Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management
Abstract
1. Introduction
2. The Phenotypic and Genotypic Components of PN
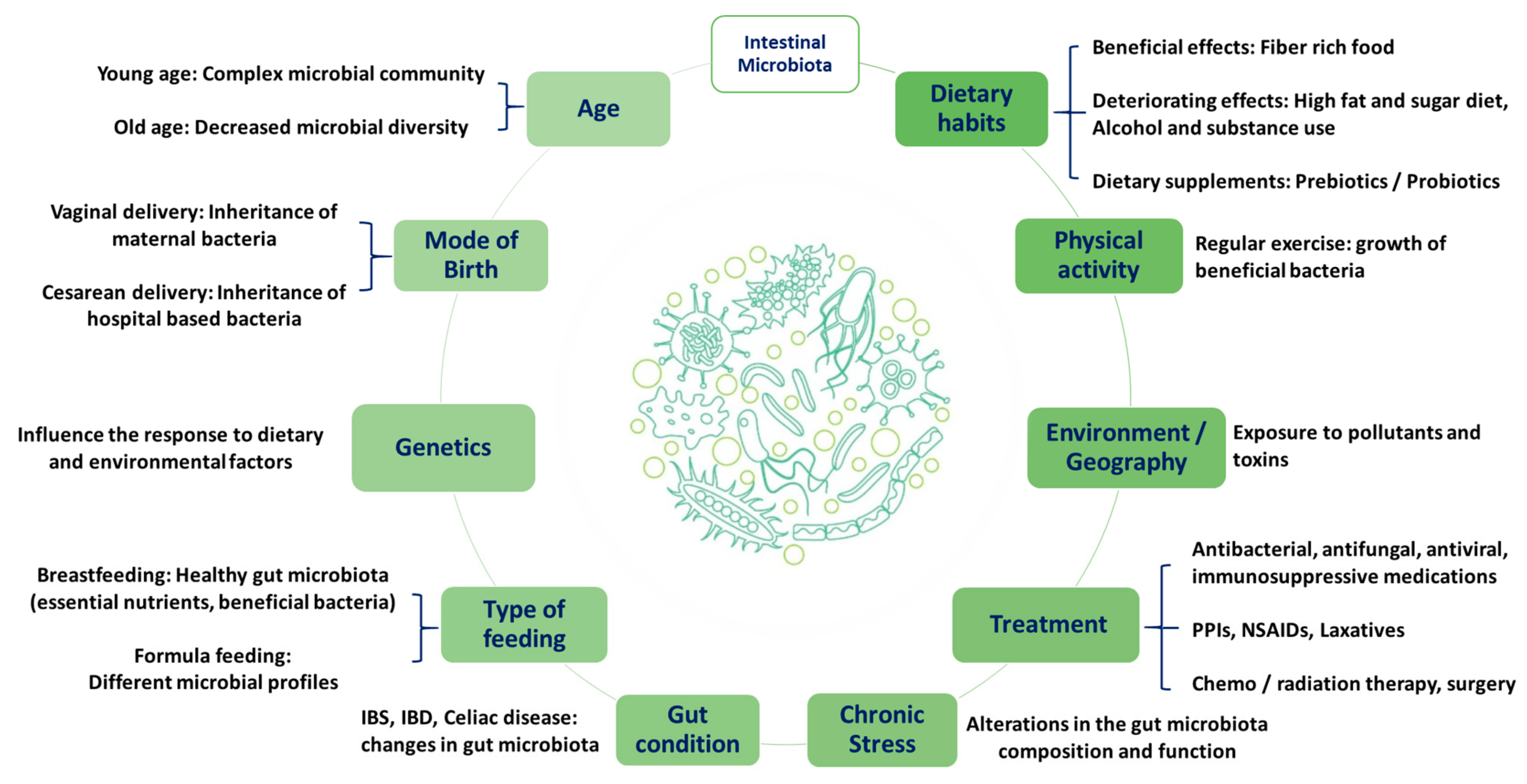
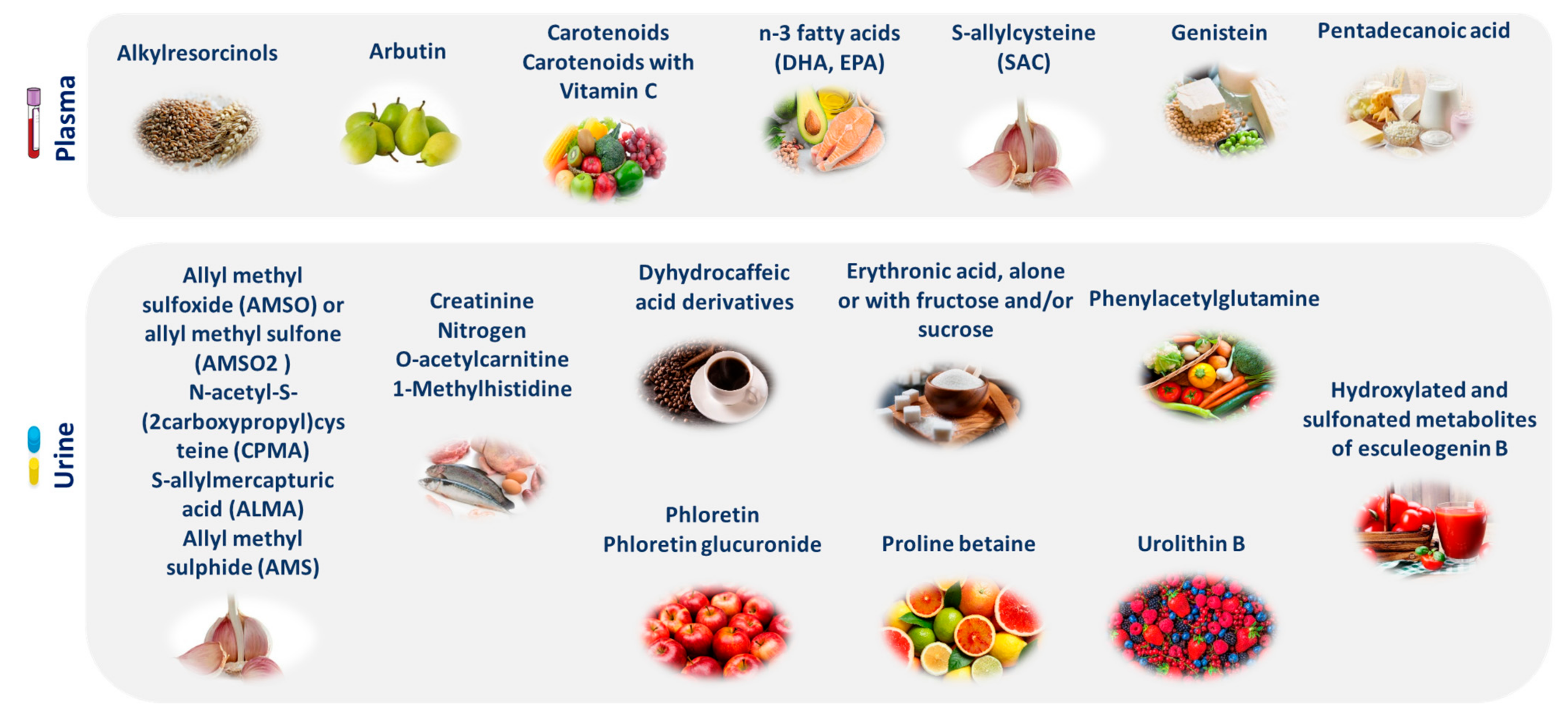
2.1. Gut Microbiota
2.2. Genetics and Metabolic Profile
2.3. Psychosocial and Socioeconomic Status
3. PN Use in the Management of Obesity
3.1. Genetic Basis of Obesity
3.2. Weight Management
3.3. Intestinal Bacterial Flora
4. Obesity-Related Complications
4.1. Diabetes
4.2. Cardiovascular Diseases
4.3. Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoo, C.S.; Knorr, D. Grand Challenges in Nutrition and Food Science Technology. Front. Nutr. 2014, 1, 4. [Google Scholar] [CrossRef]
- Head, R.J.; Buckley, J.D. Human Variation in Response to Food and Nutrients. Nutr. Rev. 2020, 78, 49–52. [Google Scholar] [CrossRef]
- Stover, P.J.; King, J.C. More Nutrition Precision, Better Decisions for the Health of Our Nation. J. Nutr. 2020, 150, 3058–3060. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- Hughes, R.L.; Kable, M.E.; Marco, M.; Keim, N.L. The Role of the Gut Microbiome in Predicting Response to Diet and the Development of Precision Nutrition Models. Part II: Results. Adv. Nutr. 2019, 10, 979–998. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Serhan, C.N.; Bazinet, R.P. The Need for Precision Nutrition, Genetic Variation and Resolution in COVID-19 Patients. Mol. Aspects Med. 2021, 77, 100943. [Google Scholar] [CrossRef]
- Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr. Nutr. Rep. 2023, 12, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M.; Corella, D. Nutritional Genomics. Annu. Rev. Genom. Hum. Genet. 2004, 5, 71–118. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M. Genotype-Phenotype Associations: Modulation by Diet and Obesity. Obesity 2008, 16, S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Marsaux, C.F.; Livingstone, K.M.; Navas-Carretero, S.; San-Cristobal, R.; Fallaize, R.; Macready, A.L.; O’Donovan, C.; Woolhead, C.; Forster, H.; et al. Can Genetic-Based Advice Help You Lose Weight? Findings from the Food4Me European Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.B.; Walsh, M.C.; Gibney, M.J.; Brennan, L.; Gibney, E.R. Knowing Your Genes: Does This Impact Behaviour Change? Proc. Nutr. Soc. 2017, 76, 182–191. [Google Scholar] [CrossRef]
- Gibney, E.R. Personalised Nutrition—Phenotypic and Genetic Variation in Response to Dietary Intervention. Proc. Nutr. Soc. 2020, 79, 236–245. [Google Scholar] [CrossRef]
- Kirwan, L.; Walsh, M.C.; Celis-Morales, C.; Marsaux, C.F.M.; Livingstone, K.M.; Navas-Carretero, S.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; et al. Phenotypic Factors Influencing the Variation in Response of Circulating Cholesterol Level to Personalised Dietary Advice in the Food4Me Study. Br. J. Nutr. 2016, 116, 2011–2019. [Google Scholar] [CrossRef]
- Shyam, S.; Lee, K.X.; Tan, A.S.W.; Khoo, T.A.; Harikrishnan, S.; Lalani, S.A.; Ramadas, A. Effect of Personalized Nutrition on Dietary, Physical Activity, and Health Outcomes: A Systematic Review of Randomized Trials. Nutrients 2022, 14, 4104. [Google Scholar] [CrossRef]
- Rankin, A.; Bunting, B.P.; Poínhos, R.; van der Lans, I.A.; Fischer, A.R.; Kuznesof, S.; Almeida, M.; Markovina, J.; Frewer, L.J.; Stewart-Knox, B.J. Food Choice Motives, Attitude towards and Intention to Adopt Personalised Nutrition. Public Health Nutr. 2018, 21, 2606–2616. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Panduro, A.; Roman, S.; Garcia Milan, R.; Torres-Reyes, L.; Aldaco, K. Chapter 10. Personalized Nutrition to Treat and Prevent Obesity and Diabetes. In Nutritional Signaling Pathway Activities in Obesity and Diabetes; ResearchGate GmbH: Berlin, Germany, 2020; pp. 272–294. ISBN 978-1-78801-557-8. [Google Scholar]
- Panduro, A.; Rivera-Iñiguez, I.; Sepulveda-Villegas, M.; Roman, S. Genes, Emotions and Gut Microbiota: The next Frontier for the Gastroenterologist. World J. Gastroenterol. 2017, 23, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards Utilization of the Human Genome and Microbiome for Personalized Nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-Microbiota Interactions and Personalized Nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Odintsova, V.E.; Revel-Muroz, A.; Tyakht, A.V. The Hallmarks of Dietary Intervention-Resilient Gut Microbiome. npj Biofilms Microbiomes 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Picu, A.; Petcu, L.; Cucu, N.; Chifiriuc, M.C. Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Front. Nutr. 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut Microbiome Diversity and High-Fibre Intake Are Related to Lower Long-Term Weight Gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the Composition of Gut Microbiota in a Population with Varied Ethnic Origins but Shared Geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the Gut Microbiota: Impact of Low Calorie Sweeteners and the Link to Insulin Resistance? Physiol. Behav. 2016, 164, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome p-450 in Male Rats. J. Toxicol. Environ. Health A 2008, 71, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front. Physiol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hemami, R.M.; Shakarami, A.; Ardekani, A.M.; Aghaii, S.; Makarem, D.; Nikrad, N.; Farhangi, M.A.; Pour Abbasi, M.S. Investigation of the Association between Habitual Dietary FODMAP Intake, Metabolic Parameters, Glycemic Status, and Anthropometric Features among Apparently Healthy Overweight and Obese Individuals. BMC Endocr. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P. When the Low FODMAP Diet Does Not Work. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 69–72. [Google Scholar] [CrossRef]
- Gibson, P.R. The Evidence Base for Efficacy of the Low FODMAP Diet in Irritable Bowel Syndrome: Is It Ready for Prime Time as a First-Line Therapy? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 32–35. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Chow, C.-E.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients 2016, 8, 684. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Costabile, A.; Bergillos-Meca, T.; Gonzalez, I.; Landriscina, L.; Ciuffreda, E.; D’Agnello, P.; Corbo, M.R.; Sinigaglia, M.; Lamacchia, C. Impact of Gluten-Friendly Bread on the Metabolism and Function of In Vitro Gut Microbiota in Healthy Human and Coeliac Subjects. PLoS ONE 2016, 11, e0162770. [Google Scholar] [CrossRef]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a Gluten-Free Diet on Gut Microbiota and Immune Function in Healthy Adult Human Subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef]
- Hansen, L.B.S.; Roager, H.M.; Søndertoft, N.B.; Gøbel, R.J.; Kristensen, M.; Vallès-Colomer, M.; Vieira-Silva, S.; Ibrügger, S.; Lind, M.V.; Mærkedahl, R.B.; et al. A Low-Gluten Diet Induces Changes in the Intestinal Microbiome of Healthy Danish Adults. Nat. Commun. 2018, 9, 4630. [Google Scholar] [CrossRef]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long Term Gluten Consumption in Adults without Celiac Disease and Risk of Coronary Heart Disease: Prospective Cohort Study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef]
- Grimm, E.R.; Steinle, N.I. Genetics of Eating Behavior: Established and Emerging Concepts. Nutr. Rev. 2011, 69, 52–60. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Allayee, H.; Chmurzynska, A.; Choi, M.S.; Curi, R.; De Caterina, R.; Ferguson, L.R.; Goni, L.; Kang, J.X.; et al. Guide for Current Nutrigenetic, Nutrigenomic, and Nutriepigenetic Approaches for Precision Nutrition Involving the Prevention and Management of Chronic Diseases Associated with Obesity. Lifestyle Genom. 2017, 10, 43–62. [Google Scholar] [CrossRef]
- Paoloni-Giacobino, A.; Grimble, R.; Pichard, C. Genetics and Nutrition. Clin. Nutr. 2003, 22, 429–435. [Google Scholar] [CrossRef]
- Fulton, J.L.; Dinas, P.C.; Carrillo, A.E.; Edsall, J.R.; Ryan, E.J.; Ryan, E.J. Impact of Genetic Variability on Physiological Responses to Caffeine in Humans: A Systematic Review. Nutrients 2018, 10, 1373. [Google Scholar] [CrossRef]
- Sachse, C.; Brockmöller, J.; Bauer, S.; Roots, I. Functional Significance of a C→A Polymorphism in Intron 1 of the Cytochrome P450 CYP1A2 Gene Tested with Caffeine. Br. J. Clin. Pharmacol. 1999, 47, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Peloso, G.; Arnett, D.K.; Demissie, S.; Cupples, L.A.; Tucker, K.; Lai, C.-Q.; Parnell, L.D.; Coltell, O.; Lee, Y.-C.; et al. APOA2, Dietary Fat, and Body Mass Index: Replication of a Gene-Diet Interaction in 3 Independent Populations. Arch. Intern. Med. 2009, 169, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. A Conceptual Framework for Studying and Investing in Precision Nutrition. Front. Genet. 