Exploring Lifestyle and Dietary Patterns in Pregnancy and Their Impact on Health: A Comparative Analysis of Two Distinct Groups 10 Years Apart
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric Assessment and Socioeconomic and Lifestyle Data
2.3. Dietary Assessment
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Nutrient Intake and Dietary Patterns
3.3. Associations between Food Groups and Dietary Patterns with Gestational Weight Gain and Pre-Gestational BMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, A.; Stephanou, A.; Rayman, M.P. Dietary Factors That Affect the Risk of Pre-Eclampsia. BMJ Nutr. Prev. Health 2022, 5, 118–133. [Google Scholar] [CrossRef]
- Li, G.; Wei, T.; Ni, W.; Zhang, A.; Zhang, J.; Xing, Y.; Xing, Q. Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front. Endocrinol. 2020, 11, 636. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, Q.; Jiang, X.; Chen, X.; Liao, Y.; Pan, Y. The Effect of Diet Quality on the Risk of Developing Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1062304. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Wibaek, R.; Poullas, M. The Dual Burden of Malnutrition Increases the Risk of Cesarean Delivery: Evidence from India. Front. Public Health 2018, 6, 292. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Addor, M.-C.; Ballardini, E.; Barisic, I.; Barrachina-Bonet, L.; Braz, P.; Cavero-Carbonell, C.; Den Hond, E.; Garne, E.; Gatt, M.; et al. Prevention of Neural Tube Defects in Europe: A Public Health Failure. Front. Pediatr. 2021, 9, 647038. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Saros, L.; Lind, A.; Setänen, S.; Tertti, K.; Koivuniemi, E.; Ahtola, A.; Haataja, L.; Shivappa, N.; Hébert, J.R.; Vahlberg, T.; et al. Maternal Obesity, Gestational Diabetes Mellitus, and Diet in Association with Neurodevelopment of 2-Year-Old Children. Pediatr. Res. 2023, 94, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Ehrhardt, R.A. Regulation of Placental Nutrient Transport and Implications for Fetal Growth. Nutr. Res. Rev. 2002, 15, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, Z.; Fallah, A.; Moravejolahkami, A.R. Maternal Dietary Patterns and Their Association with Pregnancy Outcomes. Clin. Nutr. Res. 2019, 8, 64–73. [Google Scholar] [CrossRef]
- Louvigne, M.; Rouleau, S.; Caldagues, E.; Souto, I.; Montcho, Y.; Bouvagnet, A.M.; Baud, O.; Carel, J.C.; Gascoin, G.; Coutant, R. Association of Maternal Nutrition with Transient Neonatal Hyperinsulinism. PLoS ONE 2018, 13, e0195383. [Google Scholar] [CrossRef]
- Pullar, J.; Wickramasinghe, K.; Demaio, A.R.; Roberts, N.; Perez-Blanco, K.-M.; Noonan, K.; Townsend, N. The Impact of Maternal Nutrition on Offspring’s Risk of Non-Communicable Diseases in Adulthood: A Systematic Review. J. Glob. Health 2019, 9, 020405. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.-X.; Cohen, H.J.; Wang, H.; Li, W.; Wang, T.; Xu, T.; Liu, A.; Gai, M.-Y.; Ying, S.; et al. Evidence of a Relationship between Infant Birth Weight and Later Diabetes and Impaired Glucose Regulation in a Chinese Population. Diabetes Care 2008, 31, 483–487. [Google Scholar] [CrossRef]
- Bleker, L.S.; de Rooij, S.R.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Cohort Profile: The Dutch Famine Birth Cohort (DFBC)—A Prospective Birth Cohort Study in the Netherlands. BMJ Open 2021, 11, e042078. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-X.; Wang, J.-J.; Lei, Y.-X.; Xiao, L.; Luo, Z.-C. Impact of Fetal and Infant Exposure to the Chinese Great Famine on the Risk of Hypertension in Adulthood. PLoS ONE 2012, 7, e49720. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Variyam, J.N.; Blaylock, J.; Lin, B.-H.; Ralston, K.; Smallwood, D. Mother’s Nutrition Knowledge and Children’s Dietary Intakes. Am. J. Agric. Econ. 1999, 81, 373–384. [Google Scholar] [CrossRef]
- Lobo, S.; Lucas, C.J.; Herbert, J.S.; Townsend, M.L.; Smith, M.; Kunkler, E.; Charlton, K.E. Nutrition Information in Pregnancy: Where Do Women Seek Advice and Has This Changed over Time? Nutr. Diet. 2020, 77, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.; Foster, G.D. Food Environment and Obesity. Obesity 2014, 22, 2459–2461. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Vogel, C.; Godfrey, K.M.; Baird, J.; Harvey, N.C.; Hanson, M.A.; Cooper, C.; Inskip, H.M.; Crozier, S.R. Longitudinal Dietary Trajectories from Preconception to Mid-Childhood in Women and Children in the Southampton Women’s Survey and Their Relation to Offspring Adiposity: A Group-Based Trajectory Modelling Approach. Int. J. Obes. 2022, 46, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Inskip, H.M.; Godfrey, K.M.; Robinson, S.M. Dietary Patterns in Pregnant Women: A Comparison of Food-Frequency Questionnaires and 4 d Prospective Diaries. Br. J. Nutr. 2008, 99, 869–875. [Google Scholar] [CrossRef]
