The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population
2.2. Measurement of Serum Copper
2.3. Examination of BMD
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
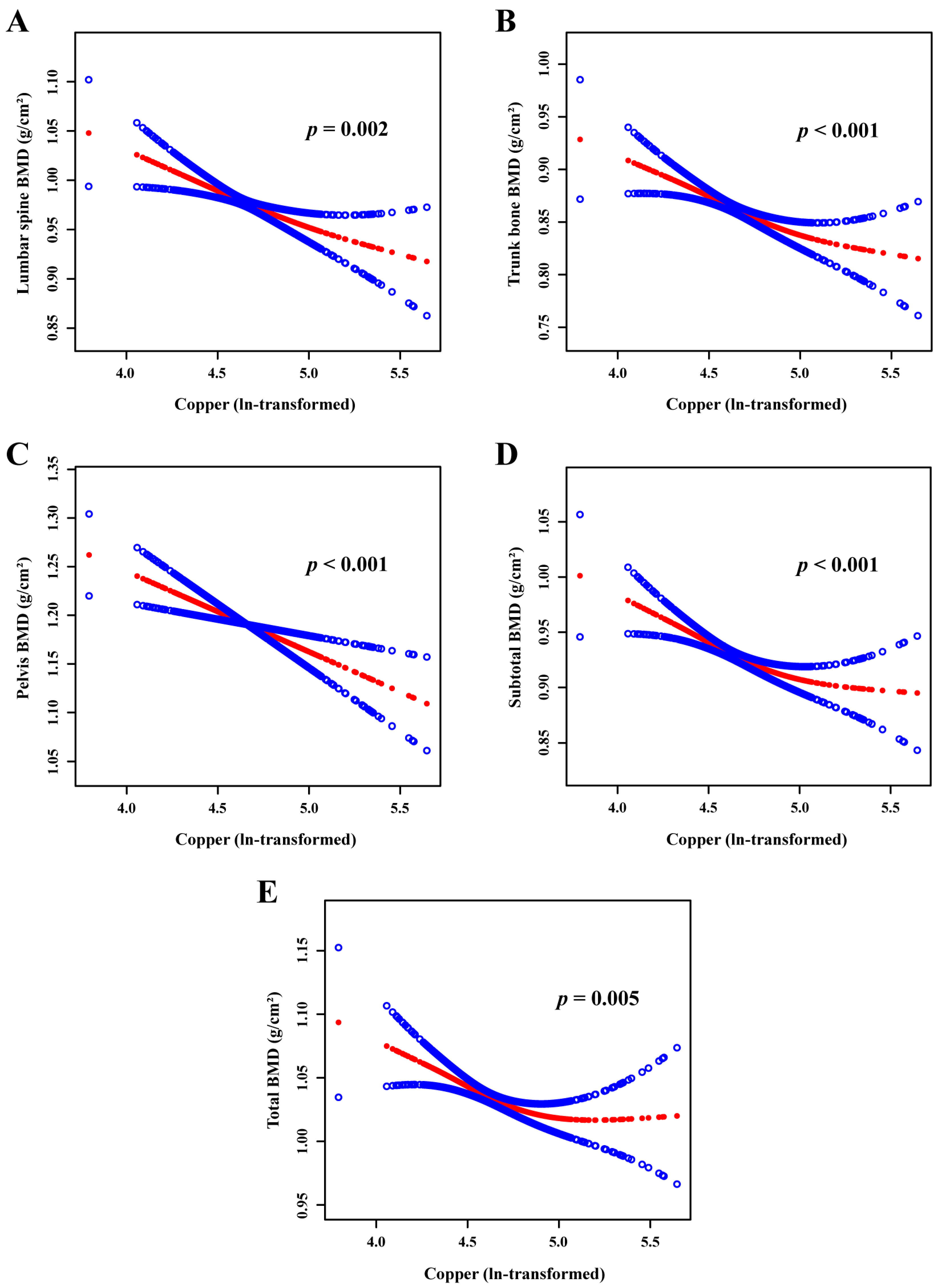
3.2. Associations between Serum Copper Level and BMD of Different Bony Sites
3.3. Subgroup Analysis of the Interactions and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Pang, L.; Yang, C.; Long, J.; Liao, Q.; Tang, P.; Huang, H.; Wei, H.; Chen, Q.; Yang, K.; et al. Effects of prenatal single and mixed bisphenol exposure on bone mineral density in preschool children: A population-based prospective cohort study. Ecotoxicol. Environ. Saf. 2023, 267, 115665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Tang, G.; Deng, P.; Qin, Y.; Han, J.; Wang, S.; Sun, X.; Li, D.; Chen, Z. The causal relationship between gut microbiota and bone mineral density: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1268935. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Kemal, O.; Lu, M.; Lix, L.M.; Leslie, W.D.; Yang, S. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005–2014. Bone 2020, 131, 115163. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, L.; An, P.; Pang, W.; Yan, X.; Deng, D.; Song, Y.; Mao, M.; Qiu, K.; Rao, Y.; et al. Low Bone Mineral Density and the Risk of Benign Paroxysmal Positional Vertigo. Otolaryngol. Head Neck Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Keirns, B.H.; Sciarrillo, C.M.; Medlin, A.R.; Hart, S.M.; Cronic, E.M.; Emerson, S.R. Whole-body bone mineral density and markers of bone homeostasis in adults with normal-weight obesity. Obes. Pillars 2023, 7, 100073. [Google Scholar] [CrossRef] [PubMed]
- Çelen, Z.E.; Onay, T. The Relationship Between Bone Mineral Density and Lumbar Disc Herniation in Postmenopausal Women. Cureus 2023, 15, e44156. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, S. Correlation between bone mineral density and sarcopenia in US adults: A population-based study. J. Orthop. Surg. Res. 2023, 18, 588. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Dong, Y.; Kang, H.; Peng, R.; Song, K.; Guo, Q.; Guan, H.; Zhu, M.; Ye, D.; Li, F. Global, Regional, and National Burden of Low Bone Mineral Density from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front. Endocrinol. 2022, 13, 870905. [Google Scholar] [CrossRef]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Peng, G.; Lu, Y.; Wang, K.; Ju, Q.; Ju, Y.; Ouyang, M. Relationship between copper and immunity: The potential role of copper in tumor immunity. Front. Oncol. 2022, 12, 1019153. [Google Scholar] [CrossRef] [PubMed]
