Global Leadership Initiative on Malnutrition-Diagnosed Malnutrition in Lung Transplant Candidates
Abstract
1. Introduction
2. Material and Methods
2.1. Diagnosis of Malnutrition Using the GLIM Criteria
2.2. Statistical Analysis
2.2.1. Percentile Estimation
2.2.2. Comparison of Nutritional Diagnostic and Screening Methods
3. Results
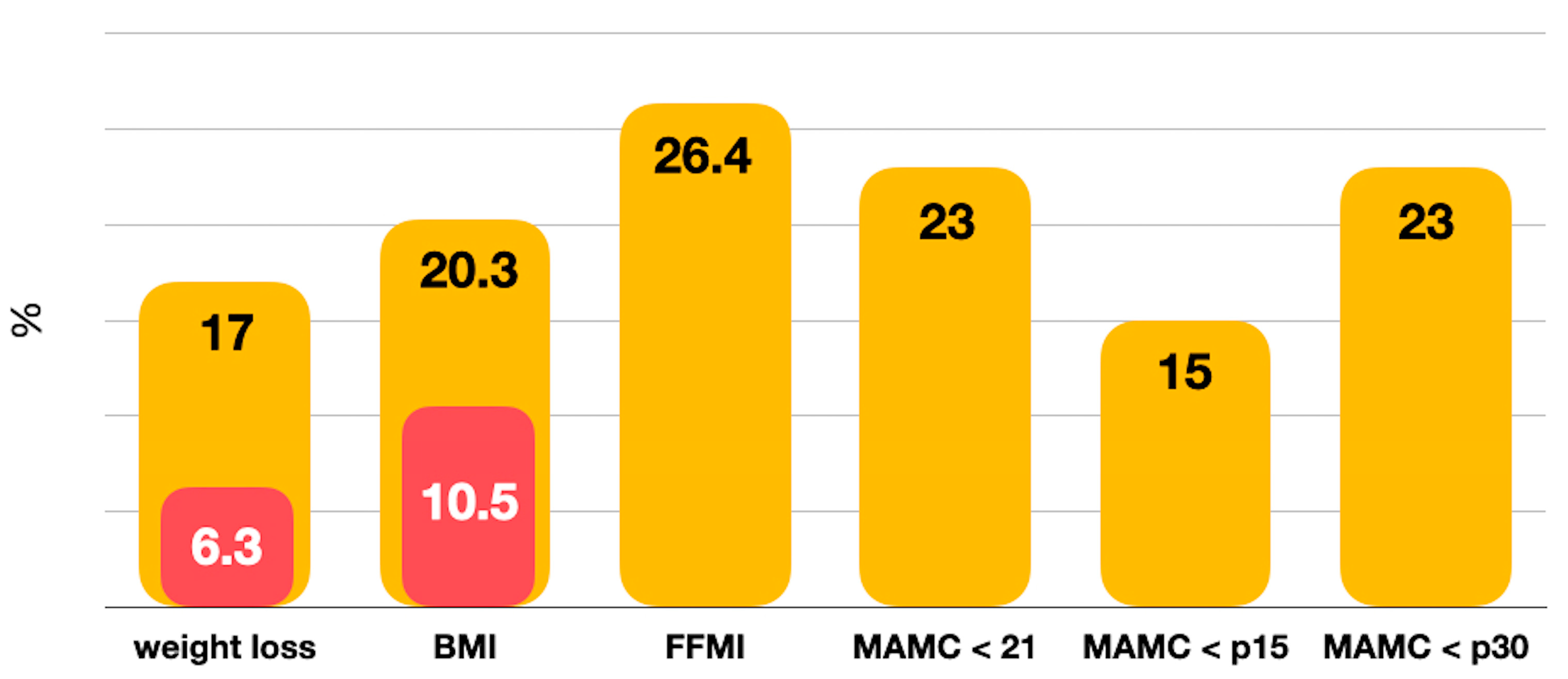
3.1. Prevalence of Nutritional Risk and Malnutrition
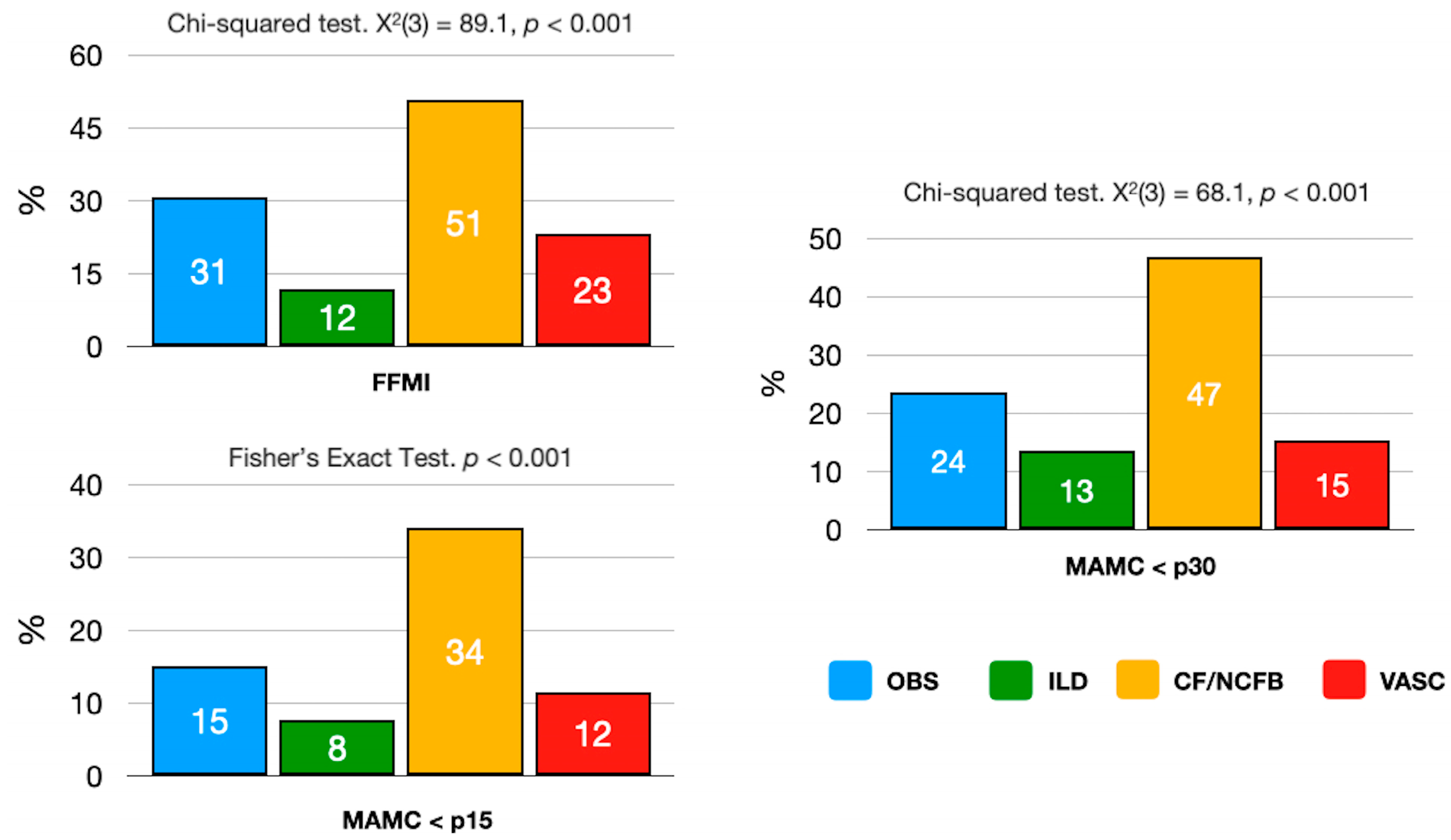
3.2. Malnutrition and Lung Disease
3.3. Lung Transplant Candidates with Malnutrition
4. Discussion
5. Limitations
6. Strengths
7. Conclusions
8. Clinical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chambers, D.C.; Cherikh, W.S.; Goldfarb, S.B.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Toll, A.E.; Khush, K.K.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transpl. 2018, 37, 1169–1183. [Google Scholar] [CrossRef]
- Emsley, C.; King, S.; Nyulasi, I.; Snell, G. A GLIMmer of insight into lung transplant nutrition: Enhanced detection of malnutrition in lung transplant patients using the GLIM criteria. Clin. Nutr. 2021, 40, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transpl. 2021, 40, 1349–1379. [Google Scholar] [CrossRef] [PubMed]
- Schwebel, C.; Pin, I.; Barnoud, D.; Devouassoux, G.; Brichon, P.Y.; Chaffanjon, P.; Chavanon, O.; Sessa, C.; Blin, D.; Guignier, M.; et al. Prevalence and consequences of nutritional depletion in lung transplant candidates. Eur. Respir. J. 2000, 16, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.J.H.C.G.; van de Bool, C.; van den Borst, B.