The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review
Abstract
1. Introduction
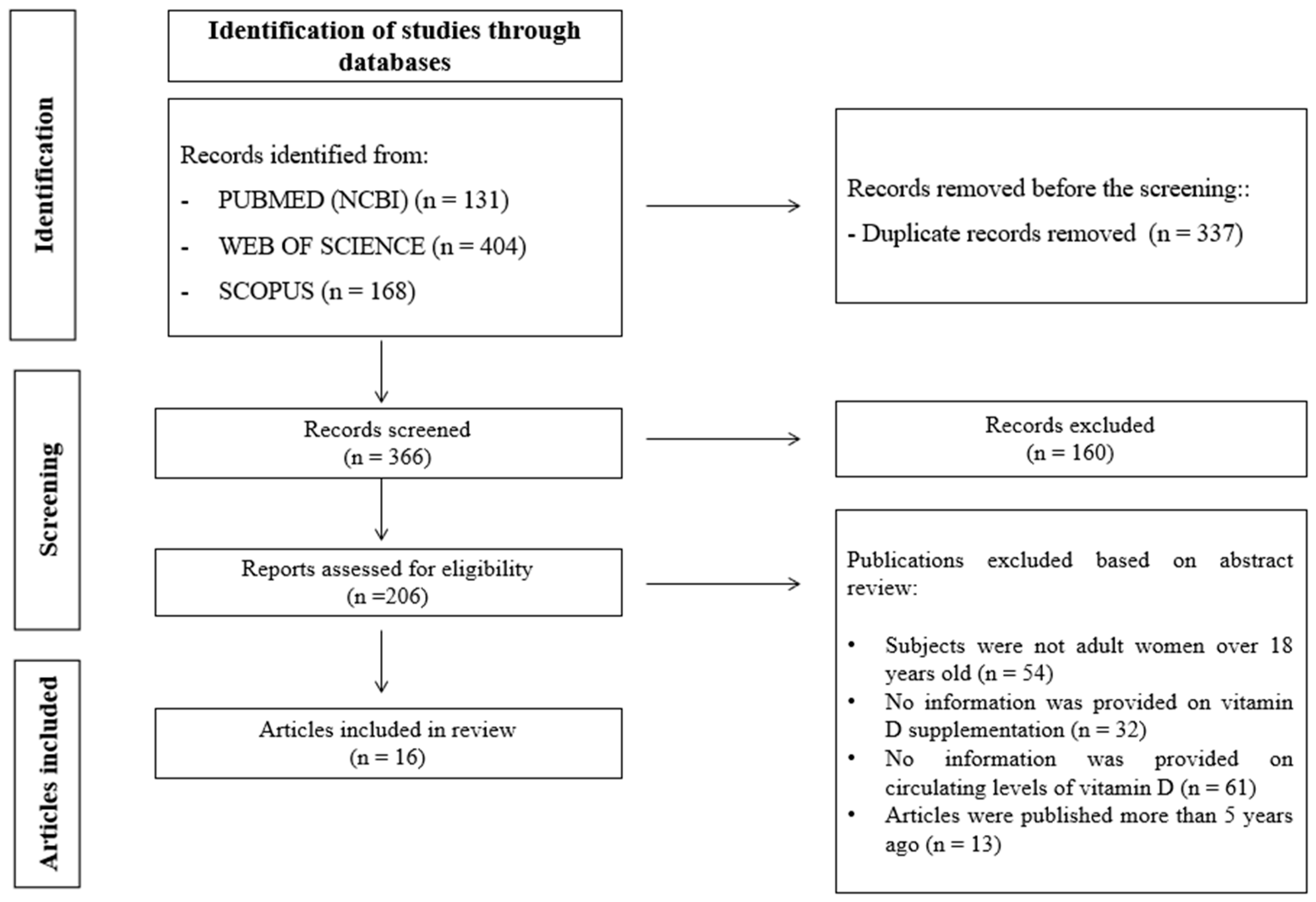
2. Materials and Methods
2.1. Search Strategy
2.2. Statistical Analysis
3. Results and Discussion
3.1. Relationship between Vitamin D Levels and Breast Cancer in Adult Women
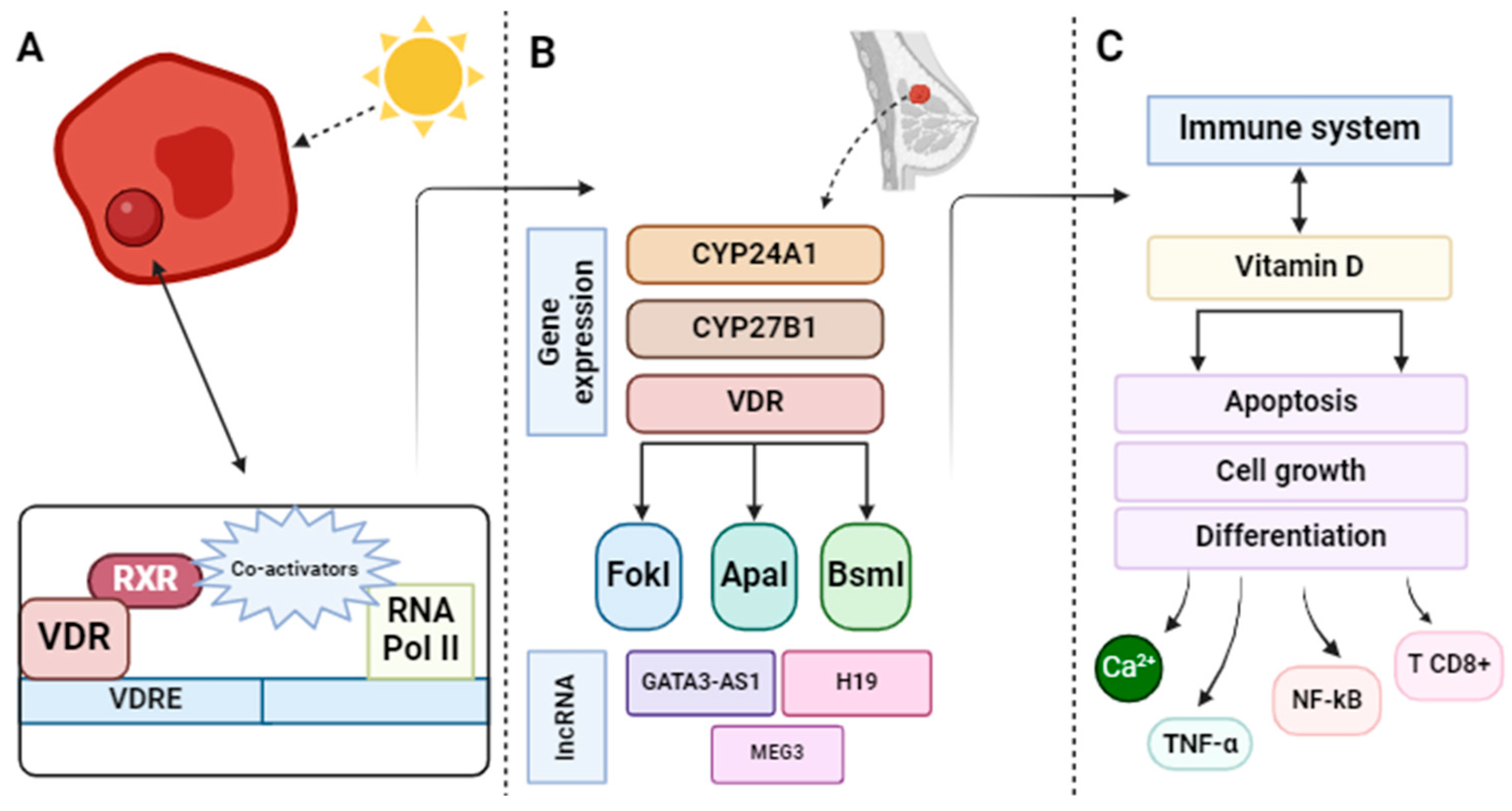
3.2. Biological Mechanism of Action Involved in the Relationship between Vitamin D and the Prevention of the Appearance of Breast Cancer
3.2.1. Vitamin D/Vitamin D Receptor (VDR) Axis
3.2.2. Regulation of Genes Related to Breast Cancer
3.2.3. Mechanisms Involved with the Immune System
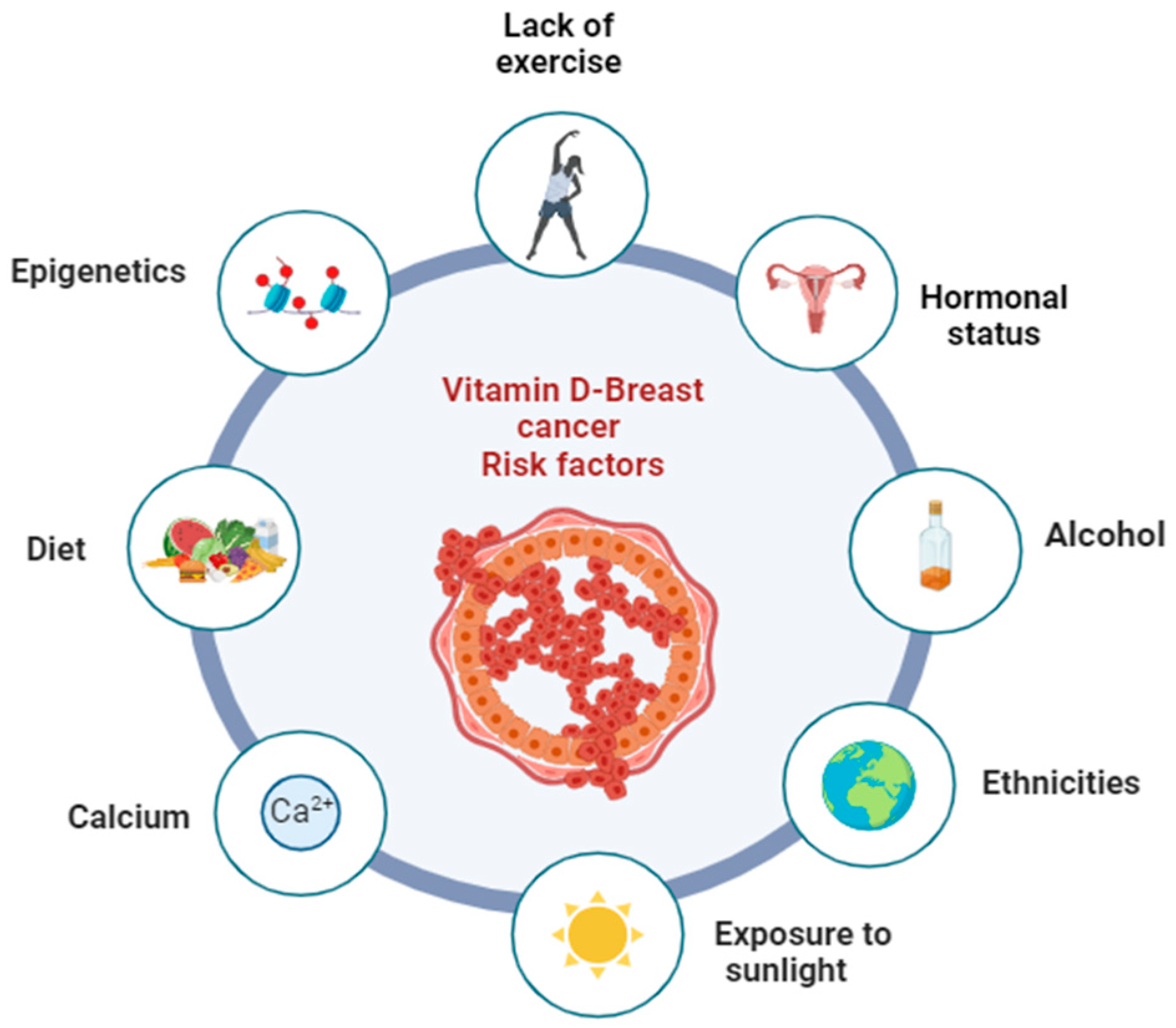
3.3. Potential Risk Factors That May Influence the Relationship between Vitamin D and Breast Cancer Prevention
3.3.1. Mechanisms Involved with the Immune System
3.3.2. Calcium
3.3.3. Other Factors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin, D; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Sanlier, N.; Guney-Coskun, M. Vitamin D, the Immune System, and Its Relationship with Diseases. Egypt. Pediatr. Assoc. Gaz. 2022, 70, 39. [Google Scholar] [CrossRef]
- Balachandar, R.; Pullakhandam, R.; Kulkarni, B.; Sachdev, H.S. Relative Efficacy of Vitamin D2 and Vitamin D3 in Improving Vitamin D Status: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R. Vitamin D: The Secosteroid Hormone and Human Reproduction. Gynecol. Endocrinol. 2007, 23, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kattner, L. Recent Developments Towards the Synthesis of Vitamin D Metabolites. Anticancer. Res. 2020, 40, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.J.; Palmieri, T.; Young, A.R. Physical Determinants of Vitamin D Photosynthesis: A Review. JBMR Plus 2021, 5, e10460. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D Fortification in the United States and Canada: Current Status and Data Needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Díaz-Muñoz, M.; García-Gaytán, A.C.; Méndez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 10 January 2024).
