Dietary Sodium Restriction and Frailty among Middle-Aged and Older Adults: An 8-Year Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables
2.3. Statistical Analysis
2.4. Robustness Tests
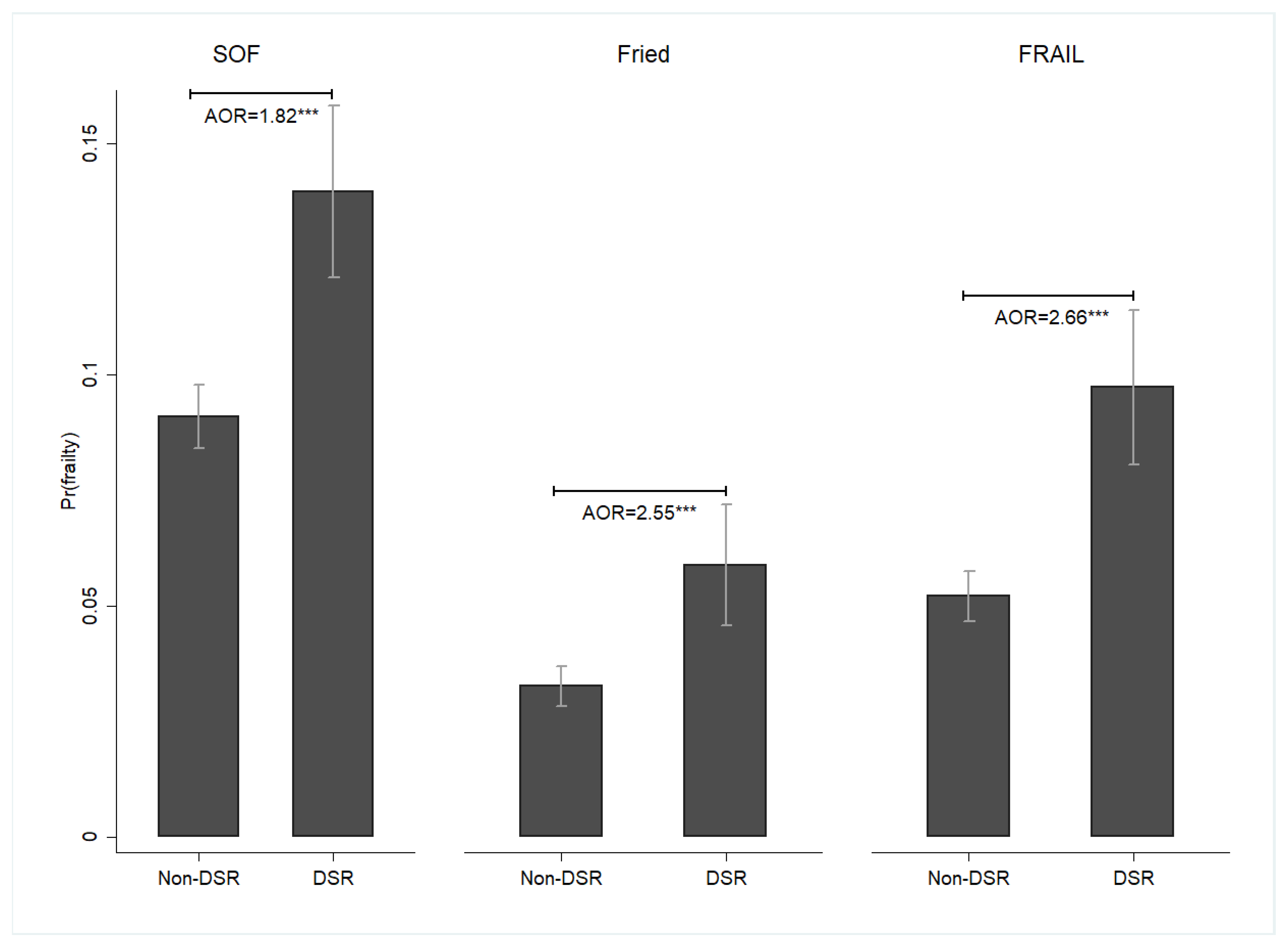
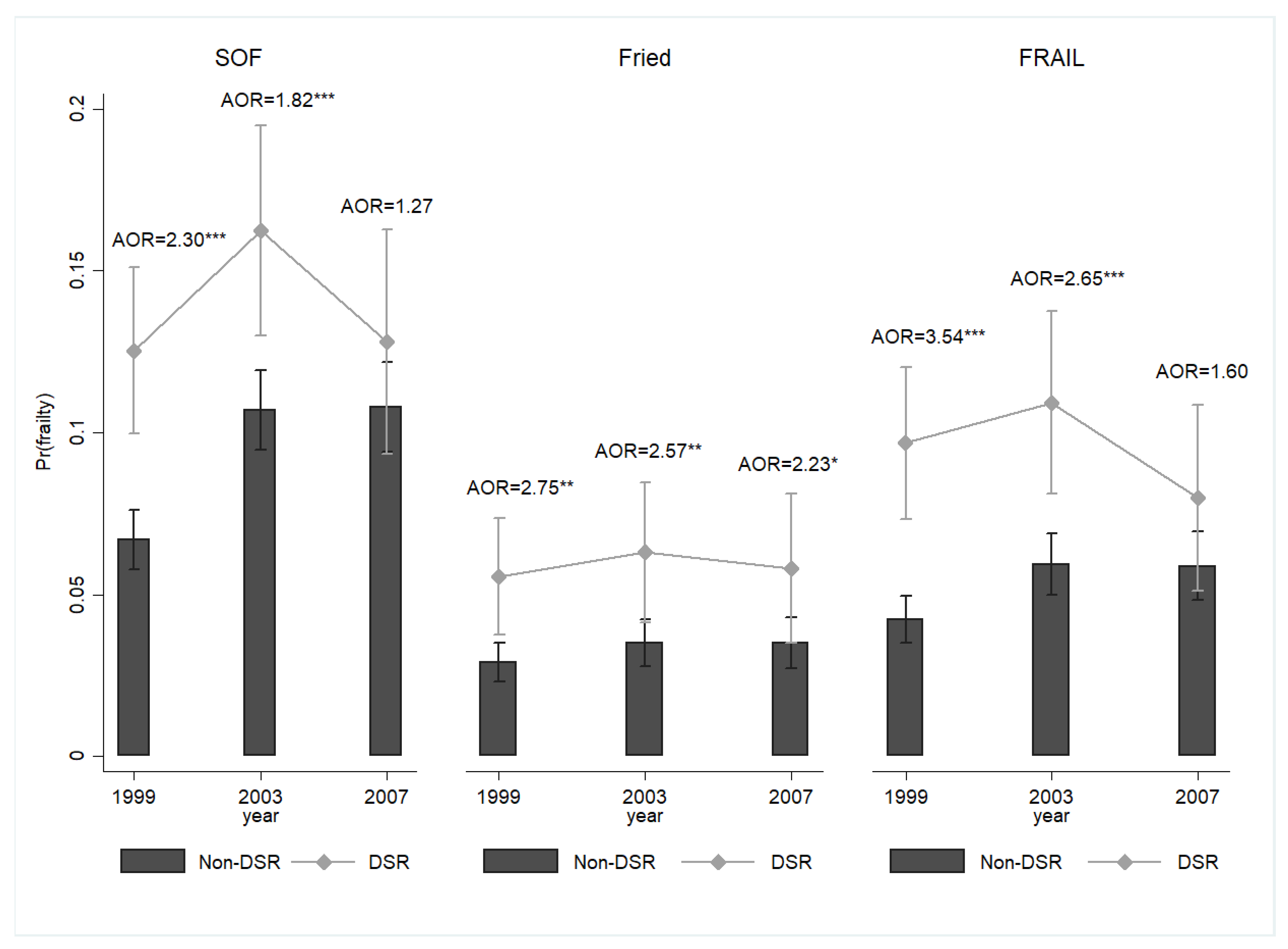
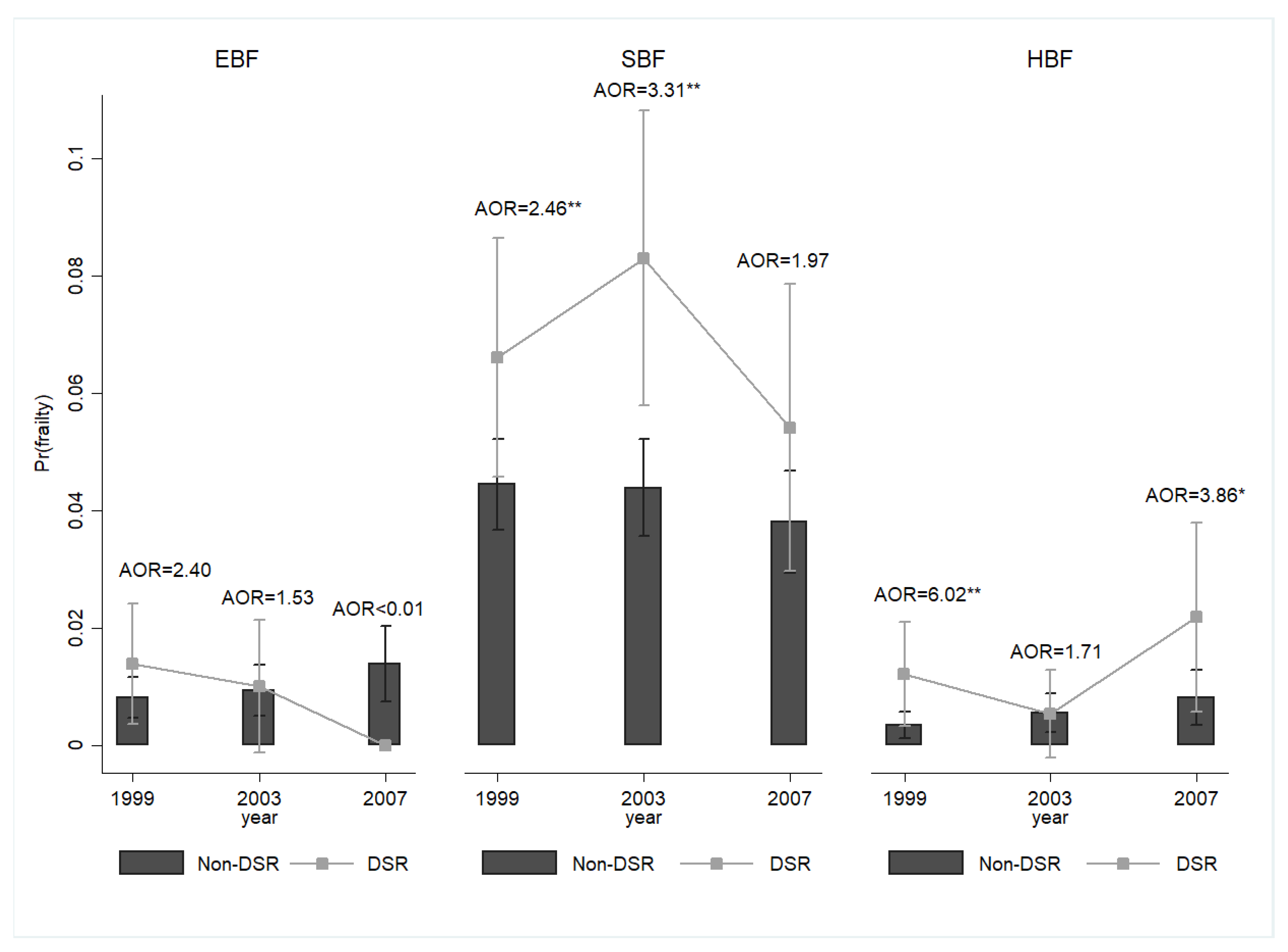
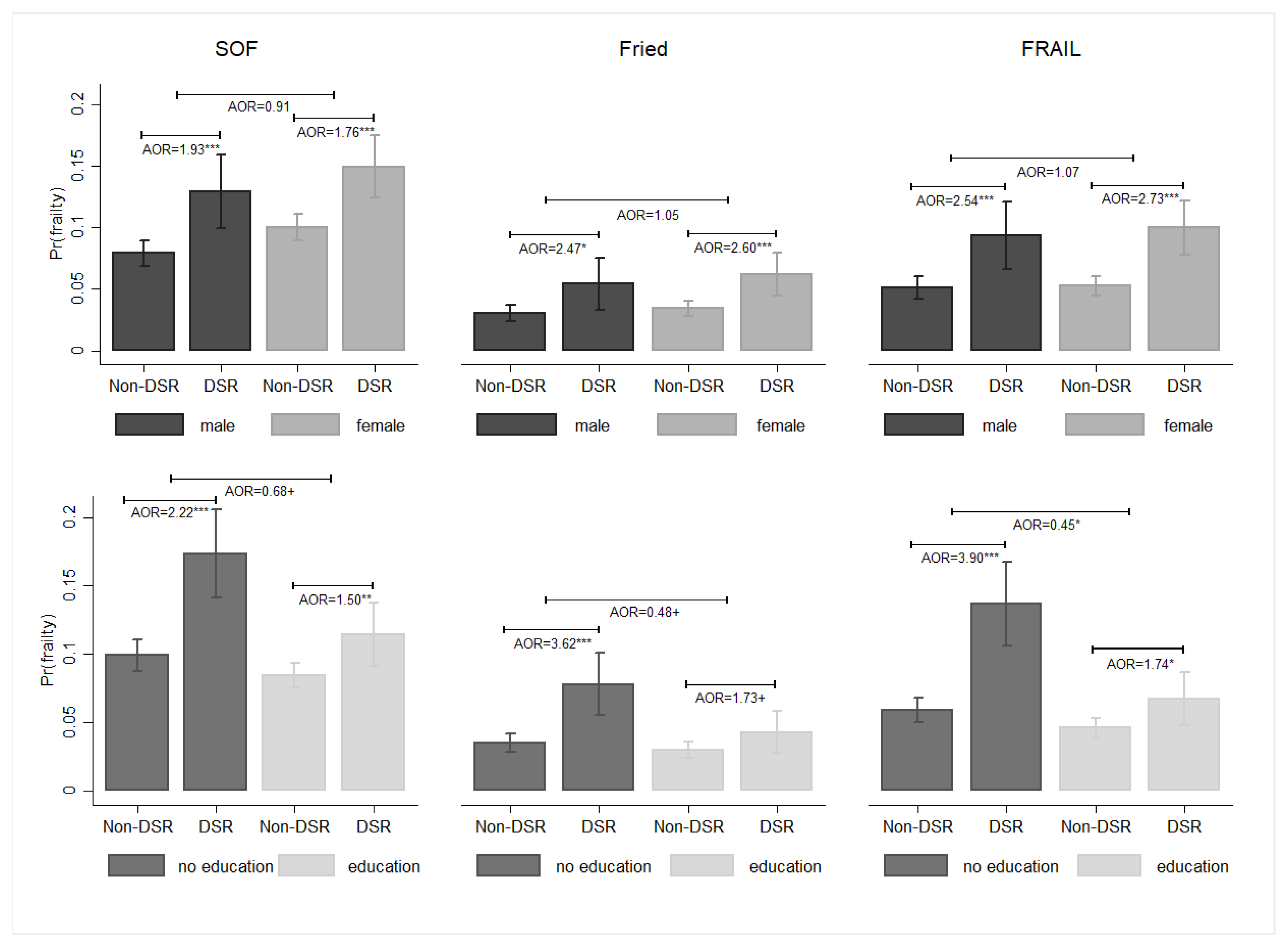
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study group. Frailty and the prediction of negative health outcomes: A meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Volkert, D.; Diekmann, R.; Kaiser, M.J.; Uter, W.; Vidal, K.; Sieber, C.C.; Bauer, J.M. Nutritional status according to the mini nutritional assessment (MNA®) and frailty in community dwelling older persons: A close relationship. J. Nutr. Health Aging 2013, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Norazman, C.W.; Adznam, S.N.; Jamaluddin, R. Malnutrition as key predictor of physical frailty among Malaysian older adults. Nutrients 2020, 12, 1713. [Google Scholar] [CrossRef]
- Wei, K.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.P. Frailty and malnutrition: Related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore Longitudinal Ageing Studies. J. Am. Med. Dir. Assoc. 2017, 18, 1019–1028. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Won, C.W.; Lee, K.E.; Chon, D. Nutritional status and frailty in community-dwelling older Korean adults: The Korean Frailty and Aging Cohort Study. J. Nutr. Health Aging 2018, 22, 774–778. [Google Scholar] [CrossRef]
- Chang, S.F.; Lin, P.L. Prefrailty in community-dwelling older adults is associated with nutrition status. J. Clin. Nurs. 2016, 25, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, sarcopenia, and malnutrition frequently (co-)occur in hospitalized older adults: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Pilleron, S.; Féart, C.; García García, F.J.; Bandinelli, S.; Gomez-Cabrero, D.; Rodriguez-Mañas, L.; Grune, T.; et al. Associations of fat-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachexia Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Insausti, H.; Pérez-Tasigchana, R.F.; López-García, E.