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A.M.W.J. Normal Weight but Low Muscle Mass and Abdominally Obese: Implications for the Cardiometabolic Risk Profile in Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2017, 18, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, L.; Li, Y.; Guo, S.; Li, X.; Zheng, J.; Chen, Y.; Huang, Y.; Chen, R.; Chen, X. Fat-Free Mass Index for Evaluating the Nutritional Status and Disease Severity in COPD. Respir. Care. 2016, 61, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, O.; Yamazaki, R.; Sano, H.; Iwanaga, T.; Higashimoto, Y.; Kume, H.; Tohda, Y. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2017, 22, 480–485. [Google Scholar] [CrossRef]
- Singer, J.P.; Peterson, E.R.; Snyder, M.E.; Katz, P.P.; Golden, J.A.; D’Ovidio, F.; Bacchetta, M.; Sonett, J.R.; Kukreja, J.; Shah, L.; et al. Body composition and mortality after adult lung transplantation in the United States. Am. J. Respir. Crit. Care Med. 2014, 190, 1012–1021. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; González, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the “malnutrition universal screening tool” (’MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Gasperini, M.L.; Proietti, L.; Muscaritoli, M. Assessing Malnutrition in Systemic Sclerosis With Global Leadership Initiative on Malnutrition and European Society of Clinical Nutrition and Metabolism Criteria. JPEN J. Parenter. Enteral Nutr. 2021, 45, 618–624. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.E.; Keller, H.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; Steiber, A.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin. Nutr. 2020, 39, 2872e80. [Google Scholar] [CrossRef]
