The Effect of Micronutrients on Obese Phenotype of Adult Mice Is Dependent on the Experimental Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Dietary Interventions
2.3. Insulin Tolerance Test (ITT) and Insulin Resistance (by-HOMA-IR)
2.4. RNA Isolation and Gene Expression
2.5. Statistical Analysis
3. Results
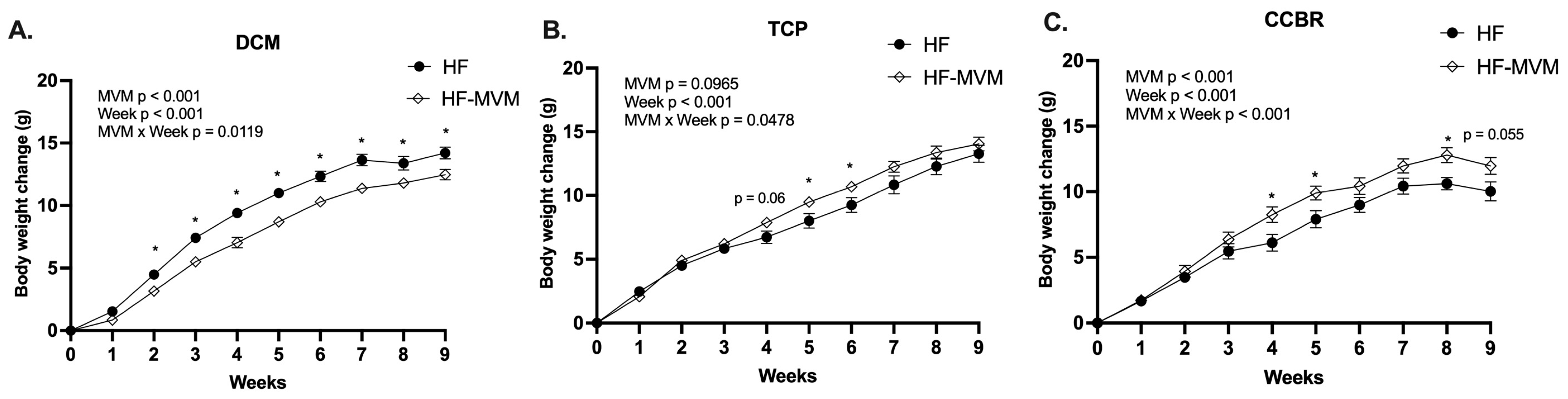
3.1. Body Weight Gain
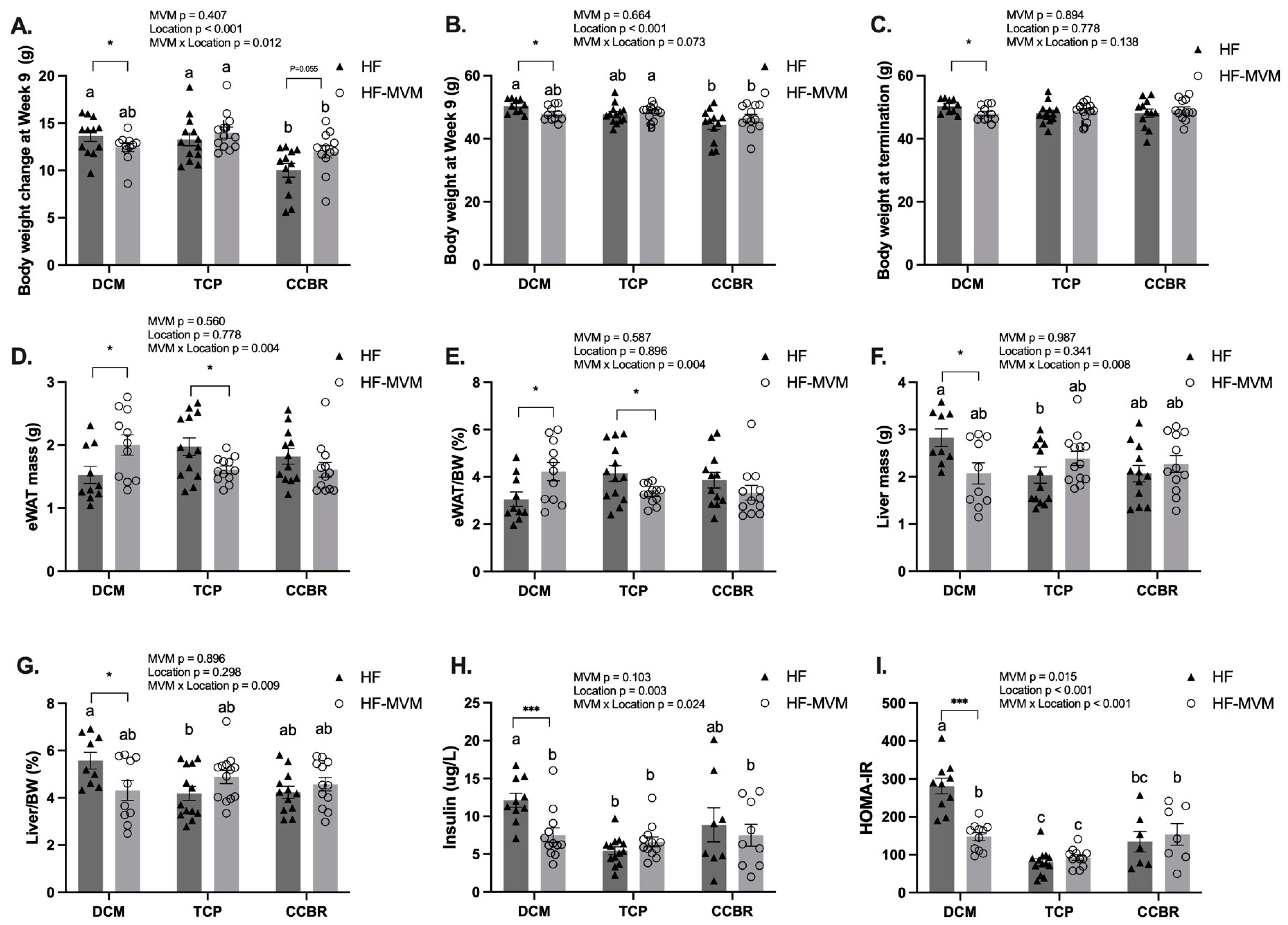
3.2. Body Composition and Insulin Resistance
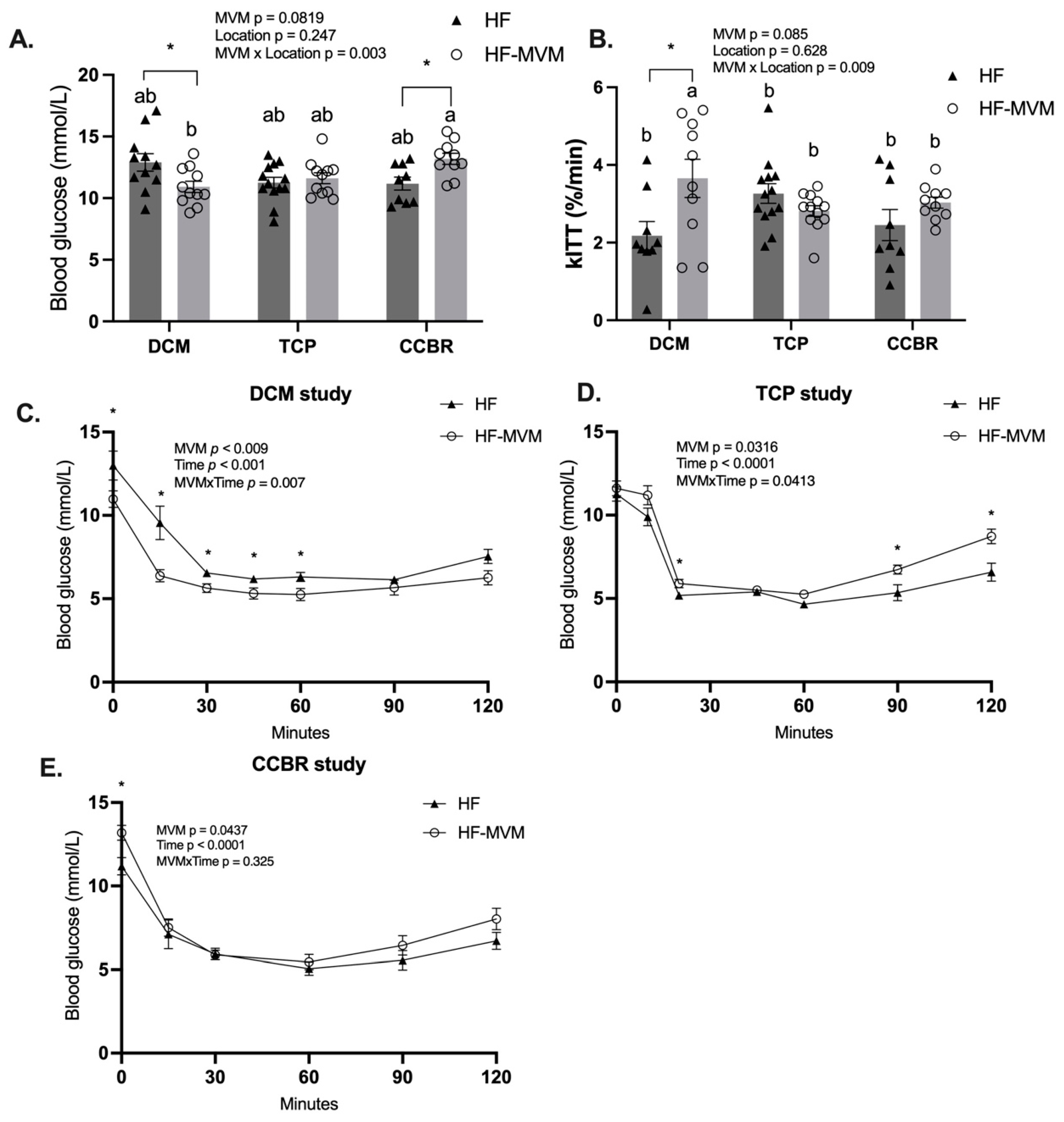
3.3. Insulin Sensitivity
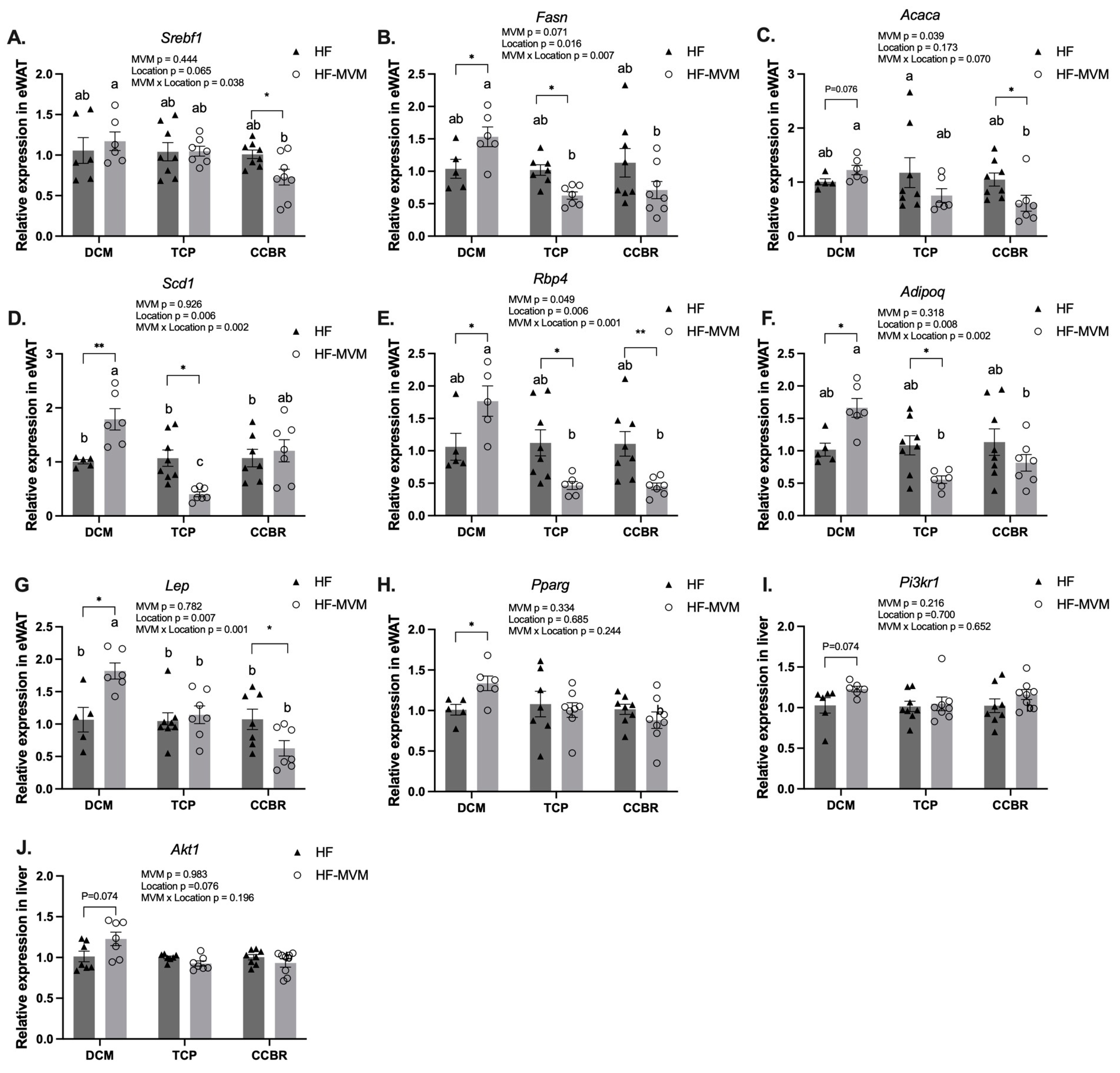
3.4. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freedman, L.P.; Cockburn, I.M.; Simcoe, T.S. The Economics of Reproducibility in Preclinical Research. PLoS Biol. 2015, 13, e1002165. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.G.; Ioannidis, J.P.A. Reproducibility in Science: Improving the Standard for Basic and Preclinical Research. Circ. Res. 2015, 116, 116–126. [Google Scholar] [CrossRef]
- Voelkl, B.; Altman, N.S.; Forsman, A.; Forstmeier, W.; Gurevitch, J.; Jaric, I.; Karp, N.A.; Kas, M.J.; Schielzeth, H.; Van de Casteele, T.; et al. Reproducibility of Animal Research in Light of Biological Variation. Nat. Rev. Neurosci. 2020, 21, 384–393. [Google Scholar] [CrossRef]
- Jaric, I.; Voelkl, B.; Clerc, M.; Schmid, M.W.; Novak, J.; Rosso, M.; Rufener, R.; von Kortzfleisch, V.T.; Richter, S.H.; Buettner, M.; et al. The Rearing Environment Persistently Modulates Mouse Phenotypes from the Molecular to the Behavioural Level. PLoS Biol. 2022, 20, e3001837. [Google Scholar] [CrossRef]