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Macronutrients intake and incident frailty in older adults: A prospective cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Shikany, J.M.; Barrett-Connor, E.; Ensrud, K.E.; Cawthon, P.M.; Lewis, C.E.; Dam, T.T.; Shannon, J.; Redden, D.T.; Osteoporotic Fractures in Men (MrOS) Research Group. Macronutrients, diet quality, and frailty in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Yildirim Borazan, F.; Citar Daziroglu, M.E.; Erdogan Govez, N.; Acar-Tek, N.; Varan, H.D. The relationship between the quantity and type of macronutrients in diet and frailty in older outpatients. Aging Clin. Exp. Res. 2023, 35, 3033–3040. [Google Scholar] [CrossRef]
- Riegel, B.; Moser, D.K.; Powell, M.; Rector, T.S.; Havranek, E.P. Nonpharmacologic care by heart failure experts. J. Card. Fail. 2006, 12, 149–153. [Google Scholar] [CrossRef]
- de Brito-Ashurst, I.; Perry, L.; Sanders, T.A.; Thomas, J.E.; Yaqoob, M.M.; Dobbie, H. Barriers and facilitators of dietary sodium restriction amongst Bangladeshi chronic kidney disease patients. J. Hum. Nutr. Diet. 2011, 24, 86–95. [Google Scholar] [CrossRef]
- Mahtani, K.R.; Heneghan, C.; Onakpoya, I.; Tierney, S.; Aronson, J.K.; Roberts, N.; Hobbs, F.D.R.; Nunan, D. Reduced salt intake for heart failure: A systematic review. JAMA Intern. Med. 2018, 178, 1693–1700. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, M.; Su, Y.; Ma, T.; Lei, X.; Hou, Y. Effect of dietary sodium restriction on the quality of life of patients with heart failure: A systematic review of randomized controlled trials. J. Cardiovasc. Nurs. 2022, 37, 570–580. [Google Scholar] [CrossRef]