- Karginova, O.; Weekley, C.M.; Raoul, A.; Alsayed, A.; Wu, T.; Lee, S.S.; He, C.; Olopade, O.I. Inhibition of Copper Transport Induces Apoptosis in Triple-Negative Breast Cancer Cells and Suppresses Tumor Angiogenesis. Mol. Cancer Ther. 2019, 18, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Wu, Y.; Qi, X.; Ling, Q.; Wu, Z.; Shi, Y.; Hu, H.; Yu, P.; Ma, J.; et al. Association between serum copper levels and stroke in the general population: A nationally representative study. J. Stroke Cerebrovasc. Dis. 2023, 33, 107473. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, X.; Zhang, B.; Jin, X.; Wan, Z.; Zhan, M.; Yan, J.; Zhang, P.; Ke, P.; Huang, X.; et al. Identification of Copper Metabolism Related Biomarkers, Polygenic Prediction Model, and Potential Therapeutic Agents in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Haberkiewicz, O.; Lipiński, P.; Starzyński, R.R.; Jończy, A.; Kurowska, P.; Ogórek, M.; Bednarz, A.; Herman, S.; Hatala, D.; Grzmil, P.; et al. Decreased Expression of the Slc31a1 Gene and Cytoplasmic Relocalization of Membrane CTR1 Protein in Renal Epithelial Cells: A Potent Protective Mechanism against Copper Nephrotoxicity in a Mouse Model of Menkes Disease. Int. J. Mol. Sci. 2022, 23, 11441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuan, Y.; Tian, J.; Long, F.; Luo, W. The associations between serum trace elements and bone mineral density in children under 3 years of age. Sci. Rep. 2021, 11, 1890. [Google Scholar] [CrossRef] [PubMed]
- Rył, A.; Miazgowski, T.; Szylińska, A.; Turoń-Skrzypińska, A.; Jurewicz, A.; Bohatyrewicz, A.; Rotter, I. Bone Health in Aging Men: Does Zinc and Cuprum Level Matter? Biomolecules 2021, 11, 237. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Yang, G.; Zhang, Y.; Wang, W.; Su, T.; Ma, W.; Yang, F.; Chen, L.; He, L.; et al. Correlation between bone mineral density and serum trace element contents of elderly males in Beijing urban area. Int. J. Clin. Exp. Med. 2015, 8, 19250–19257. [Google Scholar]