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.; Al-Jawadi, A.; Wang, S.; Moustaid-Moussa, N. Metabolic Profiling in Nutrition and Metabolic Disorders. Adv. Nutr. 2013, 4, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Gijbels, A.; Jardon, K.M.; Siebelink, E.; Hul, G.B.; Wanders, L.; Erdos, B.; Péter, S.; Singh-Povel, C.M.; de Vogel-van den Bosch, J.; et al. Cardiometabolic Health Improvements upon Dietary Intervention Are Driven by Tissue-Specific Insulin Resistance Phenotype: A Precision Nutrition Trial. Cell Metab. 2023, 35, 71–83.e5. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Newman, J.W.; Hembrooke, T.A.; Keim, N.L. Variation in Metabolic Responses to Meal Challenges Differing in Glycemic Index in Healthy Women: Is It Meaningful? Nutr. Metab. 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; De Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutr. Nutr. 2016, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688. [Google Scholar] [CrossRef]
- Rizzo, A.; Sitibondo, A. Obesity and Life History: The Hypothesis of Psychological Phenotypes. Psych 2023, 5, 866–875. [Google Scholar] [CrossRef]
- Grossniklaus, D.A.; Dunbar, S.B.; Tohill, B.C.; Gary, R.; Higgins, M.K.; Frediani, J. Psychological Factors Are Important Correlates of Dietary Pattern in Overweight Adults. J. Cardiovasc. Nurs. 2010, 25, 450–460. [Google Scholar] [CrossRef]
- Butler, M.J.; Eckel, L.A. Eating as a Motivated Behavior: Modulatory Effect of High Fat Diets on Energy Homeostasis, Reward Processing, and Neuroinflammation. Integr. Zool. 2018, 13, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Vahia, V.N. Diagnostic and Statistical Manual of Mental Disorders 5: A Quick Glance. Indian J. Psychiatry 2013, 55, 220–223. [Google Scholar] [CrossRef]
- Srp, F.; Steiger, E.; Gulledge, A.D.; Matarese, L.E.; Paysinger, J.; Roncagli, T.; Stebbins, J.; Sullivan, M. Psychosocial Issues of Nutritional Support. A Multidisciplinary Interpretation. Nurs. Clin. N. Am. 1989, 24, 447–459. [Google Scholar] [CrossRef]
- Steptoe, A.; Frank, P. Obesity and Psychological Distress. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220225. [Google Scholar] [CrossRef] [PubMed]
- Reiss, F.; Meyrose, A.-K.; Otto, C.; Lampert, T.; Klasen, F.; Ravens-Sieberer, U. Socioeconomic Status, Stressful Life Situations and Mental Health Problems in Children and Adolescents: Results of the German BELLA Cohort-Study. PLoS ONE 2019, 14, e0213700. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.; Nelson, M.; Ralph, A.; Leather, S. Socioeconomic Determinants of Health. The Contribution of Nutrition to Inequalities in Health. BMJ Br. Med. J. 1997, 314, 1545. [Google Scholar] [CrossRef] [PubMed]
- Foroozanfar, Z.; Moghadami, M.; Mohsenpour, M.A.; Houshiarrad, A.; Farmani, A.; Akbarpoor, M.A.; Shenavar, R. Socioeconomic Determinants of Nutritional Behaviors of Households in Fars Province, Iran, 2018. Front. Nutr. 2022, 9, 956293. [Google Scholar] [CrossRef]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition Quality of Food Purchases Varies by Household Income: The SHoPPER Study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of Macronutrients to Obesity: Implications for Precision Nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martínez, J.A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2015, 146, 905S–912S. [Google Scholar] [CrossRef]
- Wassel, C.L.; Pankow, J.S.; Rasmussen-Torvik, L.J.; Li, N.; Taylor, K.D.; Guo, X.; Goodarzi, M.O.; Palmas, W.R.; Post, W.S. Associations of SNPs in ADIPOQ and Subclinical Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Obesity 2011, 19, 840–847. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Mendelson, M.; Speliotes, E.K. Recent Advances in Human Genetics and Epigenetics of Adiposity: Pathway to Precision Medicine? Gastroenterology 2017, 152, 1695–1706. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. Protein Structure and Function Prediction Using I-TASSER. Curr. Protoc. Bioinform. 2015, 52, 5.8.1–5.8.15. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic Studies of Body Mass Index Yield New Insights for Obesity Biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Haro, D.; Marrero, P.F.; Relat, J. Nutritional Regulation of Gene Expression: Carbohydrate-, Fat- and Amino Acid-Dependent Modulation of Transcriptional Activity. Int. J. Mol. Sci. 2019, 20, 1386. [Google Scholar] [CrossRef]
- Bravo-Ruiz, I.; Medina, M.Á.; Martínez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Wu, X.; Bao, P.; Long, R.; Guo, X.; Ding, X.; Yan, P. The Response of Gene Expression Associated with Lipid Metabolism, Fat Deposition and Fatty Acid Profile in the Longissimus Dorsi Muscle of Gannan Yaks to Different Energy Levels of Diets. PLoS ONE 2017, 12, e0187604. [Google Scholar] [CrossRef]