- Mori, T.; Kurumazuka, D.; Matsumoto, C.; Shirakawa, H.; Kimura, S.; Kitada, K.; Kobayashi, K.; Matsuda, H.; Hayashi, T.; Kitaura, Y.; et al. Dietary salt restriction activates mineralocorticoid receptor signaling in volume-overloaded heart failure. Eur. J. Pharmacol. 2009, 623, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, Y.; Kisimori, T.; Inoue, T.; Seki, J.; Takeda, S.; Okada, M.; Kosuga, K.; Ikeguchi, S. Sodium restriction is associated with low caloric intake in patients hospitalized with acute decompensated heart failure. J. Card. Fail. 2016, 22, S226. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yan, H.T. Impact of dietary sodium restriction on falls among middle-aged and older adults: Results of an 8-year longitudinal study. Geriatr. Gerontol. Int. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Cawthon, P.M.; Fink, H.A.; Taylor, B.C.; Cauley, J.A.; Dam, T.T.; Marshall, L.M.; Orwoll, E.S.; Cummings, S.R.; et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J. Am. Geriatr. Soc. 2009, 57, 492–498. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Morley, J.E.; Silver, A.J. Anorexia in the elderly. Neurobiol. Aging 1988, 9, 9–16. [Google Scholar] [CrossRef]
- Nieuwenhuizen, W.F.; Weenen, H.; Rigby, P.; Hetherington, M.M. Older adults and patients in need of nutritional support: Review of current treatment options and factors influencing nutritional intake. Clin. Nutr. 2010, 29, 160–169. [Google Scholar] [CrossRef]
- Haberl, J.; Zollner, G.; Fickert, P.; Stadlbauer, V. To salt or not to salt?-That is the question in cirrhosis. Liver Int. 2018, 38, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Viola, A.; Cenerelli, S.; Leo, A.; Santarelli, S.; Monteburini, T. Dietary daily sodium intake lower than 1500 mg is associated with inadequately low intake of calorie, protein, iron, zinc and vitamin B1 in patients on chronic hemodialysis. Nutrients 