- Abdulrahman, G.O.; Rahman, G.A. Epidemiology of Breast Cancer in Europe and Africa. J. Cancer Epidemiol. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Olopade, O.I.; Grushko, T.A.; Nanda, R.; Huo, D. Advances in Breast Cancer: Pathways to Personalized Medicine. Clin. Cancer Res. 2008, 14, 7988–7999. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes. Dis. 2018, 5, 77. [Google Scholar] [CrossRef]
- Gajria, D.; Chandarlapaty, S. HER2-Amplified Breast Cancer: Mechanisms of Trastuzumab Resistance and Novel Targeted Therapies. Expert. Rev. Anticancer. Ther. 2011, 11, 263. [Google Scholar] [CrossRef]
- Dense Breasts: Answers to Commonly Asked Questions—NCI. Available online: https://www.cancer.gov/types/breast/breast-changes/dense-breasts (accessed on 10 January 2024).
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Starek-Świechowicz, B.; Budziszewska, B.; Starek, A. Alcohol and Breast Cancer. Pharmacol. Rep. 2023, 75, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G. Geographic Location and Vitamin D Synthesis. Mol. Aspects Med. 2008, 29, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, S.; Cyr, D.; Lynge, E.; Orsi, L.; Sabroe, S.; Merletti, F.; Gorini, G.; Morales-Suarez-Varela, M.; Ahrens, W.; Baumgardt-Elms, C.; et al. Occupation and Occupational Exposure to Endocrine Disrupting Chemicals in Male Breast Cancer: A Case-Control Study in Europe. Occup. Env. Med. 2010, 67, 837. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, R.; Bicer, U. Evaluation of Breast Cancer Risk Levels and Its Relation with Breast Self-Examination Practices in Women. J. Breast Health 2017, 13, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Merino Bonilla, J.A.; Torres Tabanera, M.; Ros Mendoza, L.H. Breast Cancer in the 21st Century: From Early Detection to New Therapies. Radiologia 2017, 59, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Garg, M.K.; Marwaha, R.K.; Khadgawat, R.; Ramot, R.; Obroi, A.K.; Mehan, N.; Gupta, N.; Madan, R. Efficacy of Vitamin D Loading Doses on Serum 25-Hydroxy Vitamin D Levels in School Going Adolescents: An Open Label Non-Randomized Prospective Trial. J. Pediatr. Endocrinol. Metab. 2013, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Khadgawat, R.; Marwaha, R.K.; Garg, M.K.; Ramot, R.; Oberoi, A.K.; Sreenivas, V.; Gahlot, M.; Mehan, N.; Mathur, P.; Gupta, N. Impact of Vitamin D Fortified Milk Supplementation on Vitamin D Status of Healthy School Children Aged 10–14 Years. Osteoporos. Int. 2013, 24, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, K.; Mondul, A.M.; Zeleniuch-Jacquotte, A.; Wang, M.; Gail, M.H.; Yaun, S.S.; Weinstein, S.J.; McCullough, M.L.; Eliassen, A.H.; Cook, N.R.; et al. Circulating Vitamin D and Breast Cancer Risk: An International Pooling Project of 17 Cohorts. Eur. J. Epidemiol. 2023, 38, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Friedrich, M.; Krämer, S.; Thill, M. Vitamin D and Breast Cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef]
- Sperati, F.; Vici, P.; Maugeri-Saccà, M.; Stranges, S.; Santesso, N.; Mariani, L.; Giordano, A.; Sergi, D.; Pizzuti, L.; Di Lauro, L.; et al. Vitamin D Supplementation and Breast Cancer Prevention: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2013, 8, e69269. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fakour, F.; Moghimi, M.; Esmaeilzadeh, A.; Kalantari, F.; Eskandari, F.; Biglari, S.; Mazloomzadeh, S. Middle East Journal of Cancer. Orig. Artic. Middle East. J. Cancer 2015, 13, 81–88. [Google Scholar]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast Cancer Risk Markedly Lower with Serum 25-Hydroxyvitamin D Concentrations ≥60 vs <20 Ng/Ml (150 vs 50 Nmol/L): Pooled Analysis of Two Randomized Trials and a Prospective Cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and Breast Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Lope, V.; Castelló, A.; Mena-Bravo, A.; Amiano, P.; Aragonés, N.; Fernández-Villa, T.; Guevara, M.; Dierssen-Sotos, T.; Fernandez-Tardón, G.; Castaño-Vinyals, G.; et al. Serum 25-Hydroxyvitamin D and Breast Cancer Risk by Pathological Subtype (MCC-Spain). J. Steroid Biochem. Mol. Biol. 2018, 182, 4–13. [Google Scholar] [CrossRef]
- Karthikayan, A.; Sureshkumar, S.; Kadambari, D.; Vijayakumar, C. Low Serum 25-Hydroxy Vitamin D Levels Are Associated with Aggressive Breast Cancer Variants and Poor Prognostic Factors in Patients with Breast Carcinoma. Arch. Endocrinol. Metab. 2018, 62, 452–459. [Google Scholar] [CrossRef]
- Welsh, J.E. Vitamin D and Breast Cancer: Past and Present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Vitamin D Baseline Levels at Diagnosis of Breast Cancer: A Systematic Review and Meta-Analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, N.; Jaleel, F.; Moosa, F.A.; Qureshi, N.A. Association between Vitamin D Deficiency and Breast Cancer. Pak. J. Med. Sci. 2017, 33, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Chlebowski, R.T.; Wactawski-Wende, J.; Robbins, J.A.; Rodabough, R.J.; Chen, Z.; Johnson, K.C.; O’Sullivan, M.J.; Jackson, R.D.; Manson, J.E. Calcium plus Vitamin D Supplementation and Health Outcomes Five Years after Active Intervention Ended: The Women’s Health Initiative. J. Womens Health 2013, 22, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.C.; Guy, M.; Mansi, J.L.; Peckitt, C.; Bliss, J.; Wilson, R.G.; Colston, K.W. Plasma 25-Hydroxy Vitamin D Concentrations, Vitamin D Receptor Genotype and Breast Cancer Risk in a UK Caucasian Population. Eur. J. Cancer 2005, 41, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Gammon, M.D.; Steck, S.E.; Hershman, D.L.; Cremers, S.; Dworakowski, E.; Shane, E.; Terry, M.B.; Desai, M.; Teitelbaum, S.L.; et al. Association between Plasma 25-Hydroxyvitamin D and Breast Cancer Risk. Cancer Prev. Res. 2009, 2, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-Analysis of Vitamin D, Calcium and the Prevention of Breast Cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Thomson, C.A.; Flatt, S.W.; Al-Delaimy, W.K.; Hibler, E.A.; Jones, L.A.; Leroy, E.C.; Newman, V.A.; Parker, B.A.; Rock, C.L.; et al. Vitamin D and Breast Cancer Recurrence in the Women’s Healthy Eating and Living (WHEL) Study 1–3. Am. J. Clin. Nutr. 2011, 93, 108–125. [Google Scholar] [CrossRef]
- Edvardsen, K.; Veierød, M.B.; Brustad, M.; Braaten, T.; Engelsen, O.; Lund, E. Vitamin D-Effective Solar UV Radiation, Dietary Vitamin D and Breast Cancer Risk. Int. J. Cancer 2011, 128, 1425–1433. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, B.; Sheng, L.; Turner, A. The Effect of Vitamin D Supplementation on the Risk of Breast Cancer: A Trial Sequential Meta-Analysis. Breast Cancer Res. Treat. 2020, 182, 1–8. [Google Scholar] [CrossRef]
