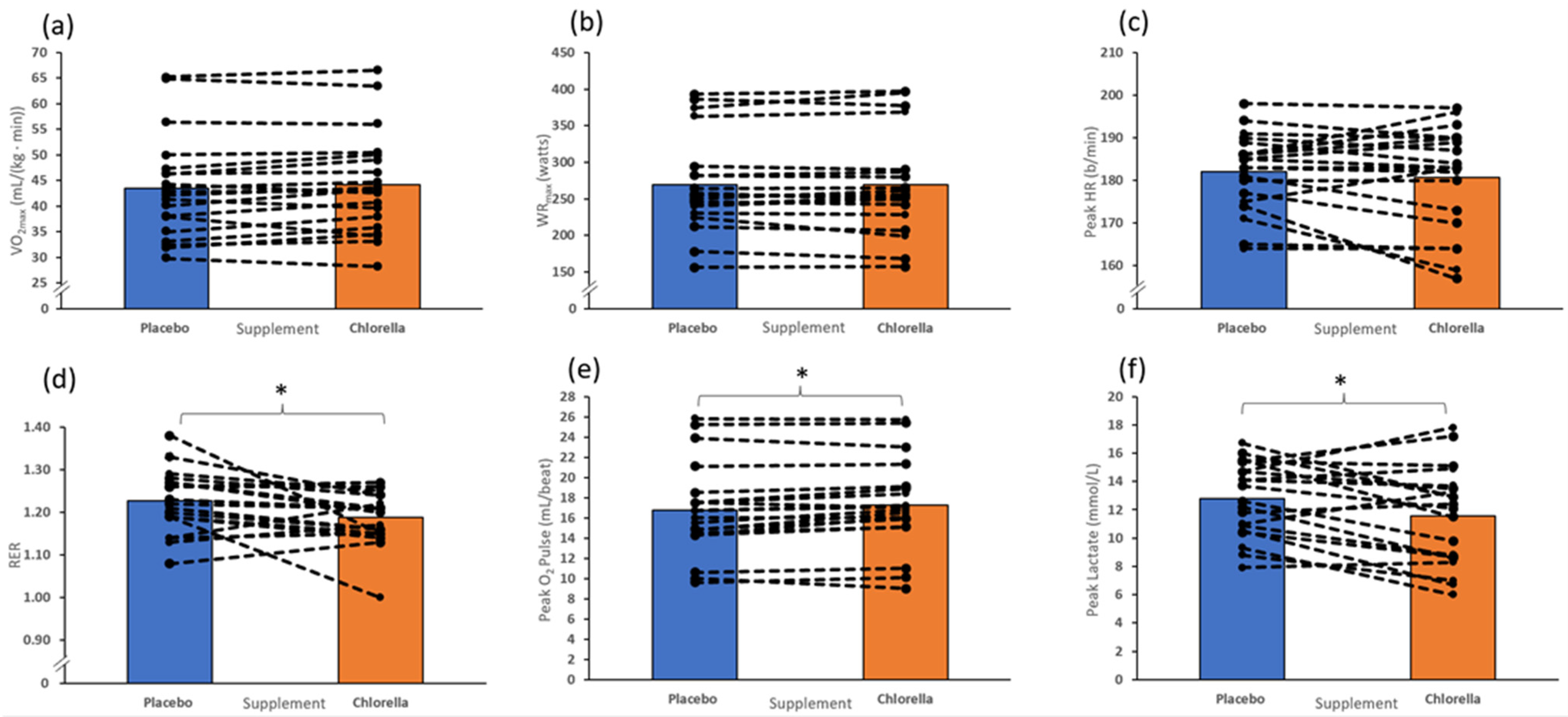
3.2. Incremental Test to Fatigue
4. Discussion
4.1. Submaximal Exercise
4.1.1. Lactate
4.1.2. O2 Pulse
4.1.3. Heart Rate
4.1.4.