2020, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; McLean, R.; Marshall, M. Dietary sodium and other nutrient intakes among patients undergoing hemodialysis in New Zealand. Nutrients 2018, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.S.; Zukeran, M.S.; Valentini Neto, J.; Romanini, C.V.; Mingardi, S.V.B.; Cipolli, G.C.; Aprahamian, I.; Ribeiro, S.M.L. Factors related to malnutrition and their association with frailty in community-dwelling older adults registered at a geriatric clinic. Exp. Gerontol. 2022, 165, 111865. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, A.; Piotrowicz, K.; Perera, I.; Gryglewska, B.; Gąsowski, J. Poor appetite in frail older persons-a systematic review. Nutrients 2023, 15, 2966. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Aging-related anorexia and its association with disability and frailty. J. Cachexia Sarcopenia Muscle 2018, 9, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Lengelé, L.; Bruyère, O.; Beaudart, C.; Reginster, J.Y.; Locquet, M. Impact of malnutrition status on muscle parameter changes over a 5-year follow-up of community-dwelling older adults from the SarcoPhAge cohort. Nutrients 2021, 13, 407. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, J.; Fu, H.; Zhang, W.; Yang, L.; Yang, M. Malnutrition in relation to muscle mass, muscle quality, and muscle strength in hospitalized older adults. J. Am. Med. Dir. Assoc. 2022, 23, 722–728. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Meskers, C.G.M.; Trappenburg, M.C.; Verlaan, S.; Reijnierse, E.M.; Whittaker, A.C.; Maier, A.B. Malnutrition is associated with dynamic physical performance. Aging Clin. Exp. Res. 2020, 32, 1085–1092. [Google Scholar] [CrossRef]
- Chew, S.T.H.; Tey, S.L.; Yalawar, M.; Liu, Z.; Baggs, G.; How, C.H.; Cheong, M.; Chow, W.L.; Low, Y.L.; Huynh, D.T.T.; et al. Prevalence and associated factors of sarcopenia in community-dwelling older adults at risk of malnutrition. BMC Geriatr. 2022, 22, 997. [Google Scholar] [CrossRef]