- Ueno, H.; Takahashi, Y.; Mori, S.; Murakami, S.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Mice Recognise Mice in Neighbouring Rearing Cages and Change Their Social Behaviour. Behav. Neurol. 2024, 2024, 9215607. [Google Scholar] [CrossRef]
- Jaric, I.; Voelkl, B.; Amrein, I.; Wolfer, D.P.; Novak, J.; Detotto, C.; Weber-Stadlbauer, U.; Meyer, U.; Manuella, F.; Mansuy, I.M.; et al. Using Mice from Different Breeding Sites Fails to Improve Replicability of Results from Single-Laboratory Studies. Lab Anim. 2024, 53, 18–22. [Google Scholar] [CrossRef]
- von Kortzfleisch, V.T.; Richter, S.H. Systematic Heterogenization Revisited: Increasing Variation in Animal Experiments to Improve Reproducibility? J. Neurosci. Methods 2024, 401, 109992. [Google Scholar] [CrossRef]
- Richter, S.H.; Garner, J.P.; Auer, C.; Kunert, J.; Würbel, H. Systematic Variation Improves Reproducibility of Animal Experiments. Nat. Methods 2010, 7, 167–168. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The Mammalian Epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef]
- Randunu, R.S.; Bertolo, R.F. The Effects of Maternal and Postnatal Dietary Methyl Nutrients on Epigenetic Changes That Lead to Non-Communicable Diseases in Adulthood. Int. J. Mol. Sci. 2020, 21, 3290. [Google Scholar] [CrossRef]
- Teske, J.A.; Perez-Leighton, C.E.; Noble, E.E.; Wang, C.; Billington, C.J.; Kotz, C.M. Effect of Housing Types on Growth, Feeding, Physical Activity, and Anxiety-Like Behavior in Male Sprague-Dawley Rats. Front. Nutr. 2016, 3, 4. [Google Scholar] [CrossRef]