- Bland, V.L.; Bea, J.W.; Blew, R.M.; Roe, D.J.; Lee, V.R.; Funk, J.L.; Going, S.B. Influence of Changes in Soft Tissue Composition on Changes in Bone Strength in Peripubertal Girls: The STAR Longitudinal Study. J. Bone Min. Res. 2021, 36, 123–132. [Google Scholar] [CrossRef]
- Wang, Z.; Du, W.; Han, M.; He, L.; Zhang, H.; Hu, J.; Quan, R. Association between creatine phosphokinase level within normal range and bone mineral density in adolescents. Medicine 2023, 102, e34724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, I.; Cha, H.; Kim, H.J.; Ryu, O.H. Inverted U-Shaped Relationship between Obesity Parameters and Bone Mineral Density in Korean Adolescents. J. Clin. Med. 2023, 12, 5869. [Google Scholar] [CrossRef] [PubMed]
- CDC. Zinc, Copper and Selenium Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/CUSEZN_I_MET.PDF (accessed on 16 January 2024).
- CDC. Body Composition Procedures Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Body_Composition_DXA.pdf (accessed on 16 January 2024).
- Cui, A.; Xiao, P.; Wei, X.; Wen, H.; Liang, S.; Wang, P.; He, J.; Zhuang, Y. Associations Between Serum Selenium and Bone Mineral Density in 8–19-year-old children and adolescents: NHANES 2013–2018. Biol. Trace. Elem. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, B.; Liu, T.; Li, J.; Xiang, Y.; Fu, Y.; Zhao, M. Association of blood mercury levels with bone mineral density in adolescents aged 12–19. Environ. Sci. Pollut. Res. Int. 2023, 30, 46933–46939. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xie, Y.; Wang, L.; Huang, G.; Cheng, Y.; Hou, D.; Liu, W.; Zhang, T.; Liu, J. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients 2022, 14, 1523. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Chen, Y.; Zhang, X.; Xia, Y.; Geng, B. Association between blood manganese and bone mineral density in US adolescents. Environ. Sci. Pollut. Res. Int. 2023, 30, 29743–29754. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Xiao, P.; Hu, B.; Ma, Y.; Fan, Z.; Wang, H.; Zhou, F.; Zhuang, Y. Blood Lead Level Is Negatively Associated With Bone Mineral Density in U.S. Children and Adolescents Aged 8–19 Years. Front. Endocrinol. 2022, 13, 928752. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2000 CDC Growth Charts for the United States: Methods and Development. Available online: https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf (accessed on 16 January 2024).
- NCHS. About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 16 January 2024).