- Flynn, A.C.; Seed, P.T.; Patel, N.; Barr, S.; Bell, R.; Briley, A.L.; Godfrey, K.M.; Nelson, S.M.; Oteng-Ntim, E.; Robinson, S.M.; et al. Dietary Patterns in Obese Pregnant Women; Influence of a Behavioral Intervention of Diet and Physical Activity in the UPBEAT Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 124. [Google Scholar] [CrossRef]
- Quaresima, P.; Visconti, F.; Interlandi, F.; Puccio, L.; Caroleo, P.; Amendola, G.; Morelli, M.; Venturella, R.; Di Carlo, C. Awareness of Gestational Diabetes Mellitus Foetal-Maternal Risks: An Italian Cohort Study on Pregnant Women. BMC Pregnancy Childbirth 2021, 21, 692. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Gillespie, S.; Satya, S.; Gaudet, L.M. Assessing the Accuracy of Pregnant Women in Recalling Pre-Pregnancy Weight and Gestational Weight Gain. J. Obstet. Gynaecol. Can. 2013, 35, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Schieve, L.A.; Perry, G.S.; Cogswell, M.E.; Scanion, K.S.; Rosenberg, D.; Carmichael, S.; Ferre, C. Validity of Self-Reported Pregnancy Delivery Weight: An Analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am. J. Epidemiol. 1999, 150, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Headen, I.; Cohen, A.K.; Mujahid, M.; Abrams, B. The Accuracy of Self-Reported Pregnancy-Related Weight: A Systematic Review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 350–369. [Google Scholar] [CrossRef]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-Mass Index and All-Cause Mortality: Individual-Participant-Data Meta-Analysis of 239 Prospective Studies in Four Continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines; The National Academies Collection: Reports funded by National Institutes of Health; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13113-1. [Google Scholar]
- Popa, A.D.; Niţă, O.; Popescu, R.M.; Gherasim, A.; Arhire, L.I.; Mihalache, L.; Graur, M. Determinants of Inadequate Weight Gain in Pregnancy. Rev. Med. Chir. Soc. Med. Nat. 2014, 118, 352–358. [Google Scholar]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. EPIC-Norfolk FFQ Study: A New Tool for Converting Food Frequency Questionnaire Data into Nutrient and Food Group Values: FETA Research Methods and Availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef]
- Gherasim, A.; Arhire, L.I.; Nita, O.; Strateanu, R.; Oprescu, A.C.; Graur, M.; Mihalache, L. Can the Epic Food Frequency Questionnaire Be Applied to the Population in Romania? Med.-Surg. J. 2015, 119, 856–863. [Google Scholar]
- Chia, A.-R.; Chen, L.-W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Schulze, M.B. Metabolic Health in Normal-Weight and Obese Individuals. Diabetologia 2019, 62, 558–566. [Google Scholar] [CrossRef]
- Fallah, F.; Pourabbas, A.; Delpisheh, A.; Veisani, Y.; Shadnoush, M. Effects of Nutrition Education on Levels of Nutritional Awareness of Pregnant Women in Western Iran. Int. J. Endocrinol. Metab. 2013, 11, 175–178. [Google Scholar] [CrossRef]
- Goodarzi-Khoigani, M.; Baghiani Moghadam, M.H.; Nadjarzadeh, A.; Mardanian, F.; Fallahzadeh, H.; Mazloomy-Mahmoodabad, S. Impact of Nutrition Education in Improving Dietary Pattern during Pregnancy Based on Pender’s Health Promotion Model: A Randomized Clinical Trial. Iran. J. Nurs. Midwifery Res. 2018, 23, 18–25. [Google Scholar] [CrossRef]
- Liu, N.; Mao, L.; Sun, X.; Liu, L.; Yao, P.; Chen, B. The Effect of Health and Nutrition Education Intervention on Women’s Postpartum Beliefs and Practices: A Randomized Controlled Trial. BMC Public Health 2009, 9, 45. [Google Scholar] [CrossRef]
- Protocol Privind Metodologia Efectuarii Consultatiei Prenatale si Postnatale Documentate in Carnetul Gravidei. Available online: https://www.insmc.ro/wp-content/uploads/2021/11/Protocol-privind-metodologia-efectuarii-consultatie-prenatala-si-postnatale.pdf (accessed on 13 January 2024).