- Wali, J.A.; Ni, D.; Facey, H.J.W.; Dodgson, T.; Pulpitel, T.J.; Senior, A.M.; Raubenheimer, D.; Macia, L.; Simpson, S.J. Determining the Metabolic Effects of Dietary Fat, Sugars and Fat-Sugar Interaction Using Nutritional Geometry in a Dietary Challenge Study with Male Mice. Nat. Commun. 2023, 14, 4409. [Google Scholar] [CrossRef]
- Georgiou, P.; Zanos, P.; Mou, T.-C.M.; An, X.; Gerhard, D.M.; Dryanovski, D.I.; Potter, L.E.; Highland, J.N.; Jenne, C.E.; Stewart, B.W.; et al. Experimenters’ Sex Modulates Mouse Behaviors and Neural Responses to Ketamine via Corticotropin Releasing Factor. Nat. Neurosci. 2022, 25, 1191–1200. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Glen, E.F. Cage Colour Preferences and Effects of Home Cage Colour on Anxiety in Laboratory Mice. Anim. Behav. 2003, 66, 1085–1092. [Google Scholar] [CrossRef]
- Zocher, S.; Overall, R.W.; Lesche, M.; Dahl, A.; Kempermann, G. Environmental Enrichment Preserves a Young DNA Methylation Landscape in the Aged Mouse Hippocampus. Nat. Commun. 2021, 12, 3892. [Google Scholar] [CrossRef]
- Yang, Z.; Kubant, R.; Cho, C.E.; Kranenburg, E.; Beaudry, J.; Bottiglieri, T.; Anderson, G.H. Micronutrients in High-Fat Diet Modify Insulin Resistance and Its Regulatory Genes in Adult Male Mice. Mol. Nutr. Food Res. 2023, 67, e2300199. [Google Scholar] [CrossRef]