- Tan, V.M.H.; Pang, B.W.J.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Chen, K.K.; Ng, T.P.; Wee, S.L. Malnutrition and sarcopenia in community-dwelling adults in Singapore: Yishun Health Study. J. Nutr. Health Aging 2021, 25, 374–381. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The underappreciated role of low muscle mass in the management of malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef]
- Hettiarachchi, J.; Reijnierse, E.M.; Soh, C.H.; Agius, B.; Fetterplace, K.; Lim, W.K.; Maier, A.B. Malnutrition is associated with poor trajectories of activities of daily living in geriatric rehabilitation inpatients: RESORT. Mech. Ageing Dev. 2021, 197, 111500. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Nishida, Y. Direct and indirect effects of nutritional status, physical function and cognitive function on activities of daily living in Japanese older adults requiring long-term care. Geriatr. Gerontol. Int. 2014, 14, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Momosaki, R. Prevalence of malnutrition assessed by the GLIM criteria and association with activities of daily living in older residents in an integrated facility for medical and long-term care. Nutrients 2022, 14, 3656. [Google Scholar] [CrossRef] [PubMed]
- Meskers, C.G.M.; Reijnierse, E.M.; Numans, S.T.; Kruizinga, R.C.; Pierik, V.D.; van Ancum, J.M.; Slee-Valentijn, M.; Scheerman, K.; Verlaan, S.; Maier, A.B. Association of handgrip strength and muscle mass with dependency in (instrumental) activities of daily living in hospitalized older adults -The EMPOWER Study. J. Nutr. Health Aging 2019, 23, 232–238. [Google Scholar] [CrossRef]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional status as a mediator of fatigue and its underlying mechanisms in older people. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef]