- Torna, E.; Fitzgerald, J.D.; Nelson, D.S.; Andrade, J.M. Perceptions, Barriers, and Strategies Towards Nutrition Counseling in Healthcare Clinics: A Survey among Health Care Providers and Adult Patients. SN Compr. Clin. Med. 2021, 3, 145–157. [Google Scholar] [CrossRef]
- Carter, C.; Harnett, J.E.; Krass, I.; Gelissen, I.C. A Review of Primary Healthcare Practitioners’ Views about Nutrition: Implications for Medical Education. Int. J. Med. Educ. 2022, 13, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Macaninch, E.; Buckner, L.; Amin, P.; Broadley, I.; Crocombe, D.; Herath, D.; Jaffee, A.; Carter, H.; Golubic, R.; Rajput-Ray, M.; et al. Time for Nutrition in Medical Education. BMJ Nutr. Prev. Health 2020, 3, 40–48. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of Neural Tube Defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.-H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in Neural-Tube Defects after Folic Acid Fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Avram, C.; Bucur, O.M.; Zazgyva, A.; Avram, L.; Ruta, F. Vitamin Supplementation in Pre-Pregnancy and Pregnancy among Women-Effects and Influencing Factors in Romania. Int. J. Environ. Res. Public Health 2022, 19, 8503. [Google Scholar] [CrossRef]
- Nelson, E.A.; Taylor, B.J. International Child Care Practices Study: Infant Sleep Position and Parental Smoking. Early Hum. Dev. 2001, 64, 7–20. [Google Scholar] [CrossRef]
- Shea, A.K.; Steiner, M. Cigarette Smoking during Pregnancy. Nicotine Tob. Res. 2008, 10, 267–278. [Google Scholar] [CrossRef]
- Wickström, R. Effects of Nicotine during Pregnancy: Human and Experimental Evidence. Curr. Neuropharmacol. 2007, 5, 213–222. [Google Scholar] [CrossRef]
- Liu, B.; Xu, G.; Sun, Y.; Qiu, X.; Ryckman, K.K.; Yu, Y.; Snetselaar, L.G.; Bao, W. Maternal Cigarette Smoking before and during Pregnancy and the Risk of Preterm Birth: A Dose-Response Analysis of 25 Million Mother-Infant Pairs. PLoS Med. 2020, 17, e1003158. [Google Scholar] [CrossRef]
- Sikorska-Jaroszyńska, M.H.J.; Mielnik-Błaszczak, M.; Krawczyk, D.; Nasiłowska-Barud, A.; Błaszczak, J. Passive Smoking as an Environmental Health Risk Factor. Ann. Agric. Environ. Med. 2012, 19, 547–550. [Google Scholar]
- Rushton, L. Health Impact of Environmental Tobacco Smoke in the Horne. Rev. Environ. Health 2021, 19, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.; Townshend, T. Obesogenic Environments: Exploring the Built and Food Environments. J. R. Soc. Promot. Health 2006, 126, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, Z.M.; Barrowman, N.; Prud’homme, D.; Walker, M.; Wen, S.W.; Rodger, M.; Adamo, K.B. Excessive Gestational Weight Gain Predicts Large for Gestational Age Neonates Independent of Maternal Body Mass Index. J. Matern.-Fetal Neonatal Med. 2012, 25, 538–542. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Currie, J. The Association between Pregnancy Weight Gain and Birthweight: A within-Family Comparison. Lancet 2010, 376, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.L.; Rahman, M.A.; Macey, S.; Atkinson, M.D.; Hill, R.A.; Khanom, A.; Paranjothy, S.; Husain, M.J.; Brophy, S.T. Obesity in Pregnancy: A Retrospective Prevalence-Based Study on Health Service Utilisation and Costs on the NHS. BMJ Open 2014, 4, e003983. [Google Scholar] [CrossRef] [PubMed]