- Qu, X.; He, Z.; Qiao, H.; Zhai, Z.; Mao, Z.; Yu, Z.; Dai, K. Serum copper levels are associated with bone mineral density and total fracture. J. Orthop. Transl. 2018, 14, 34–44. [Google Scholar] [CrossRef]
- Chaudhri, M.A.; Kemmler, W.; Harsch, I.; Watling, R.J. Plasma copper and bone mineral density in osteopenia: An indicator of bone mineral density in osteopenic females. Biol. Trace Elem. Res. 2009, 129, 94–98. [Google Scholar] [CrossRef]
- Chenbhanich, J.; Thongprayoon, C.; Atsawarungruangkit, A.; Phupitakphol, T.; Cheungpasitporn, W. Osteoporosis and bone mineral density in patients with Wilson’s disease: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 315–322. [Google Scholar] [CrossRef]
- Gaier, E.D.; Kleppinger, A.; Ralle, M.; Mains, R.E.; Kenny, A.M.; Eipper, B.A. High serum Cu and Cu/Zn ratios correlate with impairments in bone density, physical performance and overall health in a population of elderly men with frailty characteristics. Exp. Gerontol. 2012, 47, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Min. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Bane, T.; Siegel, L.; Bertels, J.; Ratz, K.; Rubessa, M.; Wheeler, M. The effect of copper on the differentiation of adipose-derived stem cells into osteoblasts. Reprod. Fertil. Dev. 2019, 31, 229–230. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Mederle, O.A.; Balas, M.; Ioanoviciu, S.D.; Gurban, C.V.; Tudor, A.; Borza, C. Correlations between bone turnover markers, serum magnesium and bone mass density in postmenopausal osteoporosis. Clin. Interv. Aging 2018, 13, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.; Faulkner, R.A.; Forwood, M.R.; Mirwald, R.L.; Bailey, D.A. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J. Bone Min. Res. 2011, 26, 1729–1739. [Google Scholar] [CrossRef]
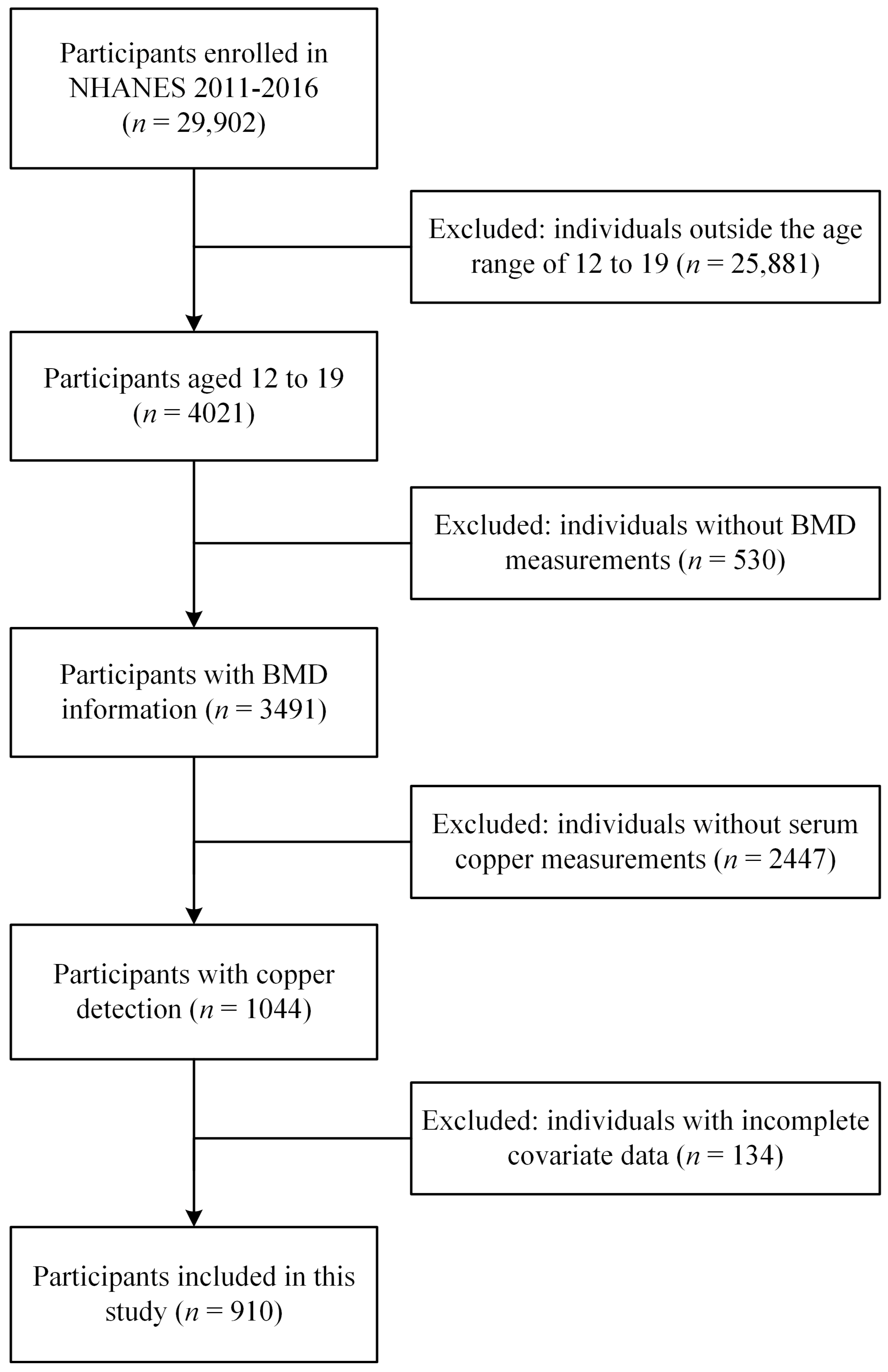
| Characteristic | Serum Copper (μg/dL) | p Value | ||||
|---|---|---|---|---|---|---|
| Total | Q1 (≤90.6) | Q2 (90.7–103.2) | Q3 (103.3–119.3) | Q4 (≥119.4) | ||
| Number of participants | 910 | 227 | 215 | 228 | 240 | |
| Age, years | 15.44 (2.15) | 15.83 (2.01) | 15.52 (2.09) | 14.84 (2.15) | 15.57 (2.24) | 0.007 |
| Gender | <0.001 | |||||
| Male | 456 (51.32) | 166 (76.56) | 109 (50.32) | 101 (48.27) | 80 (29.81) | |
| Female | 454 (48.68) | 61 (23.44) | 106(49.68) | 127 (51.73) | 160 (70.19) | |
| Race | 0.049 | |||||