4.1.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Per 100 g | Per 6 g | |
|---|---|---|
| Energy KJ/Kcal | 1448 KJ/343 Kcal | 87 KJ/21 Kcal |
| Fat | 7.8 g | 0.47 g |
| of which saturates | 3.1 g | 0.19 g |
| Carbohydrate | 6.9 g | 0.42 g |
| of which sugars | 1.6 g | 0.10 g |
| Protein | 61.3 g | 3.68 g |
| Dietary Fibre | 16.1 g | 0.97 g |
| Salt | 0.8 mg | 0.05 mg |
| Vitamin E | 8.9 mg | 0.53 mg |
| Vitamin B1 | 1.29 mg | 0.08 mg |
| Vitamin B2 | 3.1 mg | 0.19 mg |
| Vitamin B3 | 59 mg | 3.54 mg |
| Vitamin B6 | 0.96 mg | 0.06 mg |
| Folate | 2.3 mg | 0.14 mg |
| Biotin | 0.1 mg | 0.01 mg |
| Potassium | 882.5 mg | 52.95 mg |
| Calcium | 330 mg | 19.8 mg |
| Phosphorus | 1210 mg | 72.6 mg |
| Magnesium | 360 mg | 21.6 mg |
| Iron | 210 mg | 12.6 mg |
| Zinc | 69.8 mg | 4.19 mg |
| Carotenoids | 945 mg | 56.7 mg |
| Beta-carotene | 229 mg | 13.74 mg |
| Chlorophyll | 3790 mg | 227.4 mg |
| Chlorophyll-a | 1749 mg | 104.94 mg |
| Leucine | 4700 mg | 282 mg |
| Arginine | 3300 mg | 198 mg |
| Lysine | 3000 mg | 180 mg |
References
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Otsuki, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.S.; Sato, S.; Mancini-Filho, J. Antioxidant activity of the microalga Chlorella vulgaris cultered on special conditions. Boll. Chim. Farm. 2001, 140, 165–168. [Google Scholar] [PubMed]
- Chiu, H.F.; Lee, H.J.; Han, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Beneficial effect of Chlorella pyrenoidosa drink on healthy subjects: A randomized, placebo-controlled, double-blind, cross-over clinical trial. J. Food Biochem. 2021, 45, e13665. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.D.F.; Silva, A.S.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.; Araujo, L.C.; da Silva, B.A. Supplementation with Spirulina platensis Improves Tracheal Reactivity in Wistar Rats by Modulating Inflammation and Oxidative Stress. Front. Pharmacol. 2022, 13, 826649. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lepe, M.A.; Olivas-Aguirre, F.J.; Gómez-Miranda, L.M.; Hernández-Torres, R.P.; Manríquez-Torres, J.D.J.; Ramos-Jiménez, A. Systematic physical exercise and Spirulina maxima supplementation improve body composition, cardiorespiratory fitness, and blood lipid profile: Correlations of a randomized double-blind controlled trial. Antioxidants 2019, 8, 507. [Google Scholar] [CrossRef]
- Rasheed, R.; Saadaoui, I.; Bounnit, T.; Cherif, M.; Al Ghazal, G.; Al Jabri, H. Sustainable Food Production and Nutraceutical Applications from Qatar Desert Chlorella sp. (Chlorophyceae). Animals 2020, 10, 1413. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Sansawa, H.; Takahashi, M.; Tsuchikura, S.; Endo, H. Effect of chlorella and its fractions on blood pressure, cerebral stroke lesions, and life-span in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2006, 52, 457–466. [Google Scholar] [CrossRef][Green Version]
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-hypertensive effect of gamma-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Umemoto, S.; Otsuki, T. Chlorella-derived multicomponent supplementation increases aerobic endurance capacity in young individuals. J. Clin. Biochem. Nutr. 2014, 55, 143–146. [Google Scholar] [CrossRef][Green Version]
- Zempo-Miyaki, A.; Maeda, S.; Otsuki, T. Effect of Chlorella-derived multicomponent supplementation on maximal oxygen uptake and serum vitamin B(2) concentration in young men. J. Clin. Biochem. Nutr. 2017, 61, 135–139. [Google Scholar] [CrossRef][Green Version]