- Rainville, C.; Khan, Y.; Tisman, G. Case Report Triple Negative Breast Cancer Patients Presenting with Low Serum Vitamin D Levels: A Case Series. Cases J. 2009, 2, 8390. [Google Scholar] [CrossRef]
- Rollison, D.E.; Cole, A.L.; Tung, K.-H.; Slattery, M.L.; Baumgartner, K.B.; Byers, T.; Wolff, R.K.; Giuliano, A.R. Vitamin D Intake, Vitamin D Receptor Polymorphisms, and Breast Cancer Risk among Women Living in the Southwestern U.S. Breast Cancer Res. Treat. 2012, 132, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Dhasmana, A.; Bhatt, M.L.B.; Lohani, M.; Arif, J.M. Molecular Mechanism of Cancer Susceptibility Associated with Fok1 Single Nucleotide Polymorphism of VDR in Relation to Breast Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martínez, N.; Díaz, L.; Ortiz-Ortega, V.M.; Ordaz-Rosado, D.; Prado-Garcia, H.; Avila, E.; Larrea, F.; García-Becerra, R. Calcitriol Induces Estrogen Receptor α Expression through Direct Transcriptional Regulation and Epigenetic Modifications in Estrogen Receptor-Negative Breast Cancer Cells. Am. J. Cancer Res. 2021, 11, 5951–5964. [Google Scholar] [PubMed]
- Blasiak, J.; Chojnacki, J.; Pawlowska, E.; Jablkowska, A.; Chojnacki, C. Vitamin D May Protect against Breast Cancer through the Regulation of Long Noncoding RNAs by VDR Signaling. Int. J. Mol. Sci. 2022, 23, 3189. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.F.; El Askary, A.; Almehmadi, M.; Alhuthali, H.M.; Elsawy, W.H.; Allam, H.H.; Elsayyad, L.K.; Ayoub, M.A.; Shafie, A. Association of Vitamin D Deficiency and Inflammatory Cytokines with the Clinicopathological Features of Breast Cancer in Female Saudi Patients. Eur. J. Inflamm. 2022, 20, 1721727X221106507. [Google Scholar] [CrossRef]
- Karkeni, E.; Morin, S.O.; Tayeh, B.B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef]
- Welsh, J.E. Vitamin D and Breast Cancer: Mechanistic Update. JBMR Plus 2021, 5, e10582. [Google Scholar] [CrossRef]
- Eisman, J.A.; Macintyre, I.; Martin, T.J.; Moseley, J.M. 1,25-Dihydroxyvitamin-D-Receptor in Breast Cancer Cells. Lancet 1979, 2, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.A.; Bellerba, F.; Corso, F.; Gandini, S. Vitamin D Receptor Polymorphisms and Cancer. Adv. Exp. Med. Biol. 2020, 1268, 53–114. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, E.A.-E.; Ismaeil, N.A.; Abd-Alsalam, H.S. Vitamin D Receptor Gene Polymorphisms and Breast Cancer Risk among Postmenopausal Egyptian Women. Tumor Biol. 2015, 36, 6425–6431. [Google Scholar] [CrossRef]
- Jensen, T.J.; Henriksen, L.O.; Sølvsten, H.; Kragballe, K. Inhibition of the 1,25-Dihydroxyvitamin D3-Induced Increase in Vitamin D Receptor (VDR) Levels and Binding of VDR-Retinoid X Receptor (RXR) to a Direct Repeat (DR)-3 Type Response Element by an RXR-Specific Ligand in Human Keratinocyte Cultures. Biochem. Pharmacol. 1998, 55, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Peng, X.; Yuan, L.; Mehta, R.R.; von Knethen, A.; Choubey, D.; Mehta, R.G. Crosstalk between the Peroxisome Proliferator-Activated Receptor γ (PPARγ) and the Vitamin D Receptor (VDR) in Human Breast Cancer Cells: PPARγ Binds to VDR and Inhibits 1α,25-Dihydroxyvitamin D3 Mediated Transactivation. Exp. Cell Res. 2012, 318, 2490–2497. [Google Scholar] [CrossRef]
- Friedrich, M.; Axt-Fliedner, R.; Villena-Heinsen, C.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of Vitamin D-Receptor (VDR) and Retinoid X-Receptor Alpha in Breast Cancer. Histochem. J. 2002, 34, 35–40. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Warner, E.T.; Rosner, B.; Collins, L.C.; Beck, A.H.; Quintana, L.M.; Tamimi, R.M.; Hankinson, S.E. Plasma 25-Hydroxyvitamin D and Risk of Breast Cancer in Women Followed over 20 Years. Cancer Res. 2016, 76, 5423–5430. [Google Scholar] [CrossRef]
- Abe, E.; Miyaura, C.; Sakagami, H.; Takeda, M.; Konno, K.; Yamazaki, T.; Yoshiki, S.; Suda, T. Differentiation of Mouse Myeloid Leukemia Cells Induced by 1 Alpha,25-Dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1981, 78, 4990–4994. [Google Scholar] [CrossRef]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Manjer, J.; Rosendahl, A. Levels of Vitamin D and Expression of the Vitamin D Receptor in Relation to Breast Cancer Risk and Survival. Nutrients 2022, 14, 3353. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where Is the Vitamin D Receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Makonnen, E.; Fotoohi, A.; Yimer, G.; Seifu, D.; Assefa, M.; Tigeneh, W.; Aseffa, A.; Howe, R.; Aklillu, E.; et al. Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia. Nutrients 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.; Alabdali, N.; Kapila, K.; John, B.; Al-Temaimi, R.A. Vitamin D Pathway Related Polymorphisms and Vitamin D Receptor Expression in Breast Cancer. Int. J. Vitam. Nutr. Res. 2021, 91, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.M.; Esmaieli-bandboni, A.; Veisi Malekshahi, Z.; Shahbaz Sardood, M.; Hashemi, M.; Majidzadeh, K.; Kadkhodazadeh, M.; Esmaili, R.; Negahdari, B. Vitamin D Receptor Gene Polymorphisms and Risk of Breast Cancer in Iranian Women. Ann. Med. Surg. 2021, 73, 103150. [Google Scholar] [CrossRef] [PubMed]
- Dorjgochoo, T.; Delahanty, R.; Lu, W.; Long, J.; Cai, Q.; Zheng, Y.; Gu, K.; Gao, Y.-T.; Zheng, W.; Shu, X.O. Common Genetic Variants in the Vitamin D Pathway Including Genome-Wide Associated Variants Are Not Associated with Breast Cancer Risk among Chinese Women. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2313–2316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.-P.; Chang, S.-C.; Linet, M.S.; Sigurdson, A.J. Sunlight, Polymorphisms of Vitamin D-Related Genes and Risk of Breast Cancer. Anticancer. Res. 2013, 33, 543–551. [Google Scholar]
- Zhao, Z.; Cai, W.; Xing, J.; Zhao, C. Lower Vitamin D Levels and VDR Variants Are Risk Factors for Breast Cancer: An Updated Meta-Analysis. Nucleosides Nucleotides Nucleic Acids 2023, 42, 17–37. [Google Scholar] [CrossRef]
- Pineda-Moncusí, M.; Rodríguez-Sanz, M.; Servitja, S.; Díez-Pérez, G.-G. Study of the Genetic Basis of Trabecular Bone Score Reduction Related to Aromatase Inhibitors. Orig. Rev. Osteoporos. Metab. Min. 2018, 10, 82–88. [Google Scholar] [CrossRef]
- CYP27B1 Cytochrome P450 Family 27 Subfamily B Member 1 [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=1594 (accessed on 10 January 2024).