- Adamo, K.B.; Ferraro, Z.M.; Brett, K.E. Can We Modify the Intrauterine Environment to Halt the Intergenerational Cycle of Obesity? Int. J. Environ. Res. Public Health 2012, 9, 1263–1307. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Romania: Spending on Catering Services. 2022. Available online: https://www.statista.com/statistics/651483/catering-services-consumption-expenditure-volume-romania/ (accessed on 14 January 2024).
- Stanciu, S.; Oana, V.; Maria-Alexandra, S.; Bratoveanu, B. Food Retail in Romania. Market Overview. In Proceedings of the BASIQ International Conference: New Trends in Sustainable Business and Consumption, Bari, Italy, 30 May–1 June 2019. [Google Scholar]
- European Observatory on Health Systems and Policies. Romania: Country Health Profile. 2023. Available online: https://eurohealthobservatory.who.int/publications/m/romania-country-health-profile-2023 (accessed on 13 January 2024).
- Ferretti, F.; Mariani, M. Sugar-Sweetened Beverage Affordability and the Prevalence of Overweight and Obesity in a Cross Section of Countries. Glob. Health 2019, 15, 30. [Google Scholar] [CrossRef]
- Cohen, J.F.W.; Rifas-Shiman, S.L.; Young, J.; Oken, E. Associations of Prenatal and Child Sugar Intake with Child Cognition. Am. J. Prev. Med. 2018, 54, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Gamba, R.J.; Leung, C.W.; Petito, L.; Abrams, B.; Laraia, B.A. Sugar Sweetened Beverage Consumption during Pregnancy Is Associated with Lower Diet Quality and Greater Total Energy Intake. PLoS ONE 2019, 14, e0215686. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but Bitter: Focus on Fructose Impact on Brain Function in Rodent Models. Nutrients 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; López-Sánchez, P.; Ramos-Tovar, E. Fructose and the Liver. Int. J. Mol. Sci. 2021, 22, 6969. [Google Scholar] [CrossRef]
- Loy, S.-L.; Jan Mohamed, H.J.B. Relative Validity of Dietary Patterns during Pregnancy Assessed with a Food Frequency Questionnaire. Int. J. Food Sci. Nutr. 2013, 64, 668–673. [Google Scholar] [CrossRef]
- Jacques, P.F.; Tucker, K.L. Are Dietary Patterns Useful for Understanding the Role of Diet in Chronic Disease? Am. J. Clin. Nutr. 2001, 73, 1–2. [Google Scholar] [CrossRef]
- Rangel Bousquet Carrilho, T.; Rasmussen, K.M.; Rodrigues Farias, D.; Freitas Costa, N.C.; Araújo Batalha, M.; Reichenheim, M.E.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G.; Oliveira, A.E.; et al. Agreement between Self-Reported Pre-Pregnancy Weight and Measured First-Trimester Weight in Brazilian Women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar] [CrossRef] [PubMed]
| Percentiles | ||||||
|---|---|---|---|---|---|---|
| n | Median | 25th | 50th | 75th | ||
| Age (years) | 2013 | 400 | 28 | 23 | 28 | 31 |
| 2023 | 251 | 26 | 22.5 | 26 | 30 | |
| Pre-gestational BMI (kg/m2) | 2013 | 387 | 21.33 | 19.57 | 21.32 | 24.43 |