- Yin, L.; Lin, X.; Li, N.; Zhang, M.; He, X.; Liu, J.; Kang, J.; Chen, X.; Wang, C.; Wang, X.; et al. Evaluation of the Global Leadership Initiative on Malnutrition Criteria Using Different Muscle Mass Indices for Diagnosing Malnutrition and Predicting Survival in Lung Cancer Patients. JPEN J. Parenter. Enteral Nutr. 2021, 45, 607–617. [Google Scholar] [CrossRef]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Wieczorowska-Tobis, K.; Deskur-Śmielecka, E. Optimal Assessment of Nutritional Status in Older Subjects with the Chronic Obstructive Pulmonary Disease-A Comparison of Three Screening Tools Used in the GLIM Diagnostic Algorithm. Int. J. Environ. Res. Public. Health 2022, 19, 1025. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Version 4.2.1; Available online: https://www.R-project.org/ (accessed on 16 January 2024).
- Nosotti, M.; Ferrari, M. Nutritional status and lung transplantation: An intriguing problem. Ann. Transl. Med. 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Leblanc, P.; Whittom, F.; Carrier, G.; Jobin, J.; Belleau, R.; Maltais, F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Schols, A.M.; Does, J.D.; Wouters, E.F. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2000, 71, 733–738. [Google Scholar] [CrossRef] [PubMed]
- The Malnutrition Advisory Group. The MUST Report: Nutritional Screening of Adults: A Multidisciplinary Responsibility. BAPEN. 2003. Available online: https://www.bapen.org.uk/screening-and-must/must/must-report (accessed on 14 January 2021).