- Osanai, M.; Lee, G.H. CYP24A1-Induced Vitamin D Insufficiency Promotes Breast Cancer Growth. Oncol. Rep. 2016, 36, 2755–2762. [Google Scholar] [CrossRef]
- Sheng, L.; Turner, A.G.; Barratt, K.; Kremer, R.; Morris, H.A.; Callen, D.F.; Anderson, P.H.; Tarulli, G.A. Mammary-Specific Ablation of Cyp24a1 Inhibits Development, Reduces Proliferation and Increases Sensitivity to Vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 189, 240–247. [Google Scholar] [CrossRef]
- Kemmis, C.M.; Salvador, S.M.; Smith, K.M.; Welsh, J. Human Mammary Epithelial Cells Express CYP27B1 and Are Growth Inhibited by 25-Hydroxyvitamin D-3, the Major Circulating Form of Vitamin D-3. J. Nutr. 2006, 136, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Latacz, M.; Snarska, J.; Kostyra, E.; Fiedorowicz, E.; Savelkoul, H.F.J.; Grzybowski, R.; Cieślińska, A. Single Nucleotide Polymorphisms in 25-Hydroxyvitamin D3 1-Alpha-Hydroxylase (CYP27B1) Gene: The Risk of Malignant Tumors and Other Chronic Diseases. Nutrients 2020, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.; Dillon, J.; Cohen, D.J.; Halquist, M.S.; Pearcy, A.C.; Schwartz, Z.; Boyan, B.D. Local Production of Active Vitamin D3 Metabolites in Breast Cancer Cells by CYP24A1 and CYP27B1. J. Steroid Biochem. Mol. Biol. 2023, 232, 106331. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.D.; McCullough, M.L.; Ziegler, R.G.; Kraft, P.; Saltzman, B.S.; Riboli, E.; Barricarte, A.; Berg, C.D.; Bergland, G.; Bingham, S.; et al. Vitamin D Receptor Polymorphisms and Breast Cancer Risk: Results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kholghi Oskooei, V.; Geranpayeh, L.; Omrani, M.D.; Ghafouri-Fard, S. Assessment of Functional Variants and Expression of Long Noncoding RNAs in Vitamin D Receptor Signaling in Breast Cancer. Cancer Manag. Res. 2018, 10, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Dankers, W.; Colin, E.M.; Van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Vitam. D Autoimmun. Mol. Mech. Ther. Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; LaPorta, E.; Simmons, K.M.; Beaudin, S.; Welsh, J. The Impact of Vitamin D in Breast Cancer: Genomics, Pathways, Metabolism. Front. Physiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Staquicini, F.I.; Hajitou, A.; Driessen, W.H.P.; Proneth, B.; Cardó-Vila, M.; Staquicini, D.I.; Markosian, C.; Hoh, M.; Cortez, M.; Hooda-Nehra, A.; et al. Targeting a Cell Surface Vitamin D Receptor on Tumor-Associated Macrophages in Triple-Negative Breast Cancer. Elife 2021, 10, e65145. [Google Scholar] [CrossRef]
- Yao, S.; Hong, C.-C.; Mccann, S.E.; Zirpoli, G.; Quan, L.; Gong, Z.; Johnson, C.S.; Trump, D.L.; Ambrosone, C.B. Combined Effects of Circulating Levels of 25-Hydroxyvitamin D and Th1 and Th2 Cytokines on Breast Cancer Estrogen Receptor Status. Cancers 2014, 6, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The Role of Vitamin D in Cancer Prevention and Treatment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 401–418. [Google Scholar] [CrossRef]
- Koren, R.; Hadari-Naor, I.; Zuck, E.; Rotem, C.; Liberman, U.A.; Ravid, A. Vitamin D Is a Prooxidant in Breast Cancer Cells. Cancer Res. 2001, 61, 1439–1444. [Google Scholar]
- Kazemian, E.; Akbari, M.E.; Moradi, N.; Gharibzadeh, S.; Amouzegar, A.; Jamshidi-Naeini, Y.; Mondul, A.M.; Khademolmele, M.; Ghodoosi, N.; Zarins, K.R.; et al. Effect of Vitamin D Receptor Polymorphisms on Plasma Oxidative Stress and Apoptotic Biomarkers among Breast Cancer Survivors Supplemented Vitamin D3. Eur. J. Cancer Prev. 2020, 29, 433–444. [Google Scholar] [CrossRef]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast Cancer Primary Prevention and Diet: An Umbrella Review. Int. J. Env. Res. Public. Health 2020, 17, 4731. [Google Scholar] [CrossRef]
- Peila, R.; Xue, X.; Cauley, J.A.; Chlebowski, R.; Manson, J.E.; Nassir, R.; Saquib, N.; Shadyab, A.H.; Zhang, Z.; Wassertheil-Smoller, S.; et al. A Randomized Trial of Calcium Plus Vitamin D Supplementation and Risk of Ductal Carcinoma In Situ of the Breast. JNCI Cancer Spectr. 2021, 5, pkab072. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sandler, D.P.; Xu, Z.; Kinyamu, H.K.; Taylor, J.A.; Weinberg, C.R. Vitamin D, DNA Methylation, and Breast Cancer. Breast Cancer Res. 2018, 20, 70. [Google Scholar] [CrossRef]
- Nazario, C.M.; Rosario-Rosado, R.V.; Schelske-Santos, M.; Mansilla-Rivera, I.; Ramírez-Marrero, F.A.; Nie, J.; Piovanetti-Fiol, P.; Hernández-Santiago, J.; Freudenheim, J.L. Sun Exposure Is Associated with Reduced Breast Cancer Risk among Women Living in the Caribbean: The Atabey Study in Puerto Rico. Cancer Epidemiol. Biomark. Prev. 2022, 31, 430–435. [Google Scholar] [CrossRef]
- Song, D.; Deng, Y.; Liu, K.; Zhou, L.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; Wu, Y.; Zhai, Z.; et al. Vitamin D Intake, Blood Vitamin D Levels, and the Risk of Breast Cancer: A Dose-Response Meta-Analysis of Observational Studies. Aging 2019, 11, 12708–12732. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Harmon, Q.E.; Jackson, C.L.; Diaz-Santana, M.V.; Taylor, J.A.; Weinberg, C.R.; Sandler, D.P. Vitamin D Concentrations and Breast Cancer Incidence among Black/African American and Non-Black Hispanic/Latina Women. Cancer 2022, 128, 2463–2473. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A.; Evans, D.G. Can Diet and Lifestyle Prevent Breast Cancer: What Is the Evidence? Am. Soc. Clin. Oncol. Educ. Book. 2015, 35, e66–e73. [Google Scholar] [CrossRef]
- Taha, Z.; Eltom, S.E. The Role of Diet and Lifestyle in Women with Breast Cancer: An Update Review of Related Research in the Middle East. Biores Open Access 2018, 7, 73–80. [Google Scholar] [CrossRef]