- Monzillo, L.U.; Hamdy, O. Evaluation of Insulin Sensitivity in Clinical Practice and in Research Settings. Nutr. Rev. 2003, 61, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Sánchez-Hernández, D.; Reza-López, S.A.; Huot, P.S.P.; Kim, Y.-I.; Anderson, G.H. High Folate Gestational and Post-Weaning Diets Alter Hypothalamic Feeding Pathways by DNA Methylation in Wistar Rat Offspring. Epigenetics 2013, 8, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum Retinol Binding Protein 4 Contributes to Insulin Resistance in Obesity and Type 2 Diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Schwartz, M.W. Regulation of Food Intake, Energy Balance, and Body Fat Mass: Implications for the Pathogenesis and Treatment of Obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef]
- Wang, Y.; Urs, S.; Kim, S.; Soltani-Bejnood, M.; Quigley, N.; Heo, Y.-R.; Standridge, M.; Andersen, B.; Joshi, R.; Wortman, P.; et al. The Human Fatty Acid Synthase Gene and De Novo Lipogenesis Are Coordinately Regulated in Human Adipose Tissue. J. Nutr. 2004, 134, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Palou, A.; Serra, F. Methylation Analysis in Fatty-Acid-Related Genes Reveals Their Plasticity Associated with Conjugated Linoleic Acid and Calcium Supplementation in Adult Mice. Eur. J. Nutr. 2017, 56, 879–891. [Google Scholar] [CrossRef]
- Gracia, A.; Elcoroaristizabal, X.; Fernández-Quintela, A.; Miranda, J.; Bediaga, N.G.; de Pancorbo, M.M.; Rimando, A.M.; Portillo, M.P. Fatty Acid Synthase Methylation Levels in Adipose Tissue: Effects of an Obesogenic Diet and Phenol Compounds. Genes Nutr. 2014, 9, 411. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Machida, T.; Yonezawa, Y.; Noumura, T. Age-Associated Changes in Plasma Testosterone Levels in Male Mice and Their Relation to Social Dominance or Subordinance. Horm. Behav. 1981, 15, 238–245. [Google Scholar] [CrossRef]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory Exposure to Males, Including Men, Causes Stress and Related Analgesia in Rodents. Nat. Methods 2014, 11, 629–632. [Google Scholar] [CrossRef]
- Rendina-Ruedy, E.; Smith, B.J. Methodological Considerations When Studying the Skeletal Response to Glucose Intolerance Using the Diet-Induced Obesity Model. Bonekey Rep. 2016, 5, 845. [Google Scholar] [CrossRef] [PubMed]
- Rokni, D.; Hemmelder, V.; Kapoor, V.; Murthy, V.N. An Olfactory Cocktail Party: Figure-Ground Segregation of Odorants in Rodents. Nat. Neurosci. 2014, 17, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Lu, Z.; Ye, X.; Olson, D.P.; Lowell, B.B.; Buck, L.B. A Specific Area of Olfactory Cortex Involved in Stress Hormone Responses to Predator Odours. Nature 2016, 532, 103–106. [Google Scholar] [CrossRef]
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of Micronutrient Deficiencies on Obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Lapik, I.A.; Galchenko, A.V.; Gapparova, K.M. Micronutrient Status in Obese Patients: A Narrative Review. Obes. Med. 2020, 18, 100224. [Google Scholar] [CrossRef]
- Via, M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Zhu, K.; Feng, R.N.; Sun, C.H. Effects of Multivitamin and Mineral Supplementation on Adiposity, Energy Expenditure and Lipid Profiles in Obese Chinese Women. Int. J. Obes. 2010, 34, 1070–1077. [Google Scholar] [CrossRef]