- Sanayei, M.; Hajizadeh-Sharafabad, F.; Amirsasan, R.; Barzegar, A. High-intensity interval training with or without chlorella vulgaris supplementation in obese and overweight women: Effects on mitochondrial biogenesis, performance and body composition. Br. J. Nutr. 2022, 128, 200–210. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Arakawa, Y.; Kobayashi, M.; Fujishima, M. Influence of Chlorella powder intake during swimming stress in mice. Biochem. Biophys. Res. Commun. 2011, 404, 121–126. [Google Scholar] [CrossRef]
- Gurney, T.; Brouner, J.; Spendiff, O. The Efficacy of Chlorella Supplementation on Multiple Indices of Cycling Performance. J. Diet. Suppl. 2023, 21, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Silva, S.D.; Pleno de Gouveia, L.; Alexandre, A.M.R.C.; Pereira, C.V.; Pereira, A.B.; Partidário, A.C.; Silva, N.E.; Bohn, T.; Gonçalves, V.S.S.; et al. A Single Dose of Marine Chlorella vulgaris Increases Plasma Concentrations of Lutein, beta-Carotene and Zeaxanthin in Healthy Male Volunteers. Antioxidants 2021, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Aubeeluck, R.; Gurney, T. Fourteen-Days Spirulina Supplementation Increases Hemoglobin, but Does Not Provide Ergogenic Benefit in Recreationally Active Cyclists: A Double-Blinded Randomized Crossover Trial. J. Diet. Suppl. 2023, 25, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Ramos-Jimenez, A.; Hernández-Torres, R.P.; Torres-Durán, P.V.; Romero-Gonzalez, J.; Mascher, D.; Posadas-Romero, C.; Juárez-Oropeza, M.A. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin. Med. Circ. Respir. Pulm. Med. 2008, 2, 1–9. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Uchida, M.; Miyamoto-Mikami, E.; Hashimoto, T.; Tabata, I.; Iemitsu, M. High-intensity intermittent exercise training with chlorella intake accelerates exercise performance and muscle glycolytic and oxidative capacity in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R520–R528. [Google Scholar] [CrossRef]
- McCullagh, K.J.; Poole, R.C.; Halestrap, A.P.; O’brien, M.; Bonen, A. Role of the lactate transporter (MCT1) in skeletal muscles. Am. J. Physiol. 1996, 271 Pt 1, E143–E150. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Santos, G.; Pérez-Schindler, J.; Handschin, C. Skeletal muscle PGC-1alpha controls whole-body lactate homeostasis through estrogen-related receptor alpha-dependent activation of LDH B and repression of LDH A. Proc. Natl. Acad. Sci. USA 2013, 110, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Villareal, M.O.; Matsukawa, T.; Isoda, H. l-Citrulline Supplementation-Increased Skeletal Muscle PGC-1alpha Expression Is Associated with Exercise Performance and Increased Skeletal Muscle Weight. Mol. Nutr. Food Res. 2018, 62, e1701043. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Fiordelisi, A.; Spigno, L.; Boldrini, L.; Lungonelli, G.; Di Vaia, E.; Santulli, G.; Sorriento, D.; Cerasuolo, F.A.; Trimarco, V.; et al. Effects of Chronic Supplementation of l-Arginine on Physical Fitness in Water Polo Players. Oxid. Med. Cell Longev. 2021, 2021, 6684568. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Nishida, Y. Effect of lactate accumulation during exercise-induced muscle fatigue on the sensorimotor cortex. J. Phys. Ther. Sci. 2013, 25, 1637–1642. [Google Scholar] [CrossRef]
- Bernardi, M.; Guerra, E.; Rodio, A.; Dante, D.; Castellano, V.; Peluso, I.; Schena, F.; Bhambhan, Y. Assessment of Exercise Stroke Volume and Its Prediction from Oxygen Pulse in Paralympic Athletes with Locomotor Impairments: Cardiac Long-Term Adaptations Are Possible. Front. Physiol. 2019, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Van Kessel, A.L.; Burton, G.G. Interaction of physiological mechanisms during exercise. J. Appl. Physiol. 1967, 22, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Gurney, T.; Spendiff, O. Spirulina supplementation improves oxygen uptake in arm cycling exercise. Eur. J. Appl. Physiol. 2020, 120, 2657–2664. [Google Scholar] [CrossRef]