- Lenard, N.R.; Berthoud, H.-R. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity 2008, 16, S11–S22. [Google Scholar] [CrossRef] [PubMed]
- de Wouters d’Oplinter, A.; Huwart, S.J.P.; Cani, P.D.; Everard, A. Gut Microbes and Food Reward: From the Gut to the Brain. Front. Neurosci. 2022, 16, 947240. [Google Scholar] [CrossRef]
- Venkatapoorna, C.M.K.; Ayine, P.; Parra, E.P.; Koenigs, T.; Phillips, M.; Babu, J.R.; Sandey, M.; Geetha, T. Association of Salivary Amylase (AMY1) Gene Copy Number with Obesity in Alabama Elementary School Children. Nutrients 2019, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Erez, G.; Tirosh, A.; Rudich, A.; Meiner, V.; Schwarzfuchs, D.; Sharon, N.; Shpitzen, S.; Blüher, M.; Stumvoll, M.; Thiery, J.; et al. Phenotypic and Genetic Variation in Leptin as Determinants of Weight Regain. Int. J. Obes. 2011, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, Q.; Zhang, C.; Hu, F.B.; Sacks, F.M.; Qi, L. FTO Genotype and 2-Year Change in Body Composition and Fat Distribution in Response to Weight-Loss Diets. Diabetes 2012, 61, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, J.; Papandonatos, G.D.; Huggins, G.S.; Peter, I.; Kahn, S.E.; Knowler, W.C.; Hudnall, G.E.; Lipkin, E.; Kitabchi, A.E.; Wagenknecht, L.E.; et al. FTO Predicts Weight Regain in the Look AHEAD Clinical Trial. Int. J. Obes. 2013, 37, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Delahanty, L.M.; Jablonski, K.A.; Knowler, W.C.; Kahn, S.E.; Florez, J.C.; Franks, P.W.; Diabetes Prevention Program Research Group. Variation at the Melanocortin 4 Receptor Gene and Response to Weight-Loss Interventions in the Diabetes Prevention Program. Obesity 2013, 21, E520–E526. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Bray, G.A.; Smith, S.R.; Hu, F.B.; Sacks, F.M.; Qi, L. Insulin Receptor Substrate 1 (IRS1) Gene Variation Modifies Insulin Resistance Response to Weight-Loss Diets in a Two-Year Randomized Trial. Circulation 2011, 124, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Qi, Q.; Hu, F.B.; Sacks, F.M.; Qi, L. TCF7L2 Genetic Variants Modulate the Effect of Dietary Fat Intake on Changes in Body Composition during a Weight-Loss Intervention123. Am. J. Clin. Nutr. 2012, 96, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Heni, M.; Herzberg-Schäfer, S.; Machicao, F.; Häring, H.-U.; Fritsche, A. Dietary Fiber Intake Modulates the Association between Variants in TCF7L2 and Weight Loss during a Lifestyle Intervention. Diabetes Care 2012, 35, e24. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Bray, G.A.; Hu, F.B.; Sacks, F.M.; Qi, L. Weight-Loss Diets Modify Glucose-Dependent Insulinotropic Polypeptide Receptor Rs2287019 Genotype Effects on Changes in Body Weight, Fasting Glucose, and Insulin Resistance: The Preventing Overweight Using Novel Dietary Strategies Trial123. Am. J. Clin. Nutr. 2012, 95, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef]
- Qi, Q.; Xu, M.; Wu, H.; Liang, L.; Champagne, C.M.; Bray, G.A.; Sacks, F.M.; Qi, L. IRS1 Genotype Modulates Metabolic Syndrome Reversion in Response to 2-Year Weight-Loss Diet Intervention. Diabetes Care 2013, 36, 3442–3447. [Google Scholar] [CrossRef]
- Kostis, W.J.; Cabrera, J.; Hooper, W.C.; Whelton, P.K.; Espeland, M.A.; Cosgrove, N.M.; Cheng, J.Q.; Deng, Y.; De Staerck, C.; Pyle, M.; et al. Relationships Between Selected Gene Polymorphisms and Blood Pressure Sensitivity to Weight Loss in Elderly Persons With Hypertension. Hypertension 2013, 61, 857–863. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Q.; Liang, J.; Hu, F.B.; Sacks, F.M.; Qi, L. Neuropeptide Y Promoter Polymorphism Modifies Effects of a Weight-Loss Diet on 2-Year Changes of Blood Pressure: The Pounds Lost Trial. Hypertension 2012, 60, 1169–1175. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M.; Abu Dayyeh, B.; Calderon, G.; Gonzalez, D.; McRae, A.; Rossini, W.; Singh, S.; Burton, D.; Clark, M.M. Selection of Antiobesity Medications Based on Phenotypes Enhances Weight Loss: A Pragmatic Trial in an Obesity Clinic. Obesity 2021, 29, 662–671. [Google Scholar] [CrossRef]
- Cordero, P.; Li, J.; Oben, J.A. Epigenetics of Obesity: Beyond the Genome Sequence. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 361. [Google Scholar] [CrossRef]
- Rudkowska, I. Genomics and Personalized Nutrition. Nutrients 2021, 13, 1128. [Google Scholar] [CrossRef]