| 2023 | 244 | 24.35 | 21.1 | 24.35 | 27.59 | |
| Gestational weight gain (kg) | 2013 | 382 | 14 | 10 | 14 | 17 |
| 2023 | 244 | 6.7 | 2 | 6.7 | 12.23 | |
| No. of pregnancies | 2013 | 400 | 2 | 1 | 2 | 3 |
| 2023 | 251 | 1 | 1 | 1 | 2 | |
| Gestational age (weeks) | 2013 | 400 | 39 | 38 | 39 | 40 |
| 2023 | 251 | 39 | 38 | 39 | 40 | |
| Parameter | 2013 | 2023 | |
|---|---|---|---|
| n (%) | n (%) | ||
| Area of residence | urban | 217 (54.3) | 103 (41) |
| rural | 183 (45.8) | 148 (59) | |
| Education level | primary school | 16 (4) | 19 (7,6) |
| middle school | 86 (21.5) | 85 (33.9) | |
| high school | 162 (40.5) | 79 (31.5) | |
| university | 136 (34) | 68 (27.1) | |
| Smoke status | no | 354 (88.5) | 167 (66.5) |
| yes | 44 (11) | 58 (23.1) | |
| former | 2 (0.5) | 26 (10.4) | |
| Diet advice | yes | 169 (42.3) | 37 (14.7) |
| no | 231 (57.8) | 214 (85.3) | |
| Folate supplements | yes | 193 (48.3) | 20 (8) |
| no | 207 (51.7) | 231 (92) | |
| Other supplements | yes | 267 (68.8) | 84 (33.5) |
| no | 133 (31.2) | 167 (66.5) | |
| BMI | normal weight | 331 (82.75) | 142 (58.19) |
| overweight | 64 (16) | 55 (22.54) | |
| obese | 5 (1.25) | 47 (19.26) | |
| Gestational weight gain | adequate | 160 (41.88) | 150 (61) |
| inadequate | 91 (23.82) | 39 (15.9) | |
| excessive | 131 (34.29) | 57 (23.2) | |
| Year | Mean | Median | SD | p | p 1 | |
|---|---|---|---|---|---|---|
| Energy_kcal | 2013 | 1835.92 | 1796.14 | 463.46 | <0.001 | |
| 2023 | 1992.53 | 1927.44 | 537.29 | |||
| Total CH | 2013 | 212.83 | 211.20 | 60.24 | <0.001 | 0.96 |
| 2023 | 264.66 | 258.11 | 74.77 | |||
| Fiber | 2013 | 115.15 | 114.55 | 36.53 | <0.001 | 0.99 |
| 2023 | 156.82 | 154.18 | 46.55 | |||
| Total fat | 2013 | 75.54 | 72.29 | 23.41 | <0.001 | 0.93 |
| 2023 | 75.27 | 70.86 | 25.85 | |||
| MUFA | 2013 | 28.50 | 26.98 | 10.35 | 0.002 | 0.51 |
| 2023 | 26.14 | 24.36 | 10.16 | |||
| PUFA | 2013 | 11.57 | 11.13 | 3.87 | <0.001 | 0.62 |
| 2023 | 14.54 | 13.46 | 6.14 | |||
| SFA | 2013 | 28.24 | 26.82 | 8.74 | <0.001 | 0.23 |
| 2023 | 27.75 | 26.15 | 10.17 | |||
| Cholesterol | 2013 | 398.76 | 383.88 | 152.91 | 0.001 | 0.51 |
| 2023 | 362.96 | 346.04 | 139.94 | |||
| Protein | 2013 | 89.00 | 85.88 | 28.58 | <0.001 | 0.26 |
| 2023 | 80.41 | 77.87 | 21.68 | |||
| Retinol | 2013 | 2464.41 | 1254.56 | 3385.45 | <0.001 | 0.03 |
| 2023 | 755.94 | 361.65 | 975.60 | |||
| Vitamin B12 | 2013 | 11.26 | 7.86 | 11.46 | <0.001 | <0.001 |
| 2023 | 4.94 | 4.01 | 3.78 | |||
| Folate | 2013 | 247.39 | 229.64 | 81.83 | <0.001 | 0.12 |
| 2023 | 210.46 | 203.18 | 62.41 | |||
| Vitamin D | 2013 | 2.79 | 2.56 | 1.40 | <0.001 | 0.61 |
| 2023 | 2.09 | 1.89 | 1.15 | |||
| Vitamin E | 2013 | 10.79 | 9.91 | 4.88 | <0.001 | 0.42 |
| 2023 | 12.55 | 11.85 | 4.73 | |||
| Calcium | 2013 | 859.30 | 843.29 | 193.55 | <0.001 | 0.30 |
| 2023 | 812.39 | 779.39 | 318.21 | |||
| Iron | 2013 | 10.12 | 9.60 | 3.42 | 0.99 | 0.21 |
| 2023 | 9.88 | 9.83 | 2.60 | |||
| Iodine | 2013 | 131.39 | 128.72 | 33.04 | 0.81 | 0.40 |
| 2023 | 134.33 | 127.60 | 52.42 | |||
| Magnesium | 2013 | 248.59 | 240.93 | 64.95 | 0.83 | 0.15 |