- Dallmeier, D.; Braisch, U.; Rapp, K.; Klenk, J.; Rothenbacher, D.; Denkinger, M.; ActiFE Study Group. Frailty index and sex-specific 6-year mortality in community-dwelling older people: The ActiFE study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 366–373. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Fouweather, T.; Jagger, C. Cross-national disparities in sex differences in life expectancy with and without frailty. Age Ageing 2014, 43, 222–228. [Google Scholar] [CrossRef]
- Saum, K.U.; Dieffenbach, A.K.; Müller, H.; Holleczek, B.; Hauer, K.; Brenner, H. Frailty prevalence and 10-year survival in community-dwelling older adults: Results from the ESTHER cohort study. Eur. J. Epidemiol. 2014, 29, 171–179. [Google Scholar] [CrossRef]
- Etman, A.; Kamphuis, C.B.; van der Cammen, T.J.; Burdorf, A.; van Lenthe, F.J. Do lifestyle, health and social participation mediate educational inequalities in frailty worsening? Eur. J. Public Health 2015, 25, 345–350. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; van Hout, H.P.; Heymans, M.W.; van der Horst, H.E.; Frijters, D.H.; Broese van Groenou, M.I.; Deeg, D.J.; Huisman, M. Explaining the association between educational level and frailty in older adults: Results from a 13-year longitudinal study in the Netherlands. Ann. Epidemiol. 2014, 24, 538–544.e2. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Q.L.; Odden, M.C.; Chen, X.; Wu, C. Linking early life risk factors to frailty in old age: Evidence from the China Health and Retirement Longitudinal Study. Age Ageing 2020, 49, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Sardella, A.; Catalano, A.; Lenzo, V.; Bellone, F.; Corica, F.; Quattropani, M.C.; Basile, G. Association between cognitive reserve dimensions and frailty among older adults: A structured narrative review. Geriatr. Gerontol. Int. 2020, 20, 1005–1023. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Manganello, J.A.; Richards, M. A life course approach to health literacy: The role of gender, educational attainment and lifetime cognitive capability. Age Ageing 2017, 46, 493–499. [Google Scholar] [CrossRef]