- Jenzer, H.; Sadeghi-Reeves, L. Nutrigenomics-Associated Impacts of Nutrients on Genes and Enzymes With Special Consideration of Aromatase. Front. Nutr. 2020, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Arkadianos, I.; Valdes, A.M.; Marinos, E.; Florou, A.; Gill, R.D.; Grimaldi, K.A. Improved Weight Management Using Genetic Information to Personalize a Calorie Controlled Diet. Nutr. J. 2007, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brusaferro, A.; Cozzali, R.; Orabona, C.; Biscarini, A.; Farinelli, E.; Cavalli, E.; Grohmann, U.; Principi, N.; Esposito, S. Is It Time to Use Probiotics to Prevent or Treat Obesity? Nutrients 2018, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 2019, e3291367. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean Diet in the Management and Prevention of Obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary Macronutrients and Food Consumption as Determinants of Long-Term Weight Change in Adult Populations: A Systematic Literature Review. Food Nutr. Res. 2012, 56, 19103. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in Intake of Protein Foods, Carbohydrate Amount and Quality, and Long-Term Weight Change: Results from 3 Prospective Cohorts1234. Am. J. Clin. Nutr. 2015, 101, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity—A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Young, A.F.; Hodge, A. Diet Quality Is Associated with Higher Nutrient Intake and Self-Rated Health in Mid-Aged Women. J. Am. Coll. Nutr. 2008, 27, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, Olfactory and Food Texture Reward Processing in the Brain and the Control of Appetite. Proc. Nutr. Soc. 2012, 71, 488–501. [Google Scholar] [CrossRef]
- Popp, C.J.; Hu, L.; Kharmats, A.Y.; Curran, M.; Berube, L.; Wang, C.; Pompeii, M.L.; Illiano, P.; St-Jules, D.E.; Mottern, M.; et al. Effect of a Personalized Diet to Reduce Postprandial Glycemic Response vs a Low-Fat Diet on Weight Loss in Adults With Abnormal Glucose Metabolism and Obesity. JAMA Netw. Open 2022, 5, e2233760. [Google Scholar] [CrossRef]
- Macleod, M.; Gregor, A.; Barnett, C.; Magee, E.; Thompson, J.; Anderson, A.S. Provision of Weight Management Advice for Obese Women during Pregnancy: A Survey of Current Practice and Midwives’ Views on Future Approaches. Matern. Child. Nutr. 2012, 9, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of Personalized Nutrition on Health-Related Behaviour Change: Evidence from the Food4Me European Randomized Controlled Trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Zenun Franco, R.; Fallaize, R.; Weech, M.; Hwang, F.; Lovegrove, J.A. Effectiveness of Web-Based Personalized Nutrition Advice for Adults Using the eNutri Web App: Evidence From the EatWellUK Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e29088. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.E.; Shih, S.; El-Sohemy, A. Perceptions of Genetic Testing for Personalized Nutrition: A Randomized Trial of DNA-Based Dietary Advice. J. Nutr. Nutr. 2014, 7, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Madill, J.; Gilliland, J. Incorporating the “Theory of Planned Behavior” into Personalized Healthcare Behavior Change Research: A Call to Action. Pers. Med. 2017, 14, 521–529. [Google Scholar] [CrossRef]
- Drabsch, T.; Holzapfel, C. A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Gilliland, J.; O’Connor, C.; Seabrook, J.; Madill, J. Enhanced Long-Term Dietary Change and Adherence in a Nutrigenomics-Guided Lifestyle Intervention Compared to a Population-Based (GLB/DPP) Lifestyle Intervention for Weight Management: Results from the NOW Randomised Controlled Trial. BMJ Nutr. Prev. Health 2020, 3, 49–59. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical Activity and Incident Type 2 Diabetes Mellitus: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Hurley, J.C.; Todd, M.; Bhuiyan, N.; Jarrett, C.L.; Tucker, W.J.; Hollingshead, K.E.; Angadi, S.S. Adaptive Goal Setting and Financial Incentives: A 2 × 2 Factorial Randomized Controlled Trial to Increase Adults’ Physical Activity. BMC Public Health 2017, 17, 286. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Davis, K.K.; Rogers, R.J.; King, W.C.; Marcus, M.D.; Helsel, D.; Rickman, A.D.; Wahed, A.S.; Belle, S.H. Effect of Wearable Technology Combined with a Lifestyle Intervention on Long-Term Weight Loss: The IDEA Randomized Clinical Trial. JAMA 2016, 316, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.P.; Keller, C.; Adams, M.A.; Ainsworth, B.E. Print versus a Culturally-Relevant Facebook and Text Message Delivered Intervention to Promote Physical Activity in African American Women: A Randomized Pilot Trial. BMC Womens Health 2015, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Marsaux, C.F.; Celis-Morales, C.; Fallaize, R.; Macready, A.L.; Kolossa, S.; Woolhead, C.; O’Donovan, C.B.; Forster, H.; Navas-Carretero, S.; San-Cristobal, R.; et al. Effects of a Web-Based Personalized Intervention on Physical Activity in European Adults: A Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e231. [Google Scholar] [CrossRef]