| Non-Hispanic White | 239 (54.50) | 67 (56.24) | 61 (58.97) | 56 (53.69) | 55 (49.10) | |
| Non-Hispanic Black | 219 (13.58) | 38 (8.99) | 41 (9.97) | 54 (13.08) | 86 (22.29) | |
| Hispanic | 297 (22.23) | 79 (23.74) | 71 (22.92) | 78 (23.13) | 69 (19.11) | |
| Other | 155 (9.70) | 43 (11.02) | 42 (8.13) | 40 (10.10) | 30 (9.50) | |
| Poverty income ratio | 2.39 (1.53) | 2.39 (1.54) | 2.56 (1.49) | 2.34 (1.55) | 2.29 (1.55) | 0.438 |
| BMI, Kg/m2 | <0.001 | |||||
| Underweight | 23 (2.94) | 7 (3.69) | 9 (5.96) | 6 (1.57) | 1 (0.56) | |
| Normal | 512 (56.87) | 161 (69.81) | 141 (60.68) | 125 (55.44) | 85 (41.39) | |
| Overweight | 171 (18.77) | 34 (16.95) | 40 (20.07) | 43 (16.09) | 54 (22.03) | |
| Obese | 204 (21.42) | 25 (9.54) | 25 (13.28) | 54 (26.89) | 100 (36.02) | |
| Physical activity | 0.336 | |||||
| Low | 313 (30.29) | 62 (23.90) | 76 (31.61) | 81 (29.77) | 94 (35.97) | |
| Medium | 125 (13.03) | 34 (12.14) | 30 (11.94) | 32 (15.94) | 29 (12.10) | |
| High | 472 (56.68) | 131 (63.95) | 109 (56.44) | 115 (54.29) | 117 (51.93) | |
| Total calcium, mg/dL | 9.61 (0.28) | 9.61 (0.28) | 9.63 (0.27) | 9.65 (0.29) | 9.55 (0.29) | 0.063 |
| Phosphorus, mg/dL | 4.31 (0.65) | 4.28 (0.66) | 4.27 (0.64) | 4.47 (0.64) | 4.21 (0.63) | 0.004 |
| Total protein, g/dL | 7.23 (0.40) | 7.17 (0.37) | 7.26 (0.42) | 7.26 (0.41) | 7.24 (0.39) | 0.158 |
| Total cholesterol, mg/dL | 157.55 (31.38) | 150.53 (29.00) | 156.81 (34.15) | 157.05 (28.51) | 165.92 (31.87) | <0.001 |
| Lumbar spine BMD, g/cm2 | 0.97 (0.15) | 0.99 (0.15) | 0.96 (0.15) | 0.94 (0.16) | 1.00 (0.16) | 0.023 |
| Trunk bone BMD, g/cm2 | 0.86 (0.12) | 0.89 (0.12) | 0.85 (0.13) | 0.84 (0.12) | 0.86 (0.12) | 0.008 |
| Pelvis BMD, g/cm2 | 1.19 (0.18) | 1.20 (0.18) | 1.18 (0.19) | 1.17 (0.19) | 1.21 (0.18) | 0.209 |
| Subtotal BMD, g/cm2 | 0.93 (0.12) | 0.96 (0.12) | 0.92 (0.12) | 0.90 (0.11) | 0.92 (0.10) | <0.001 |
| Total BMD, g/cm2 | 1.03 (0.12) | 1.06 (0.12) | 1.02 (0.12) | 1.01 (0.12) | 1.04 (0.11) | 0.006 |
| Variable | Model 1 β (95% CI) | Model 2 β (95% CI) |
|---|---|---|
| Lumbar spine BMD | ||
| Continuous variable | −0.048 (−0.095, −0.002) * | −0.057 (−0.109, −0.005) * |
| Categorical variable | ||
| Q1 | Reference | Reference |
| Q2 | −0.042 (−0.070, −0.013) ** | −0.046 (−0.074, −0.017) ** |
| Q3 | −0.043 (−0.075, −0.010) * | −0.043 (−0.074, −0.012) ** |
| Q4 | −0.032 (−0.066, 0.001) | −0.043 (−0.079, −0.007) * |