- Romieu, I.I.; Amadou, A.; Chajes, V. The Role of Diet, Physical Activity, Body Fatness, and Breastfeeding in Breast Cancer in Young Women: Epidemiological Evidence. Rev. De. Investig. Clínica 2017, 69, 193–203. [Google Scholar] [CrossRef]
- Rossi, R.E.; Pericleous, M.; Mandair, D.; Whyand, T.; Caplin, M.E. The Role of Dietary Factors in Prevention and Progression of Breast Cancer. Anticancer. Res. 2014, 34, 6861–6875. [Google Scholar]
- Smith-Warner, S.A.; Spiegelman, D.; Adami, H.O.; Beeson, W.L.; van den Brandt, P.A.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; Graham, S.; et al. Types of Dietary Fat and Breast Cancer: A Pooled Analysis of Cohort Studies. Int. J. Cancer 2001, 92, 767–774. [Google Scholar] [CrossRef]
- Michels, K.B.; Mohllajee, A.P.; Roset-Bahmanyar, E.; Beehler, G.P.; Moysich, K.B. Diet and Breast Cancer: A Review of the Prospective Observational Studies. Cancer 2007, 109, 2712–2749. [Google Scholar] [CrossRef]
- Laudisio, D.; Castellucci, B.; Barrea, L.; Pugliese, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Mediterranean Diet and Breast Cancer Risk: A Narrative Review. Minerva Endocrinol. 2021, 46, 441–452. [Google Scholar] [CrossRef]
- Montagnese, C.; Porciello, G.; Vitale, S.; Palumbo, E.; Crispo, A.; Grimaldi, M.; Calabrese, I.; Pica, R.; Prete, M.; Falzone, L.; et al. Quality of Life in Women Diagnosed with Breast Cancer after a 12-Month Treatment of Lifestyle Modifications. Nutrients 2020, 13, 136. [Google Scholar] [CrossRef]
- Holick, F.M. Vitamin D, Sunlight and Cancer Connection. Anti-Cancer Agents Med. Chem. Anti-Cancer Agents 2012, 13, 70–82. [Google Scholar] [CrossRef]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Cashman, K.D.; O’Neill, C.M. Strategic Food Vehicles for Vitamin D Fortification and Effects on Vitamin D Status: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Steroid Biochem. Mol. Biol. 2023, 238, 106448. [Google Scholar] [CrossRef]
- Navarro Valverde, C.; Manuel, J.; Gómez, Q. Vitamina D, Determinante de La Salud Ósea y Extra Ósea; Importancia de Su Suplementación En La Leche y Derivados. Nutr. Hosp. 2015, 31, 18–25. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Nzekoue, F.K.; Alesi, A.; Ricciutelli, M.; Sagratini, G.; Suo, X.; Torregiani, E.; Vittori, S.; Caprioli, G. Development of Innovative Vitamin D Enrichment Designs for Two Typical Italian Fresh Cheeses: Burrata and Giuncata. Molecules 2023, 28, 1049. [Google Scholar] [CrossRef]
- Naderi, M.; Kordestani, H.; Sahebi, Z.; Khedmati Zare, V.; Amani-Shalamzari, S.; Kaviani, M.; Wiskemann, J.; Molanouri Shamsi, M. Serum and Gene Expression Profile of Cytokines Following Combination of Yoga Training and Vitamin D Supplementation in Breast Cancer Survivors: A Randomized Controlled Trial. BMC Womens Health 2022, 22, 90. [Google Scholar] [CrossRef]
- Campbell, K.L.; Foster-Schubert, K.E.; Alfano, C.M.; Wang, C.C.; Wang, C.Y.; Duggan, C.R.; Mason, C.; Imayama, I.; Kong, A.; Xiao, L.; et al. Reduced-Calorie Dietary Weight Loss, Exercise, and Sex Hormones in Postmenopausal Women: Randomized Controlled Trial. J. Clin. Oncol. 2012, 30, 2314–2326. [Google Scholar] [CrossRef]
- Oliveira-Sediyama, C.M.N.; Dos Santos Dias, M.M.; Pessoa, M.C.; Queiroz, A.R.; Suhett, L.G.; Freitas, R.N.; De Paula, S.O.; Gouveia Peluzio, M.D.C. Lifestyle and Vitamin D Dosage in Women with Breast Cancer. Nutr. Hosp. 2016, 33, 1179–1186. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D and Calcium Interactions: Functional Outcomes. Am. J. Clin. Nutr. 2008, 88, 541S–544S. [Google Scholar] [CrossRef]
- Krusinska, B.; Wadolowska, L.; Biernacki, M.; Slowinska, M.A.; Drozdowski, M. Serum “Vitamin-Mineral” Profiles: Associations with Postmenopausal Breast Cancer Risk Including Dietary Patterns and Supplementation. A Case-Control Study. Nutrients 2019, 11, 2244. [Google Scholar] [CrossRef]
- Lin, J.; Manson, J.A.E.; Lee, I.M.; Cook, N.R.; Buring, J.E.; Zhang, S.M. Intakes of Calcium and Vitamin D and Breast Cancer Risk in Women. Arch. Intern. Med. 2007, 167, 1050–1059. [Google Scholar] [CrossRef]
- Lipkin, M.; Newmark, H.L. Vitamin D, Calcium and Prevention of Breast Cancer: A Review. J. Am. Coll. Nutr. 1999, 18, 392S–397S. [Google Scholar] [CrossRef]
- Qin, B.; Xu, B.; Ji, N.; Yao, S.; Pawlish, K.; Llanos, A.A.; Lin, Y.; Demissie, K.; Ambrosone, C.B.; Hong, C.-C.; et al. Intake of Vitamin D and Calcium, Sun Exposure, and Risk of Breast Cancer Subtypes among Black Women. Am. J. Clin. Nutr. 2020, 111, 396–405. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, C.I.; Romanos-Nanclares, A.; Sánchez-Bayona, R.; Gea, A.; Sayon-Orea, C.; Martinez-Gonzalez, M.A.; Toledo, E. Dietary Calcium, Vitamin D, and Breast Cancer Risk in Women: Findings from the SUN Cohort. Eur. J. Nutr. 2021, 60, 3783–3797. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; McTiernan, A.; Thomson, C.A.; Wactawski-Wende, J.; Aragaki, A.K.; Rohan, T.E.; Vitolins, M.Z.; Tamimi, R.M.; Johnson, K.C.; Lane, D.; et al. Vitamin D and Calcium Supplementation and One-Year Change in Mammographic Density in the Women’s Health Initiative Calcium and Vitamin D Trial. Cancer Epidemiol. Biomark. Prev. 2012, 21, 462–473. [Google Scholar] [CrossRef]