- Anthony, P.A. Nutrition screening tool for hospitalized patients. Nutr. Clin. Pract. 2008, 23, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Coltman, A.; Tomesko, J.; Charney, P.; Porcari, J.; Piemonte, T.A.; Handu, D.; Cheng, F.W. Position of the Academy of Nutrition and Dietetics: Malnutrition (Undernutrition) Screening Tools for All Adults. J. Acad. Nutr. Diet. 2020, 120, 709–713. [Google Scholar] [CrossRef]
- Lengelé, L.; Bruyère, O.; Beaudart, C.; Reginster, J.Y.; Locquet, M. Malnutrition, assessed by the Global Leadership Initiative on Malnutrition (GLIM) criteria but not by the mini nutritional assessment (MNA), predicts the incidence of sarcopenia over a 5-year in the SarcoPhAge cohort. Aging Clin. Exp. Res. 2021, 33, 1507–1517. [Google Scholar] [CrossRef]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Lewandowicz, M.; Deskur-Śmielecka, E.; Stachnik, K.; Wieczorowska-Tobis, K. Diagnostic Performance and Accuracy of the MNA-SF against GLIM Criteria in Community-Dwelling Older Adults from Poland. Nutrients 2021, 13, 2183. [Google Scholar] [CrossRef]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Duran, X.; Meza-Valderrama, D.; Rodríguez, D.A.; Muñoz, E.; Tejero-Sánchez, M.; Muns, M.D.; Guillén-Solà, A.; et al. Malnutrition According to GLIM Criteria Is Associated with Mortality and Hospitalizations in Rehabilitation Patients with Stable Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, L.; Wang, H.; Hao, Q.; Dong, B.; Yang, M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci. Rep. 2017, 7, 3171. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Usmani, S.E.; Azhie, A.; Woo, M. Metabolic Consequences of Solid Organ Transplantation. Endocr. Rev. 2021, 42, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Chamogeorgakis, T.; Mason, D.P.; Murthy, S.C.; Thuita, L.; Raymond, D.P.; Pettersson, G.B.; Blackstone, E.H. Impact of nutritional state on lung transplant outcomes. J. Heart Lung Transpl. 2013, 32, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Madill, J.; Gutierrez, C.; Grossman, J.; Allard, J.; Chan, C.; Hutcheon, M.; Keshavjee, S.H.; Toronto Lung Transplant Program. Nutritional assessment of the lung transplant patient: Body mass index as a predictor of 90-day mortality following transplantation. J. Heart Lung Transpl. 2001, 20, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Arnaoutakis, G.J.; Weiss, E.S.; Merlo, C.A.; Conte, J.V.; Shah, A.S. The impact of recipient body mass index on survival after lung transplantation. J. Heart Lung Transpl. 2010, 29, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ruttens, D.; Verleden, S.; Vandermeulen, E.; Vos, R.; van Raemdonck, D.; Vanaudenaerde, B.; Verleden, G. Body mass index in lung transplant candidates: A contraindication to transplant or not? Transpl. Proc. 2014, 46, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Weig, T.; Milger, K.; Langhans, B.; Janitza, S.; Sisic, A.; Kenn, K.; Irlbeck, T.; Pomschar, A.; Johnson, T.; Irlbeck, M.; et al. Core Muscle Size Predicts Postoperative Outcome in Lung Transplant Candidates. Ann. Thorac. Surg. 2016, 101, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28, Erratum in: Nutr. Clin. Pract. 