- Godino, J.G.; van Sluijs, E.M.F.; Marteau, T.M.; Sutton, S.; Sharp, S.J.; Griffin, S.J. Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial. PLoS Med. 2016, 13, e1002185. [Google Scholar] [CrossRef]
- Nielsen, D.E.; Carere, D.A.; Wang, C.; Roberts, J.S.; Green, R.C.; Green, R.C.; Krier, J.B.; Kalia, S.S.; Christensen, K.D.; Nielsen, D.E.; et al. Diet and Exercise Changes Following Direct-to-Consumer Personal Genomic Testing. BMC Med. Genom. 2017, 10, 24. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Li, T.; Gao, X.; Yan, H.; Wang, Y.; Wang, J.; Wang, S.; et al. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front. Microbiol. 2021, 12, 682721. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef]
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.-R.; Hoseini-Tavassol, Z.; Ejtahed, H.-S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 6688450. [Google Scholar] [CrossRef]
- Hijová, E. Synbiotic Supplements in the Prevention of Obesity and Obesity-Related Diseases. Metabolites 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic Kurir, T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut Microbiota, and Metabolome: From Pathophysiology to Nutritional Interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-En-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult Obesity Complications: Challenges and Clinical Impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; de Courcy, J.; de Laguiche, E.; Faurby, M.; Haase, C.L.; Matthiessen, K.S.; Moore, A.; Pearson-Stuttard, J. Obesity-Related Complications, Healthcare Resource Use and Weight Loss Strategies in Six European Countries: The RESOURCE Survey. Int. J. Obes. 2023, 47, 750–757. [Google Scholar] [CrossRef]
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of Obesity. QJM Int. J. Med. 2018, 111, 437–443. [Google Scholar] [CrossRef]
- Stephenson, J.; Smith, C.M.; Kearns, B.; Haywood, A.; Bissell, P. The Association between Obesity and Quality of Life: A Retrospective Analysis of a Large-Scale Population-Based Cohort Study. BMC Public Health 2021, 21, 1990. [Google Scholar] [CrossRef]
- Katz, D.A.; McHorney, C.A.; Atkinson, R.L. Impact of Obesity on Health-Related Quality of Life in Patients with Chronic Illness. J. Gen. Intern. Med. 2000, 15, 789. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Bawady, N.; Aldafrawy, O.; ElZobair, E.M.; Suliman, W.; Alzaabi, A.; Ahmed, S.H. Prevalence of Overweight and Obesity in Type 2 Diabetic Patients Visiting PHC in the Dubai Health Authority. Dubai Diabetes Endocrinol. J. 2021, 28, 20–24. [Google Scholar] [CrossRef]
- Grant, B.; Sandelson, M.; Agyemang-Prempeh, B.; Zalin, A. Managing Obesity in People with Type 2 Diabetes. Clin. Med. 2021, 21, e327–e231. [Google Scholar] [CrossRef]
- Dunn, T.N.; Adams, S.H. Relations between Metabolic Homeostasis, Diet, and Peripheral Afferent Neuron Biology. Adv. Nutr. 2014, 5, 386–393. [Google Scholar] [CrossRef]
- Kheriji, N.; Boukhalfa, W.; Mahjoub, F.; Hechmi, M.; Dakhlaoui, T.; Mrad, M.; Hadj Salah Bahlous, A.; Ben Amor, N.; Jamoussi, H.; Kefi, R. The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis. Nutrients 2022, 14, 2132. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.; Sang, D.; Gao, Q.; Li, Q. The Role of Nutrition in the Prevention and Intervention of Type 2 Diabetes. Front. Bioeng. Biotechnol. 2020, 8, 575442. [Google Scholar] [CrossRef]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N. Biologic and Methodologic Issues for Nutritional Biomarkers. J. Nutr. 2003, 133 (Suppl. S3), 875S–880S. [Google Scholar] [CrossRef] [PubMed]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; Martínez-González, M.Á.; Salas-Salvadó, J.; Covas, M.-I.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Associations of the FTO Rs9939609 and the MC4R Rs17782313 Polymorphisms with Type 2 Diabetes Are Modulated by Diet, Being Higher When Adherence to the Mediterranean Diet Pattern Is Low. Cardiovasc. Diabetol. 