- Guyonnet, E.; Kim, S.J.; Pullella, K.; Zhang, C.X.W.; McCuaig, J.M.; Armel, S.; Narod, S.A.; Kotsopoulos, J. Vitamin D and Calcium Supplement Use and High-Risk Breast Cancer: A Case-Control Study among BRCA1 and BRCA2 Mutation Carriers. Cancers 2023, 15, 2790. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and Calcium Supplementation Reduces Cancer Risk: Results of a Randomized Trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, Mechanisms and Clinical Perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Marik, R.; Fackler, M.; Gabrielson, E.; Zeiger, M.A.; Sukumar, S.; Stearns, V.; Umbricht, C.B. DNA Methylation-Related Vitamin D Receptor Insensitivity in Breast Cancer) DNA Methylation-Related Vitamin D Receptor Insensitivity in Breast Cancer. Cancer Biol. Ther. 2010, 10, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Muñoz, A. An Update on Vitamin D Signaling and Cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Cai, X. Identification of Epigenetic Modulators in Human Breast Cancer by Integrated Analysis of DNA Methylation and RNA-Seq Data. Epigenetics 2018, 13, 473–489. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Long, Z.; Du, J.; Li, S.; Yin, H.; Xie, K.; Wu, Z.; Chen, Y.; Volontovich, D.; et al. Are Dietary Factors Involved in the Association of CDH4 Methylation and Breast Cancer Risk? Br. J. Nutr. 2022, 127, 1868–1877. [Google Scholar] [CrossRef]
- Xi, Y.; Shi, J.; Li, W.; Tanaka, K.; Allton, K.L.; Richardson, D.; Li, J.; Franco, H.L.; Nagari, A.; Malladi, V.S.; et al. Histone Modification Profiling in Breast Cancer Cell Lines Highlights Commonalities and Differences among Subtypes. BMC Genom. 2018, 19, 150. [Google Scholar] [CrossRef]
- Shahrzad, M.K.; Gharehgozlou, R.; Fadaei, S.; Hajian, P.; Mirzaei, H.R. Vitamin D and Non-Coding RNAs: New Insights into the Regulation of Breast Cancer. Curr. Mol. Med. 2021, 21, 194–210. [Google Scholar] [CrossRef]
- Singh, T.; Adams, B.D. The Regulatory Role of MiRNAs on VDR in Breast Cancer. Transcription 2017, 8, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Nehal, A.S.; Mona, R.; Nadia, A.E.M.; Sanaa, S.; Maher, K. The Prognostic Value of Vitamin D Receptor and Its Up-Stream MiR-27b and MiR-125a Expression in Breast Cancer Patients. Gene Rep. 2021, 23, 101121. [Google Scholar] [CrossRef]
- Engel, P.; Fagherazzi, G.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Joint Effects of Dietary Vitamin D and Sun Exposure on Breast Cancer Risk: Results from the French E3N Cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hiller, T.W.R.; O’Sullivan, D.E.; Brenner, D.R.; Peters, C.E.; King, W.D. Solar Ultraviolet Radiation and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Env. Health Perspect. 2020, 128, 16002. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.N.; Cotterchio, M.; Cole, D.E.C.; Knight, J.A. Vitamin D-Related Genetic Variants, Interactions with Vitamin D Exposure, and Breast Cancer Risk among Caucasian Women in Ontario. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1708–1717. [Google Scholar] [CrossRef]
- Saucedo, G.M.G.; Salido Vallejo, R.; Carlos, J.; Giménez, M. Efectos de La Radiación Solar y Actualización En Fotoprotección. An. Pediatría 2020, 92, 1–377. [Google Scholar] [CrossRef]
- Porojnicu, A.C.; Lagunova, Z.; Robsahm, T.E.; Berg, J.P.; Dahlback, A.; Moan, J. Changes in Risk of Death from Breast Cancer with Season and Latitude: Sun Exposure and Breast Cancer Survival in Norway. Breast Cancer Res. Treat. 2007, 102, 323–328. [Google Scholar] [CrossRef]
- Kim, Y.; Franke, A.A.; Shvetsov, Y.B.; Wilkens, L.R.; Cooney, R.V.; Lurie, G.; Maskarinec, G.; Hernandez, B.Y.; Le Marchand, L.; Henderson, B.E.; et al. Plasma 25-Hydroxyvitamin D3 Is Associated with Decreased Risk of Postmenopausal Breast Cancer in Whites: A Nested Case–Control Study in the Multiethnic Cohort Study. BMC Cancer 2014, 14, 29. [Google Scholar] [CrossRef]
- Hysaj, O.; Karavasiloglou, N.; Limam, M.; Wanner, M.; Korol, D.; Rohrmann, S. Is Season of Diagnosis a Predictor of Cancer Survival? Results from the Zurich Cancer Registry. Nutrients 2022, 14, 4291. [Google Scholar] [CrossRef]
- David Vantman, B.; Margarita Vega, B. Reproductive Physiology and Evolutive Changes with Women Age. Rev. Medica Clin. Las. Condes 2010, 21, 348–362. [Google Scholar] [CrossRef]
- Chu, C.; Tsuprykov, O.; Chen, X.; Elitok, S.; Krämer, B.K.; Hocher, B. Relationship Between Vitamin D and Hormones Important for Human Fertility in Reproductive-Aged Women. Front. Endocrinol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D Exposure and Risk of Breast Cancer: A Meta-Analysis OPEN. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.R.; Hankinson, S.E.; Bertone-Johnson, E.R.; Ding, E.L. Plasma Vitamin D Levels, Menopause, and Risk of Breast Cancer: Dose-Response Meta-Analysis of Prospective Studies. Medicine 2013, 92, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Robien, K.; Cutler, G.J.; Lazovich, D. Vitamin D Intake and Breast Cancer Risk in Postmenopausal Women: The Iowa Women’s Health Study. Cancer Causes Control 2007, 18, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Purrington, K.S.; Raychaudhuri, S.; Simon, M.S.; Clark, J.; Ratliff, V.; Dyson, G.; Craig, D.B.; Boerner, J.L.; Beebe-Dimmer, J.L.; Schwartz, A.G. Heritable Susceptibility to Breast Cancer among African-American Women in the Detroit Research on Cancer Survivors Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Henderson, S.L.; Adams, J.S.; Holick, M.F. Increased Skin Pigment Reduces the Capacity of Skin to Synthesise Vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Yao, S.; Ambrosone, C.B. Associations between Vitamin D Deficiency and Risk of Aggressive Breast Cancer in African-American Women. J. Steroid Biochem. Mol. Biol. 2013, 136, 337–341. [Google Scholar] [CrossRef]
- Mishra, D.K.; Wu, Y.; Sarkissyan, M.; Sarkissyan, S.; Chen, Z.; Shang, X.; Ong, M.; Heber, D.; Koeffler, H.P.; Vadgama, J.V. Vitamin D Receptor Gene Polymorphisms and Prognosis of Breast Cancer among African-American and Hispanic Women. PLoS ONE 2013, 8, e57967. [Google Scholar] [CrossRef]