| 2023 | 251.13 | 239.62 | 70.40 | |||
| Sodium | 2013 | 2577.43 | 2527.03 | 781.61 | <0.001 | 0.74 |
| 2023 | 2960.45 | 2923.41 | 984.57 | |||
| Selenium | 2013 | 84.60 | 84.02 | 28.38 | <0.001 | 0.94 |
| 2023 | 77.68 | 76.93 | 21.99 | |||
| Zinc | 2013 | 9.43 | 9.24 | 2.94 | <0.001 | 0.65 |
| 2023 | 8.34 | 7.92 | 2.45 |
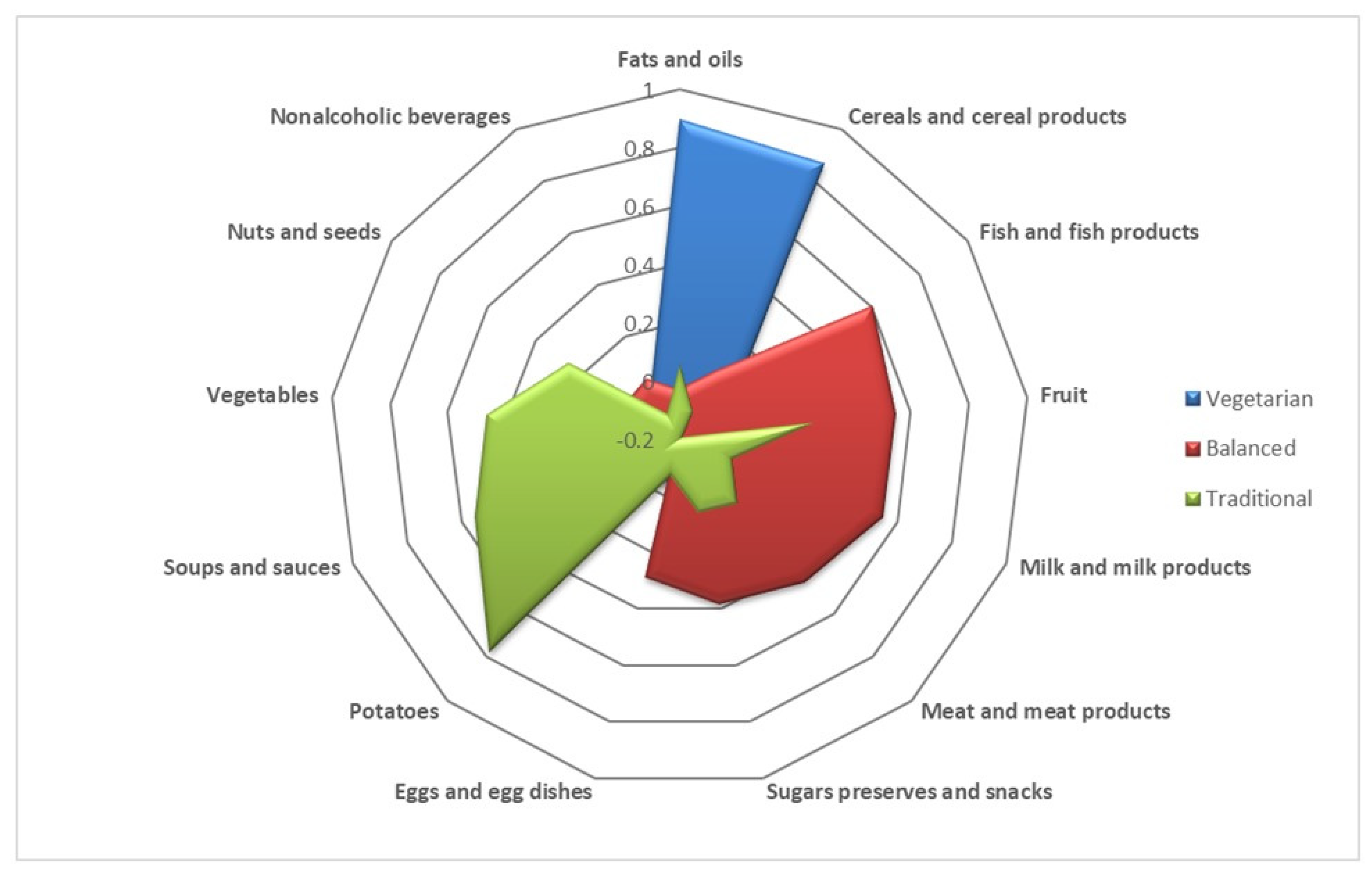
| Food Groups | Dietary Pattern | ||
|---|---|---|---|
| Vegetarian | Balanced | Traditional | |
| Fats and oils | 0.89811 | −0.0264 | 0.05277 |
| Cereals and cereal products | 0.87099 | 0.0691 | −0.10012 |
| Fish and fish products | 0.01653 | 0.6058 | −0.19065 |
| Fruit | 0.11378 | 0.5486 | 0.26453 |
| Milk and milk products | −0.03476 | 0.5477 | −0.00698 |
| Meat and meat products | 0.07633 | 0.4522 | 0.09564 |
| Sugars, preserves, and snacks | 0.28113 | 0.3819 | 0.06046 |
| Eggs and egg dishes | −0.05401 | 0.2871 | −0.06703 |
| Potatoes | −0.00119 | −0.1888 | 0.77581 |
| Soups and sauces | 0.15452 | 0.1628 | 0.54711 |
| Vegetables | −0.14215 | 0.2807 | 0.46199 |
| Nuts and seeds | −0.0121 | 0.0207 | 0.25932 |
| Non-alcoholic beverages | 0.00446 | 0.0373 | −0.10501 |
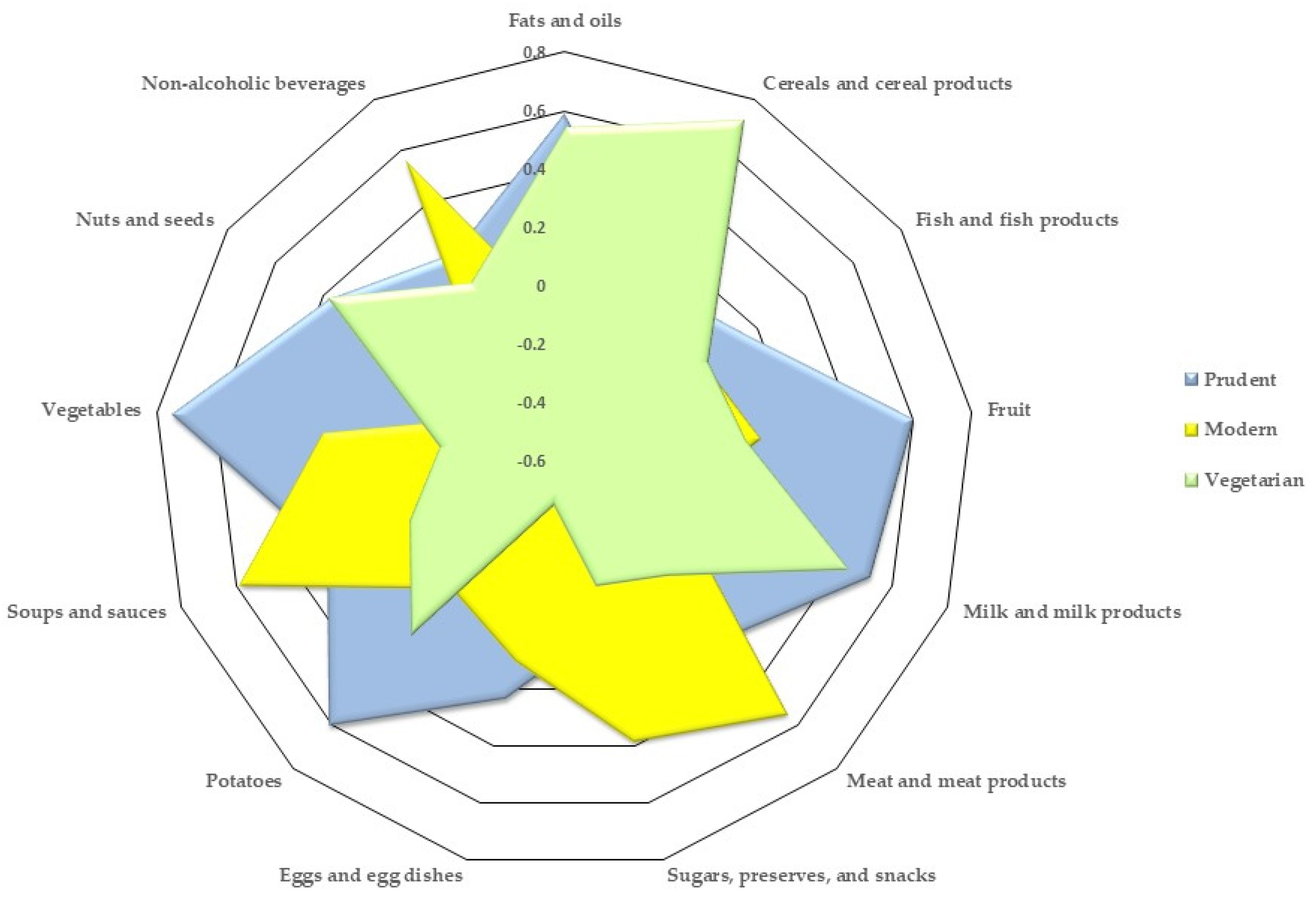
| Food Groups | Dietary Pattern | ||
|---|---|---|---|
| Prudent | Modern | Vegetarian | |
| Vegetables | 0.7489 | 0.2259 | −0.17117 |
| Fruit | 0.6015 | 0.0773 | 0.0177 |
| Soups and sauces | 0.5866 | 0.2647 | −0.03526 |
| Milk and milk products | 0.5201 | −0.1906 | 0.43748 |
| Fish and fish products | 0.1603 | −0.0155 | −0.00433 |
| Potatoes | −0.0211 | 0.605 | 0.19334 |
| Fats and oils | −0.1141 | 0.5879 | 0.54456 |
| Non-alcoholic beverages | 0.2045 | 0.5634 | 0.08878 |
| Meat and meat products | 0.2135 | 0.5495 | −0.07521 |
| Sugars, preserves, and snacks | −0.0193 | 0.3839 | −0.16185 |
| Cereals and cereal products | 0.0805 | 0.2474 | 0.71934 |
| Eggs and egg dishes | 0.2361 | 0.0962 | −0.445 |
| Nuts and seeds | 0.3759 | −0.3285 | 0.38559 |
| Parameters | 2013 | 2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Gestational BMI | Gestational Weight Gain | Pre-Gestational BMI | Gestational Weight Gain | |||||
| r | p | r | p | r | p | r | p | |
| Cereals and cereal products | 0.052 | 0.309 | 0.098 | 0.057 | 0.125 | 0.051 | −0.003 | 0.964 |
| Eggs and egg dishes | 0.043 | 0.397 | 0.138 | 0.007 | 0.133 | 0.038 | 0.031 | 0.630 |
| Fats and oils | 0.049 | 0.340 | 0.088 | 0.085 | 0.076 | 0.236 | 0.200 | 0.002 |
| Fish and fish products | 0.002 | 0.970 | 0.141 | 0.006 | 0.045 | 0.487 | −0.044 | 0.492 |
| Fruit | 0.068 | 0.180 | 0.073 | 0.154 | 0.005 | 0.938 | 0.032 | 0.617 |
| Meat and meat products | 0.031 | 0.540 | 0.052 | 0.310 | 0.050 | 0.439 | 0.021 | 0.742 |
| Milk and milk products | 0.041 | 0.416 | 0.120 | 0.019 | −0.091 | 0.157 | 0.062 | 0.338 |
| Non-alcoholic beverages | −0.004 | 0.931 | −0.026 | 0.616 | 0.001 | 0.987 | 0.142 | 0.027 |
| Nuts and seeds | 0.072 | 0.156 | −0.069 | 0.177 | 0.082 | 0.203 | 0.006 | 0.926 |
| Potatoes | 0.102 | 0.046 | −0.066 | 0.200 | 0.035 | 0.588 | 0.036 | 0.573 |
| Soups and sauces | 0.049 | 0.337 | 0.006 | 0.909 | −0.015 | 0.810 | −0.019 | 0.768 |