2012, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Shaat, N.; Lernmark, A.; Karlsson, E.; Ivarsson, S.; Parikh, H.; Berntorp, K.; Groop, L. A Variant in the Transcription Factor 7-like 2 (TCF7L2) Gene Is Associated with an Increased Risk of Gestational Diabetes Mellitus. Diabetologia 2007, 50, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)Genomic Data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Gojda, J.; Cahova, M. Gut Microbiota as the Link between Elevated BCAA Serum Levels and Insulin Resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; de Vos, W.M.; Salonen, A. Gut Microbiota Signatures Predict Host and Microbiota Responses to Dietary Interventions in Obese Individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Elfaki, I.; Javid, J.; Barnawi, J.; Altayar, M.A.; Albalawi, S.O.; Jalal, M.M.; Tayeb, F.J.; Yousif, A.; Ullah, M.F.; et al. Genetic Determinants of Cardiovascular Disease: The Endothelial Nitric Oxide Synthase 3 (eNOS3), Krüppel-Like Factor-14 (KLF-14), Methylenetetrahydrofolate Reductase (MTHFR), MiRNAs27a and Their Association with the Predisposition and Susceptibility to Coronary Artery Disease. Life 2022, 12, 1905. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Wickman, B.E.; Enkhmaa, B.; Ridberg, R.; Romero, E.; Cadeiras, M.; Meyers, F.; Steinberg, F. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients 2021, 13, 4424. [Google Scholar] [CrossRef]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Consistency with the DASH Diet and Incidence of Heart Failure. Arch. Intern. Med. 2009, 169, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- King, D.E. Dietary Fiber, Inflammation, and Cardiovascular Disease. Mol. Nutr. Food Res. 2005, 49, 594–600. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Liu, B.; Zhong, V.W.; Deng, Y.; Luo, D.; Gao, C.; Bao, W.; Rong, S. Frequency of Adding Salt at the Table and Risk of Incident Cardiovascular Disease and All-Cause Mortality: A Prospective Cohort Study. BMC Med. 2022, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhou, J.; Huang, Y.; Feng, X.; Dang, P.; Li, G.; Yuan, Z. A Proinflammatory Diet Is Associated with Higher Risk of Peripheral Artery Disease. Nutrients 2022, 14, 3490. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The Dietary Inflammatory Index, Obesity, Type 2 Diabetes, and Cardiovascular Risk Factors and Diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Syed Soffian, S.S.; Mohammed Nawi, A.; Hod, R.; Ja’afar, M.H.; Isa, Z.M.; Chan, H.-K.; Hassan, M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022, 14, 1555. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality—A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef]
- Chen, C.; Yang, T.; Wang, C. The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey. Nutrients 2022, 14, 2841. [Google Scholar] [CrossRef]
- Jia, G.; Wu, C.-C.; Su, C.-H. Dietary Inflammatory Index and Metabolic Syndrome in US Children and Adolescents: Evidence from NHANES 2001–2018. Nutr. Metab. 2022, 19, 39. [Google Scholar] [CrossRef]
- Motamedi, A.; Askari, M.; Mozaffari, H.; Homayounfrar, R.; Nikparast, A.; Ghazi, M.L.; Nejad, M.M.; Alizadeh, S. Dietary Inflammatory Index in Relation to Type 2 Diabetes: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 9953115. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, S.; Bian, H.; Tian, B.; Wang, H.; Tu, X.; Cai, B.; Jin, K.; Zheng, X.; Yang, L.; et al. Association between Dietary Inflammatory Index and Kidney Stones in US Adults: Data from the National Health and Nutrition Examination Survey (NHANES) 2007–2016. Public Health Nutr. 2021, 24, 6113–6121. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.Z.; Davis, R.B.; Mukamal, K.J. Nutrient Intake and Peripheral Artery Disease in Adults: Key Considerations in Cross-Sectional Studies. Clin. Nutr. 2014, 33, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, S.; Demopoulos, C.A. Protective Effect of Olive Oil Microconstituents in Atherosclerosis: Emphasis on PAF Implicated Atherosclerosis Theory. Biomolecules 2023, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Krieghoff-Henning, E.; Folkerts, J.; Penzkofer, A.; Weg-Remers, S. Cancer—An Overview. Med. Monatsschr. Pharm. 2017, 40, 48–54. [Google Scholar] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Spyrou, N.; Mantzoros, C.S. Body Fatness Associations with Cancer: Evidence from Recent Epidemiological Studies and Future Directions. Metabolism 2022, 137, 155326. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef] [PubMed]