| Objective | Search Strategy | Total Results |
|---|---|---|
| 1 | “Vitamin D” AND “Breast cancer” AND “Prevention” | n = 467 |
| “Vitamin D” AND “Breast cancer” AND “Prevention” AND “Optimal levels” | n = 14 | |
| 2 | Vitamin D” AND “Breast cancer” AND “Prevention” AND “Action mechanism” | n = 30 |
| 3 | “Vitamin D” AND “Breast cancer” AND “Prevention” AND “Risk factors” | n = 192 |
| N = 703 |
| Sample Size | Design | Significant Serum Vitamin D Levels (ng/mL) | Results | References |
|---|---|---|---|---|
| 144 (71 cases/73 controls) | Case–control study | 63.34 | The average level of vitamin D is 39.04 ng/mL and 63.34 ng/mL in cases and controls, respectively (p = 0.046). An inverse and independent association between vitamin D and BC is observed. | [32] |
| 5038 (77 cases/4961 cases) | Combined analysis of two randomized clinical trials and one prospective cohort | 42.5 | 80% less risk of BC at concentrations >60 ng/mL compared to <20 ng/ml. | [33] |
| 123,044 (25,515 cases/97,529 controls) | Meta-analysis | 27.07 | Direct association between vitamin D deficiency and BC (RR = 1.91; 95% CI = 1.5 to 2.41; p < 0.001). | [34] |
| 1104 (546 cases/558 controls) | Multicase–control study | 31 | Vitamin D levels above 27 ng/mL were associated with a 12% lower risk of postmenopausal BC. Significant dose–response trends (OR per 10 nmol/L = 0.88, 95% CI = 0.82–0.94). More pronounced protection in triple-negative tumors. | [35] |
| 156 (78 cases/78 controls) | Case–control study | 37.41 | Mean serum levels of vitamin D in cases are significantly lower compared to controls (22.33 ± 8.19 vs. 37.41 ± 12.9 ng/mL, respectively; p = 0.0001). | [36] |
| Average | 40.26 ± 14.19 ng/ml | |||
| Biological Mechanism | Objective | Conclusion | References |
|---|---|---|---|
| Vitamin D/VDR axis | To study the distribution of the FokI polymorphism (rs2228570) in VDR and its association with BC | The risk and pathogenesis of BC can be influenced by various VDR SNPs, including FokI | [49] |
| Vitamin D/VDR axis | To investigate the mechanisms involved in the expression of ERα-negative | The induction of ERα dependent on 1,25(OH)2D in ERα-negative BC cells results from the binding of the VDR-RXR complex to VDRE in the promoter region of the ERα gene, including negative regulation of enzymes with chromatin remodeling activities | [50] |
| Regulation of genes related to BC | Providing arguments that vitamin D/VD3 may include protective effects on BC through the modulation of lncRNA that are important for BC pathogenesis | The involvement of different lncRNAs in the interplay between vitamin D3/VDR signaling and the pathogenesis of BC has been identified, such as GATA3-AS1, H19, or MEG3 | [51] |
| Mechanisms involved with the immune system | Studying the association between serum vitamin D, calcium, IL-6, TNF-α, and chemerin and the progression of BC | Higher levels of IL-6, TNF-α, and chemerin were significantly associated with the presence of BC, particularly in its more advanced stages | [52] |
| Mechanisms involved with the immune system | Evaluating the impact of vitamin D on the progression of BC and the microenvironment of the mammary tumor | Vitamin D can modulate the growth and inflammation of BC tumors in the in vivo tumor microenvironment, as an increase in CD8+ T cells was observed | [53] |
| Risk Factor | Objective | Conclusion | References |
|---|---|---|---|
| Lifestyle | Study on health policies and healthy dietary practices for the prevention of BC | A healthy lifestyle may be associated with a significant reduction in the risk of BC | [88] |
| Calcium | Studying the effect of CaD supplementation on the risk of DCIS | Supplementation with CaD in postmenopausal women is associated with a reduced risk of DCIS | [89] |
| Epigenetics | Studying the relationship between vitamin D, DNA methylation, and BC | The methylation of CpG sites in genes related to vitamin D may interact with 25(OH)D to influence the risk of BC | [90] |
| Exposure to sunlight | Examining the relationship between exposure to sunlight and a lower risk of BC | Greater exposure to solar radiation is associated with a decreased risk of BC | [91] |
| Hormonal status | Assessing the association between vitamin D levels and BC in premenopausal and postmenopausal women | BC risk is inversely related to vitamin D levels, with no differences between premenopausal and postmenopausal women | [92] |
| Ethnicities | Studying the association between vitamin D and BC in African American/Black and Hispanic/Latina women | Vitamin D may have a protective effect against BC, including in African American/Black and Hispanic/Latina women | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Cameselle, C.; Otero, P.; Simal-Gandara, J. The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review. Nutrients 2024, 16, 573. https://doi.org/10.3390/nu16050573
Torres A, Cameselle C, Otero P, Simal-Gandara J. The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review. Nutrients. 2024; 16(5):573. https://doi.org/10.3390/nu16050573
Chicago/Turabian StyleTorres, Antía, Carla Cameselle, Paz Otero, and Jesus Simal-Gandara. 2024. "The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review" Nutrients 16, no. 5: 573. https://doi.org/10.3390/nu16050573
APA StyleTorres, A., Cameselle, C., Otero, P., & Simal-Gandara, J. (2024). The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review. Nutrients, 16(5), 573. https://doi.org/10.3390/nu16050573