- Gunasekara, P.; Hettiarachchi, M.; Liyanage, C.; Lekamwasam, S. Effects of Zinc and Multimineral Vitamin Supplementation on Glycemic and Lipid Control in Adult Diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 53–60. [Google Scholar] [CrossRef][Green Version]
- Lee, C.Y. Effects of Dietary Vitamins on Obesity-Related Metabolic Parameters. J. Nutr. Sci. 2023, 12, e47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Kubant, R.; Kranenburg, E.; Cho, C.E.; Anderson, G.H. The Effect of Micronutrients on Obese Phenotype of Adult Mice Is Dependent on the Experimental Environment. Nutrients 2024, 16, 696. https://doi.org/10.3390/nu16050696
Yang Z, Kubant R, Kranenburg E, Cho CE, Anderson GH. The Effect of Micronutrients on Obese Phenotype of Adult Mice Is Dependent on the Experimental Environment. Nutrients. 2024; 16(5):696. https://doi.org/10.3390/nu16050696
Chicago/Turabian StyleYang, Zeyu, Ruslan Kubant, Eva Kranenburg, Clara E. Cho, and G. Harvey Anderson. 2024. "The Effect of Micronutrients on Obese Phenotype of Adult Mice Is Dependent on the Experimental Environment" Nutrients 16, no. 5: 696. https://doi.org/10.3390/nu16050696
APA StyleYang, Z., Kubant, R., Kranenburg, E., Cho, C. E., & Anderson, G. H. (2024). The Effect of Micronutrients on Obese Phenotype of Adult Mice Is Dependent on the Experimental Environment. Nutrients, 16(5), 696. https://doi.org/10.3390/nu16050696