2021, 36, 909. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Plochl, W.; Pezawas, L.; Artemiou, O.; Grimm, M.; Klepetko, W.; Hiesmayr, M. Nutritional status, ICU duration and ICU mortality in lung transplant recipients. Intensive Care Med. 1996, 22, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Ramos, K.J.; Kapnadak, S.G.; Bradford, M.C.; Somayaji, R.; Morrell, E.D.; Pilewski, J.M.; Lease, E.D.; Mulligan, M.S.; Aitken, M.L.; Gries, C.J.; et al. Underweight patients with cystic fibrosis have acceptable survival following lung transplantation: A United Network for Organ Sharing Registry Study. Chest. 2020, 157, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.; Mogensen, K.M. Key approaches to diagnosing malnutrition in adults. Nutr. Clin. Pract. 2022, 37, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Boslooper-Meulenbelt, K.; van Vliet, I.M.Y.; Gomes-Neto, A.W.; de Jong, M.F.C.; Bakker, S.J.L.; Jager-Wittenaar, H.; Navis, G.J. Malnutrition according to GLIM criteria in stable renal transplant recipients: Reduced muscle mass as predominant phenotypic criterion. Clin. Nutr. 2021, 40, 3522–3530. [Google Scholar] [CrossRef]
- Eren, T.; Burcu, B.; Tombalak, E.; Ozdemir, T.; Leblebici, M.; Ozemir, I.A.; Ziyade, S.; Alimoglu, O. Clinical Significance of the Glasgow Prognostic Score for Survival after Colorectal Cancer Surgery. J. Gastrointest. Surg. 2016, 20, 1231–1238. [Google Scholar] [CrossRef]
- Kang, H.S.; Cho, K.W.; Kwon, S.S.; Kim, Y.H. Prognostic significance of Glasgow prognostic score in patients with acute exacerbation of idiopathic pulmonary fibrosis. Respirology 2018, 23, 206–212. [Google Scholar] [CrossRef]
- Wilson, A.; Altman, K.; Schindler, T.; Schwarzenberg, S.J. Updates in Nutrition Management of Cystic Fibrosis in the Highly Effective Modulator Era. Clin. Chest Med. 2022, 43, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Mielus, M.; Sands, D.; Woynarowski, M. Improving nutrition in cystic fibrosis: A systematic literature review. Nutrition 2022, 102, 111725. [Google Scholar] [CrossRef] [PubMed]
| n (%) | |
|---|---|
| OBSTRUCTIVE Emphysema Chronic bronchitis Combined pulmonary fibrosis and emphysema Others: Langerhans cells histiocytosis, bronchiolitis obliterans, graft-versus-host disease | 483 (45.6%) |
| INTERSTITIAL Idiopathic pulmonary fibrosis Desquamative interstitial pneumonia Idiopathic nonspecific interstitial pneumonia Respiratory bronchiolitis-associated interstitial lung disease Cryptogenic organizing pneumonia Acute interstitial pneumonia Idiopathic lymphoid interstitial pneumonia Idiopathic pleuroparenchymal fibroelastosis Unclassifiable idiopathic interstitial pneumonias Hypersensitivity pneumonitis Autoimmune interstitial lung diseases Others: lymphangioleiomyomatosis, sarcoidosis, drug-associated ILDs, vasculitis/granulomatosis ILDs, proteinosis, alveolar microlithiasis, pneumoconiosis and other rare ILDs | 388 (36.6%) |
| CYSTIC FIBROSIS NON-CYSTIC FIBROSIS BRONCHIECTASIS | 163 (15.4%) |
| VASCULAR Pulmonary arterial hypertension Others | 26 (2.4%) |
| 1060 |
| Balanced Accuracy | Sensitivity | Specificity | PPV | NPV | Cohen’s Kappa | |