| Sugars, preserves, and snacks | −0.027 | 0.597 | 0.044 | 0.394 | −0.065 | 0.310 | 0.075 | 0.246 |
| Vegetables | 0.044 | 0.390 | 0.069 | 0.176 | −0.063 | 0.325 | 0.076 | 0.238 |
| Vegetarian 2013 | 0.039 | 0.439 | 0.086 | 0.092 | - | - | - | - |
| Balanced 2013 | 0.94 | 0.066 | 0.180 | <0.001 | - | - | - | - |
| Traditional 2013 | 0.128 | 0.012 | −0.049 | 0.338 | - | - | - | - |
| Prudent 2023 | - | - | - | - | −0.016 | 0.808 | 0.064 | 0.318 |
| Modern 2023 | - | - | - | - | 0.051 | 0.429 | 0.136 | 0.034 |
| Vegetarian 2023 | - | - | - | - | 0.069 | 0.284 | 0.018 | 0.777 |
| GWG | 2013 | 2023 | ||||
|---|---|---|---|---|---|---|
| p | OR (95% CI) | p | OR (95% CI) | |||
| Model 1 | ||||||
| Inadequate | vegetarian vs. traditional pattern | 0.822 | 0.929 (0.48–1.77) | prudent vs. vegetarian pattern | 0.094 | 0.487 (0.21–1.13) |
| balanced vs. traditional pattern | 0.898 | 0.96 (0.51–1.80) | modern vs. vegetarian pattern | 0.012 | 0.317 (0.12–0.77) | |
| Excessive | vegetarian vs. traditional pattern | 0.489 | 1.229 (0.68–2.20) | prudent vs. vegetarian pattern | 0.225 | 0.626 (0.29–1.33) |
| balanced vs. traditional pattern | 0.242 | 1.402 (0.79–2.47) | modern vs. vegetarian pattern | 0.11 | 0.543 (0.25–1.14) | |
| Model 2 | ||||||
| Inadequate | environment | 0.572 | 1.196 (0.64–2.22) | environment | 0.752 | 1.136 (0.51–2.50) |
| age | 0.064 | 0.946 (0.89–1.00) | age | 0.428 | 0.969 (0.89–1.04) | |
| education | 0.66 | 0.912 (0.60–1.37) | education | 0.992 | 0.998 (0.65–1.52) | |
| smoking status | 0.37 | 1.505 (0.61–3.68) | smoking status | 0.726 | 0.903 (0.51–1.59) | |
| dietary advice | 0.787 | 1.079 (0.62–1.87) | dietary advice | 0.051 | 2.599 (0.99–6.79) | |
| multiparous | 0.035 | 1.941 (1.04–3.6) | multiparous | 0.813 | 1.144 (0.37–3.49) | |
| vegetarian vs. traditional pattern | 0.907 | 1.04 (0.53–2.02) | prudent vs. vegetarian pattern | 0.062 | 0.432 (0.17–1.04) | |
| balanced vs. traditional pattern | 0.676 | 1.151 (0.59–2.22) | modern vs. vegetarian pattern | 0.014 | 0.316 (0.12–0.79) | |
| Excessive | environment | 0.166 | 0.672 (0.38–1.17) | environment | 0.179 | 1.606 (0.80–1.13) |
| age | 0.495 | 1.017 (0.96–1.06) | age | 0.55 | 0.98 (0.21–3.20) | |
| education | 0.879 | 1.029 (0.70–1.49) | education | 0.147 | 1.31 (0.90–1.88) | |
| smoking status | 0.543 | 0.803 (0.39–1.63) | smoking status | 0.984 | 0.995 (0.62–1.59) | |
| dietary advice | 0.924 | 1.024 (0.63–1.64) | dietary advice | 0.834 | 0.9 (0.33–2.40) | |
| multiparous | 0.849 | 1.055 (0.60–1.82) | multiparous | 0.895 | 0.936 (0.35–2.50) | |
| vegetarian vs. traditional pattern | 0.624 | 1.16 (0.64–2.09) | prudent vs. vegetarian pattern | 0.186 | 0.59 (0.27–1.29) | |
| balanced vs. traditional pattern | 0.375 | 1.303 (0.72–2.33) | modern vs. vegetarian pattern | 0.099 | 0.521 (0.24–1.13) | |
| Model 3 | ||||||
| Inadequate | environment | 0.542 | 1.22 (0.63–2.37) | environment | 0.878 | 0.938 (0.41–2.12) |
| age | 0.059 | 0.94 (0.89–1) | age | 0.267 | 0.956 (0.88–1.03) | |
| education | 0.545 | 0.89 (0.59–1.32) | education | 0.661 | 0.907 (0.58–1.40) | |