| Trunk bone BMD | ||
| Continuous variable | −0.057 (−0.095, −0.019) ** | −0.068 (−0.110, −0.026) ** |
| Categorical variable | ||
| Q1 | Reference | Reference |
| Q2 | −0.026 (−0.048, −0.005) * | −0.030 (−0.053, −0.007) * |
| Q3 | −0.035 (−0.062, −0.008) * | −0.036 (−0.060, −0.012) ** |
| Q4 | −0.041 (−0.067, −0.015) ** | −0.052 (−0.079, −0.024) *** |
| Pelvis BMD | ||
| Continuous variable | −0.067 (−0.121, −0.013) * | −0.085 (−0.145, −0.024) ** |
| Categorical variable | ||
| Q1 | Reference | Reference |
| Q2 | −0.023 (−0.057, 0.011) | −0.030 (−0.062, 0.003) |
| Q3 | −0.033 (−0.070, 0.005) | −0.036 (−0.068, −0.004) * |
| Q4 | −0.047 (−0.086, −0.007) * | −0.062 (−0.102, −0.022)** |
| Subtotal BMD | ||
| Continuous variable | −0.063 (−0.100, −0.026) ** | −0.072 (−0.111, −0.033) *** |
| Categorical variable | ||
| Q1 | Reference | Reference |
| Q2 | −0.029 (−0.048, −0.009) ** | −0.031 (−0.051, −0.010) ** |
| Q3 | −0.038 (−0.060, −0.015) ** | −0.037 (−0.057, −0.016) ** |
| Q4 | −0.045 (−0.069, −0.021) *** | −0.053 (−0.077, −0.028) *** |
| Total BMD | ||
| Continuous variable | −0.042 (−0.073, −0.011) ** | −0.051 (−0.087, −0.016) ** |
| Categorical variable | ||
| Q1 | Reference | Reference |
| Q2 | −0.030 (−0.051, −0.010) ** | −0.032 (−0.053, −0.011) ** |
| Q3 | −0.032 (−0.054, −0.009) ** | −0.030 (−0.051, −0.009) ** |
| Q4 | −0.034 (−0.058, −0.011) ** | −0.043 (−0.068, −0.018) ** |
| Characteristic | Lumbar Spine BMD | Trunk Bone BMD | Pelvis BMD | Subtotal BMD | Total BMD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | Pinteraction | β (95% CI) | Pinteraction | β (95% CI) | Pinteraction | β (95% CI) | Pinteraction | β (95% CI) | Pinteraction | |
| Age (years) | 0.302 | 0.022 | 0.018 | 0.103 | 0.160 | |||||
| 12–15 (n = 473) | −0.105 (−0.185, −0.025) | −0.124 (−0.182, −0.067) | −0.165 (−0.248, −0.082) | −0.115 (−0.175, −0.054) | −0.088 (−0.149, −0.028) | |||||
| 16–19 (n = 437) | −0.055 (−0.119, 0.009) | −0.055 (−0.104, −0.007) | −0.060 (−0.126, 0.006) | −0.069 (−0.109, −0.029) | −0.045 (−0.085, −0.005) | |||||
| Gender | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Male (n = 456) | −0.177 (−0.245, −0.109) | −0.173 (−0.225, −0.122) | −0.211 (−0.283, −0.140) | −0.182 (−0.229, −0.134) | −0.161 (−0.204, −0.117) | |||||
| Female (n = 454) | 0.005 (−0.049, 0.059) | −0.011 (−0.058, 0.035) | −0.015 (−0.088, 0.057) | −0.017 (−0.059, 0.025) | 0.004 (−0.038, 0.046) | |||||