- Tiller, D.; Herzog, B.; Kluttig, A.; Haerting, J. Health literacy in an urban elderly East-German population—Results from the population-based CARLA study. BMC Public Health 2015, 15, 883. [Google Scholar] [CrossRef]
- Wister, A.V.; Malloy-Weir, L.J.; Rootman, I.; Desjardins, R. Lifelong educational practices and resources in enabling health literacy among older adults. J. Aging Health 2010, 22, 827–854. [Google Scholar] [CrossRef] [PubMed]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in older adults: A nationally representative profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Niederstrasser, N.G.; Rogers, N.T.; Bandelow, S. Determinants of frailty development and progression using a multidimensional frailty index: Evidence from the English Longitudinal Study of Ageing. PLoS ONE 2019, 14, e0223799. [Google Scholar] [CrossRef]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef]


| Frailty (SOF) | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| N | 7539 | 818 | |
| (%) | (90.21) | (9.79) | |
| Age | <0.001 | ||
| 50–64 | 2466 | 86 | |
| (32.71) | (10.51) | ||
| 65–79 | 4106 | 476 | |
| (54.46) | (58.19) | ||
| ≥80 | 967 | 256 | |
| (12.83) | (31.30) | ||
| Age (continuous) | <0.001 | ||
| Sex | <0.001 | ||
| Female | 3285 | 515 | |
| (43.57) | (62.96) | ||
| Male | 4254 | 303 | |
| (56.43) | (37.04) | ||
| Education (years) | <0.001 | ||
| 0 | 2318 | 411 | |
| (30.75) | (50.24) | ||
| 1–6 | 3205 | 290 | |
| (42.51) | (35.45) | ||
| 7–12 | 1483 | 87 | |
| (19.67) | (10.64) | ||
| ≥13 | 533 | 30 | |
| (7.07) | (3.67) | ||
| Marital status a | <0.001 | ||
| Having a spouse | 5296 | 432 | |
| (70.25) | (52.81) | ||