- Dimou, N.L.; Papadimitriou, N.; Mariosa, D.; Johansson, M.; Brennan, P.; Peters, U.; Chanock, S.J.; Purdue, M.; Bishop, D.T.; Gago-Dominquez, M.; et al. Circulating Adipokine Concentrations and Risk of Five Obesity-related Cancers: A Mendelian Randomization Study. Int. J. Cancer 2021, 148, 1625–1636. [Google Scholar] [CrossRef]
- Reglero, C.; Reglero, G. Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients 2019, 11, 2799. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Surangkul, D.; Srikummool, M.; Richert, L.; Pekthong, D.; Parhira, S.; Somran, J.; Srisawang, P. Epigallocatechin Gallate Triggers Apoptosis by Suppressing de Novo Lipogenesis in Colorectal Carcinoma Cells. FEBS Open Bio 2022, 12, 937–958. [Google Scholar] [CrossRef]
- Mossine, V.V.; Mawhinney, T.P.; Giovannucci, E.L. Dried Fruit Intake and Cancer: A Systematic Review of Observational Studies. Adv. Nutr. 2020, 11, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Apoptosis by Dietary Factors: The Suicide Solution for Delaying Cancer Growth. Carcinogenesis 2007, 28, 233–239. [Google Scholar] [CrossRef]

| Type of Illness | Type of Intervention | n | Genetic Component | Key Finding | Ref. |
|---|---|---|---|---|---|
| Obesity | 2 years of dietary intervention | 322 | Leptin (LEP) SNPs * | Possibility of regaining weight from 7 to 24 months | [81] |
| 2 years of dietary intervention | 742 | Fat mass and obesity-associated gene (FTO) SNP rs1558902 | A high-protein diet in the presence of FTO genotype: (1) Remarkable weight loss (2) Amelioration in body composition and lipid distribution | [82] | |
| 4 years of lifestyle-based intervention | 3899 | SNPs | Following weight loss, a risk of regaining weight was linked to FTO and BDNF loci | [83] | |
| 2 years of lifestyle-based intervention and metformin use | 3819 | Melanocortin 4 Receptor gene (MC4R) SNPs | In the intervention group, the rs17066866 marker was associated with: (1) Less short-term weight loss (first 6-month period) (2) Less long-term weight loss (first 2-year period) | [84] | |
| Diabetes | 2 years of dietary intervention | 738 | Insulin receptor substrate 1 gene (IRS1) rs2943641 | Altered intrinsic effect of dietary carbohydrate on weight loss and insulin resistance | [85] |
| 2 years of dietary intervention | 591 | Transcription Factor 7-Like 2 (TCF7L2) SNP rs7903146 | Interaction between dietary fat intake and TCF7L2 🡪 modified BMI as well as total and trunk fat mass | [86] | |
| 9 months of dietary intervention | 304 | TCF7L2 SNP rs7903146 | Interaction between high-fibre dietary intake and CC genotype 🡪 improved weight loss | [87] | |
| 2 years of dietary intervention | 737 | Glucose-dependent insulinotropic polypeptide receptor (GIPR) SNP rs2287019 | Interaction of dietary carbohydrates with GIPR genotype 🡪 modifications in body weight, fasting glucose, and insulin resistance | [88,89] | |
| 2 years of dietary intervention | 738 | IRS1 SNP rs1522813 | Changes in dietary fat effects based on both IRS1 genotype and MetS status | [89,90] | |
| Obesity, diabetes, or hypertension | 4 months of dietary and medical intervention | 722 | 21 SNPs | Interaction between dietary intervention and SNPs leads to changes in blood pressure | [91] |
| Hypertension | 2 years of dietary intervention | 723 | Neuropeptide Y Promoter (NPY) SNP rs16147 | Interaction between dietary fat and NPY genotype, modifying blood pressure | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, S.; Alkhaaldi, S.M.I.; Sammanasunathan, A.F.; Ibrahim, S.; Farhat, J.; Al-Omari, B. Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management. Nutrients 2024, 16, 581. https://doi.org/10.3390/nu16050581
Mansour S, Alkhaaldi SMI, Sammanasunathan AF, Ibrahim S, Farhat J, Al-Omari B. Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management. Nutrients. 2024; 16(5):581. https://doi.org/10.3390/nu16050581
Chicago/Turabian StyleMansour, Samy, Saif M. I. Alkhaaldi, Ashwin F. Sammanasunathan, Saleh Ibrahim, Joviana Farhat, and Basem Al-Omari. 2024. "Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management" Nutrients 16, no. 5: 581. https://doi.org/10.3390/nu16050581
APA StyleMansour, S., Alkhaaldi, S. M. I., Sammanasunathan, A. F., Ibrahim, S., Farhat, J., & Al-Omari, B. (2024). Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management. Nutrients, 16(5), 581. https://doi.org/10.3390/nu16050581