| smoking status | 0.465 | 1.41 (0.55–3.6) | smoking status | 0.795 | 0.926 (0.51–1.65) | |
| dietary advice | 0.783 | 1.08 (0.62–1.88) | dietary advice | 0.027 | 3.045 (1.13–8.18) | |
| multiparous | 0.035 | 1.946 (1.04–3.62) | multiparous | 0.774 | 1.18 (0.38–3.66) | |
| vegetarian vs. traditional pattern | 0.92 | 1.03 (0.52–2.01) | prudent vs. vegetarian pattern | 0.09 | 0.456 (0.18–1.13) | |
| balanced vs. traditional pattern | 0.64 | 1.17 (0.60–2.28) | modern vs. vegetarian pattern | 0.03 | 0.352 (0.13–0.90) | |
| normal weight vs. obesity | 0.256 | 0.40 (0.08–1.92) | normal weight vs. obesity | 0.789 | 0.856 (0.27–2.68) | |
| overweight vs. obesity | 0.014 | 0.09 (0.01–0.62) | overweight vs. obesity | 0.109 | 2.65 (0.80–8.73) | |
| Excessive | environment | 0.065 | 0.568 (0.31–1.03) | environment | 0.256 | 1.514 (0.74–3.09) |
| age | 0.938 | 0.998 (0.94–1.05) | age | 0.482 | 0.975 (0.90–1.04) | |
| education | 0.762 | 1.061 (0.72–1.55) | education | 0.18 | 1.291 (0.24–1.13) | |
| smoking status | 0.569 | 0.80 (0.37–1.71) | smoking status | 0.65 | 1.118 (0.69–1.81) | |
| dietary advice | 0.815 | 1.060 (0.64–1.72) | dietary advice | 0.9 | 0.938 (0.34–2.54) | |
| multiparous | 0.981 | 1.006 (0.57–1.77) | multiparous | 0.893 | 0.933 (0.33–2.57) | |
| vegetarian vs. traditional pattern | 0.596 | 1.179 (0.64–2.17) | prudent vs. vegetarian pattern | 0.207 | 0.598 (0.26–1.32) | |
| balanced vs. traditional pattern | 0.178 | 1.523 (0.82–2.8) | modern vs. vegetarian pattern | 0.137 | 0.547 (0.24–1.21) | |
| normal weight vs. obesity | 0.004 | 0.142 (0.03–0.53) | normal weight vs. obesity | 0.012 | 0.375 (0.17–0.80) | |
| overweight vs. obesity | 0.093 | 0.305 (0.07–1.20) | overweight vs. obesity | 0.016 | 0.286 (0.10–0.79) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, A.-M.; Gherasim, A.; Niță, O.; Mihalache, L.; Arhire, L.I.; Cioancă, O.; Gafițanu, D.; Popa, A.D. Exploring Lifestyle and Dietary Patterns in Pregnancy and Their Impact on Health: A Comparative Analysis of Two Distinct Groups 10 Years Apart. Nutrients 2024, 16, 377. https://doi.org/10.3390/nu16030377
Mitran A-M, Gherasim A, Niță O, Mihalache L, Arhire LI, Cioancă O, Gafițanu D, Popa AD. Exploring Lifestyle and Dietary Patterns in Pregnancy and Their Impact on Health: A Comparative Analysis of Two Distinct Groups 10 Years Apart. Nutrients. 2024; 16(3):377. https://doi.org/10.3390/nu16030377
Chicago/Turabian StyleMitran, Andreea-Maria, Andreea Gherasim, Otilia Niță, Laura Mihalache, Lidia Iuliana Arhire, Oana Cioancă, Dumitru Gafițanu, and Alina Delia Popa. 2024. "Exploring Lifestyle and Dietary Patterns in Pregnancy and Their Impact on Health: A Comparative Analysis of Two Distinct Groups 10 Years Apart" Nutrients 16, no. 3: 377. https://doi.org/10.3390/nu16030377
APA StyleMitran, A.-M., Gherasim, A., Niță, O., Mihalache, L., Arhire, L. I., Cioancă, O., Gafițanu, D., & Popa, A. D. (2024). Exploring Lifestyle and Dietary Patterns in Pregnancy and Their Impact on Health: A Comparative Analysis of Two Distinct Groups 10 Years Apart. Nutrients, 16(3), 377. https://doi.org/10.3390/nu16030377