| Not having a spouse | 2243 | 386 | |
| (29.75) | (47.19) | ||
| Current living status | 0.413 | ||
| Living with spouse, child, etc. | 6803 | 731 | |
| (90.26) | (89.36) | ||
| Living alone | 734 | 87 | |
| (9.74) | (10.64) | ||
| Smoking status | <0.001 | ||
| Non-smoker | 5784 | 730 | |
| (76.72) | (89.24) | ||
| Smoker | 1755 | 88 | |
| (23.28) | (10.76) | ||
| Alcohol intake | <0.001 | ||
| Non-alcohol drinker | 5410 | 734 | |
| (71.76) | (89.73) | ||
| Alcohol drinker | 2129 | 84 | |
| (28.24) | (10.27) | ||
| Frequency of exercise (times per week) | <0.001 | ||
| 0 | 2475 | 449 | |
| (32.83) | (54.89) | ||
| 1 | 481 | 33 | |
| (6.38) | (4.03) | ||
| 2 | 860 | 84 | |
| (11.41) | (10.27) | ||
| ≥3 | 3722 | 252 | |
| (49.38) | (30.81) | ||
| Dietary sodium restriction | <0.001 | ||
| No | 6436 | 614 | |
| (86.66) | (76.37) | ||
| Yes | 991 | 190 | |
| (13.34) | (23.63) | ||
| Year | <0.001 | ||
| 1999 | 3336 | 257 | |
| (44.25) | (31.42) | ||
| 2003 | 2362 | 307 | |
| (31.33) | (37.53) | ||
| 2007 | 1841 | 254 | |
| (24.42) | (31.05) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Yan, H.-T. Dietary Sodium Restriction and Frailty among Middle-Aged and Older Adults: An 8-Year Longitudinal Study. Nutrients 2024, 16, 580. https://doi.org/10.3390/nu16050580
Lin Y-C, Yan H-T. Dietary Sodium Restriction and Frailty among Middle-Aged and Older Adults: An 8-Year Longitudinal Study. Nutrients. 2024; 16(5):580. https://doi.org/10.3390/nu16050580
Chicago/Turabian StyleLin, Yu-Chun, and Huang-Ting Yan. 2024. "Dietary Sodium Restriction and Frailty among Middle-Aged and Older Adults: An 8-Year Longitudinal Study" Nutrients 16, no. 5: 580. https://doi.org/10.3390/nu16050580
APA StyleLin, Y.-C., & Yan, H.-T. (2024). Dietary Sodium Restriction and Frailty among Middle-Aged and Older Adults: An 8-Year Longitudinal Study. Nutrients, 16(5), 580. https://doi.org/10.3390/nu16050580