|---|---|---|---|---|---|---|
| GLIM (FFMI) vs. ESPEN | 0.882 | 1 | 0.763 | 0.417 | 1 | 0.483 |
| GLIM (MAMC < 21 cm) vs. ESPEN | 0.888 | 1 | 0.775 | 0.456 | 1 | 0.523 |
| GLIM (MAMC < p15) vs. ESPEN | 0.899 | 1 | 0.797 | 0.469 | 1 | 0.544 |
| GLIM (MAMC < p30) vs. ESPEN | 0.873 | 1 | 0.746 | 0.414 | 1 | 0.471 |
| GLIM | |
|---|---|
| CF/NCFB (n = 155) | 84.5% (n = 131) |
| OBSTRUCTIVE (n = 463) | 45.4% (n = 210) |
| INTERSTITIAL (n = 377) | 31.3% (n = 118) |
| VASCULAR (n = 24) | 58.3% (n = 14) |
| Malnourished | Not Malnourished | p | |
|---|---|---|---|
| Age | 46.7 (15.1) | 55.1 (8.9) | <0.001 |
| Male/Female | 41.2%/60.1% | 58.8%/39.9% | <0.001 |
| FEV1 (%) | 30.0 (17.1) | 38.4 (17.9) | <0.001 |
| TLC (%) | 104.1 (42.1) | 86.4 (39.9) | <0.01 |
| pCO2 (mmHg) | 46.3 (8.7) | 43.5 (6.9) | <0.01 |
| WL 6 months (yes) | 84.2% | 15.8% | <0.001 |
| WL 6 months (kg) | 5.1 (3.3) | 2.5 (1.2) | <0.001 |
| WL 6 months (%) | 8.0 (4.6) | 3.1 (1.1) | <0.001 |
| Oral nutritional supplements (yes) | 91% | 8.6% | <0.001 |
| Weight (kg) | 58.2 (12.2) | 78.0 (12.4) | <0.001 |
| BMI (kg/m2) | 21.2 (3.9) | 27.9 (3.6) | <0.001 |
| TSF (mm) | 13.3 (6.8) | 16.3 (6.3) | <0.001 |
| SSF (mm) | 12.9 (6.4) | 20.1 (6.2) | <0.001 |
| AC (cm) | 25.4 (3.7) | 30.9 (2.9) | <0.001 |
| MAMC (cm) | 21.2 (2.9) | 25.8 (2.3) | <0.001 |
| FM (kg) | 13.9 (8.0) | 24.4 (8.1) | <0.001 |
| FFM (kg) | 44.4 (8.3) | 53.9 (8.7) | <0.001 |
| Albumin (g/dL) | 3.9 (0.5) | 4.0 (0.4) | <0.01 |
| Prealbumin (mg/dL) | 22.2 (7.4) | 24.0 (5.8) | <0.001 |
| Cholesterol (mg/dL) | 179.4 (51.0) | 198.5 (40.2) | <0.001 |
| HDL cholesterol (mg/dL) | 49.6 (16.3) | 47.9 (16.8) | <0.001 |
| LDL cholesterol (mg/dL) | 111.1 (40.3) | 124.6 (35.5) | <0.001 |
| Triglycerides (mg/dL) | 106.0 (50.8) | 127.6 (57.2) | <0.001 |
| CRP (mg/L) | 12.8 (22.6) | 9.0 (16.3) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calañas-Continente, A.; Gutiérrez-Botella, J.; García-Currás, J.; Cobos, M.J.; Vaquero, J.M.; Herrera, A.; Molina, M.J.; Gálvez, M.Á. Global Leadership Initiative on Malnutrition-Diagnosed Malnutrition in Lung Transplant Candidates. Nutrients 2024, 16, 376. https://doi.org/10.3390/nu16030376
Calañas-Continente A, Gutiérrez-Botella J, García-Currás J, Cobos MJ, Vaquero JM, Herrera A, Molina MJ, Gálvez MÁ. Global Leadership Initiative on Malnutrition-Diagnosed Malnutrition in Lung Transplant Candidates. Nutrients. 2024; 16(3):376. https://doi.org/10.3390/nu16030376
Chicago/Turabian StyleCalañas-Continente, Alfonso, Jesús Gutiérrez-Botella, Julia García-Currás, Mª Jesús Cobos, José Manuel Vaquero, Aura Herrera, Mª José Molina, and Mª Ángeles Gálvez. 2024. "Global Leadership Initiative on Malnutrition-Diagnosed Malnutrition in Lung Transplant Candidates" Nutrients 16, no. 3: 376. https://doi.org/10.3390/nu16030376
APA StyleCalañas-Continente, A., Gutiérrez-Botella, J., García-Currás, J., Cobos, M. J., Vaquero, J. M., Herrera, A., Molina, M. J., & Gálvez, M. Á. (2024). Global Leadership Initiative on Malnutrition-Diagnosed Malnutrition in Lung Transplant Candidates. Nutrients, 16(3), 376. https://doi.org/10.3390/nu16030376