| Race | 0.566 | 0.589 | 0.815 | 0.379 | 0.255 | |||||
| Non-Hispanic White (n = 239) | −0.065 (−0.142, 0.012) | −0.087 (−0.149, −0.025) | −0.103 (−0.189, −0.016) | −0.093 (−0.157, −0.030) | −0.065 (−0.122, −0.008) | |||||
| Non-Hispanic Black (n = 219) | −0.049 (−0.116, 0.017) | −0.063 (−0.120, −0.007) | −0.113 (−0.193, −0.033) | −0.052 (−0.097, −0.007) | −0.031 (−0.087, 0.026) | |||||
| Hispanic (n = 297) | −0.122 (−0.197, −0.046) | −0.106 (−0.168, −0.044) | −0.112 (−0.197, −0.027) | −0.111 (−0.159, −0.063) | −0.102 (−0.156, −0.049) | |||||
| Other (n = 155) | −0.086 (−0.191, 0.020) | −0.044 (−0.121, 0.034) | −0.053 (−0.143, 0.036) | −0.054 (−0.115, 0.006) | −0.016 (−0.089, 0.058) | |||||
| BMI | 0.661 | 0.457 | 0.496 | 0.590 | 0.765 | |||||
| Underweight (n = 23) | −0.125 (−0.292, 0.043) | −0.036 (−0.197, 0.126) | 0.090 (−0.208, 0.389) | −0.078 (−0.251, 0.095) | −0.089 (−0.264, 0.086) | |||||
| Normal (n = 512) | −0.058 (−0.116, −0.001) | −0.063 (−0.109, −0.017) | −0.086 (−0.151, −0.021) | −0.068 (−0.111, −0.025) | −0.048 (−0.090, −0.005) | |||||
| Overweight (n = 171) | −0.124 (−0.235, −0.014) | −0.132 (−0.216, −0.048) | −0.140 (−0.226, −0.054) | −0.112 (−0.195, −0.029) | −0.080 (−0.176, 0.015) | |||||
| Obese (n = 204) | −0.071 (−0.198, 0.057) | −0.100 (−0.207, 0.008) | −0.132 (−0.291, 0.026) | −0.117 (−0.213, −0.021) | −0.088 (−0.187, 0.010) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Bao, M.; Liu, M.; Deng, F.; Wen, X.; Wan, P.; Lin, X.; Dong, G.; Li, Z.; Han, J. The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States. Nutrients 2024, 16, 453. https://doi.org/10.3390/nu16030453
Liu H, Bao M, Liu M, Deng F, Wen X, Wan P, Lin X, Dong G, Li Z, Han J. The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States. Nutrients. 2024; 16(3):453. https://doi.org/10.3390/nu16030453
Chicago/Turabian StyleLiu, Haobiao, Miaoye Bao, Mian Liu, Feidan Deng, Xinyue Wen, Ping Wan, Xue Lin, Guoqiang Dong, Zhaoyang Li, and Jing Han. 2024. "The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States" Nutrients 16, no. 3: 453. https://doi.org/10.3390/nu16030453
APA StyleLiu, H., Bao, M., Liu, M., Deng, F., Wen, X., Wan, P., Lin, X., Dong, G., Li, Z., & Han, J. (2024). The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States. Nutrients, 16(3), 453. https://doi.org/10.3390/nu16030453