- Scribbans, T.D.; Vecsey, S.; Hankinson, P.B.; Foster, W.S.; Gurd, B.J. The Effect of Training Intensity on VO2max in Young Healthy Adults: A Meta-Regression and Meta-Analysis. Int. J. Exerc. Sci. 2016, 9, 230–247. [Google Scholar]
- Delong, C.; Sharma, S. Physiology, Peripheral Vascular Resistance; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kagota, S.N.M.; Morikawa, K.; Maruyama-Fumoto, K.; Yamada, S.; Shinozuka, K. Vasorelaxant Effects of Chlorella on Blood Circulation in Healthy Rats. Austin J. Nutr. Food Sci. 2020, 8, 1142. [Google Scholar]
- Alvares, T.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M.F. Acute l-Arginine supplementation does not increase nitric oxide production in healthy subjects. Nutr. Metab. 2012, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E239–E245. [Google Scholar]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Pokan, R.; Hofmann, P.; von Duvillard, S.P.; Smekal, G.; Wonisch, M.; Lettner, K.; Schmid, P.; Shechter, M.; Silver, B.; Bachl, N. Oral magnesium therapy, exercise heart rate, exercise tolerance, and myocardial function in coronary artery disease patients. Br. J. Sports Med. 2006, 40, 773–778. [Google Scholar] [CrossRef]
- Zheng, D.; Upton, R.N.; Ludbrook, G.L.; Martinez, A. Acute cardiovascular effects of magnesium and their relationship to systemic and myocardial magnesium concentrations after short infusion in awake sheep. J. Pharmacol. Exp. Ther. 2001, 297, 1176–1183. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. beta-Carotene and lycopene affect endothelial response to TNF-alpha reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gurney, T.; Brouner, J.; Spendiff, O. Twenty-one days of spirulina supplementation lowers heart rate during submaximal cycling and augments power output during repeated sprints in trained cyclists. Appl. Physiol. Nutr. Metab. 2021, 47, 18–26. [Google Scholar] [CrossRef]
- Chowdhary, S.; Nuttall, S.L.; Coote, J.H.; Townend, J.N. l-arginine augments cardiac vagal control in healthy human subjects. Hypertension 2002, 39, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, E.A.; de Moraes, A.V.V.; Esteves-Filho, A.; Bacal, F.; Auler, J.O.; Carmona, M.J.; Bellotti, G.; Ramires, A.F. l-arginine reduces heart rate and improves hemodynamics in severe congestive heart failure. Clin. Cardiol. 2000, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Terrio, P.R.; Stiffler, D.F. Cardiovascular responses to metabolic and respiratory acidosis and anesthesia in larval Ambystoma tigrinum. J. Comp. Physiol. B 1994, 164, 272–277. [Google Scholar] [CrossRef]
- Valen, E.L.; Engeset, D.; Øverby, N.C.; Hillesund, E.R. StudentKost: A cross-sectional study assessing college students’ diets: Reason for concern? J. Nutr. Sci. 2020, 9, e39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, H.; Gurney, T. Chlorella Supplementation Reduces Blood Lactate Concentration and Increases O2 Pulse during Submaximal and Maximal Cycling in Young Healthy Adults. Nutrients 2024, 16, 697. https://doi.org/10.3390/nu16050697
White H, Gurney T. Chlorella Supplementation Reduces Blood Lactate Concentration and Increases O2 Pulse during Submaximal and Maximal Cycling in Young Healthy Adults. Nutrients. 2024; 16(5):697. https://doi.org/10.3390/nu16050697
Chicago/Turabian StyleWhite, Harry, and Tom Gurney. 2024. "Chlorella Supplementation Reduces Blood Lactate Concentration and Increases O2 Pulse during Submaximal and Maximal Cycling in Young Healthy Adults" Nutrients 16, no. 5: 697. https://doi.org/10.3390/nu16050697
APA StyleWhite, H., & Gurney, T. (2024). Chlorella Supplementation Reduces Blood Lactate Concentration and Increases O2 Pulse during Submaximal and Maximal Cycling in Young Healthy Adults. Nutrients, 16(5), 697. https://doi.org/10.3390/nu16050697





