Abstract
Hip fracture is a common condition in older adults, leading to disability and mortality. Several studies have demonstrated the association between nutritional status and the risk of a negative health outcome after fractures. In this systematic review, we evaluated the association between malnutrition and mortality, changes in mobility/living arrangements, and postoperative complications, such as delirium, in older patients with hip fractures. A literature search on the PubMed, Web of Science, and Scopus databases, up to September 2023, was conducted to identify all studies involving older subjects that reported an association between MNA/GNRI/PNI/CONUT and health outcome after hip fracture. Meta-analysis was performed by a random-effects model using risk values (RR, OR, and HR) extracted from the 14 eligible selected studies. Malnutrition significantly increased the risk of any analyzed adverse outcome by 70% at 1 month, and up to 250% at 1 year. Malnutrition significantly increased delirium risk by 275% (OR = 2.75; 95% CI 1.80–4.18; p ≤ 0.05), mortality risk by 342% (OR = 3.42; 95% CI 2.14–5.48; p ≤ 0.05), mortality hazard risk by 351% (HR = 3.51; 95% CI 1.63–7.55; p ≤ 0.05) at 1 month, and transfer-to-more-supported-living-arrangements risk by 218% (OR = 2.18; 95% CI 1.58–3.01; p ≤ 0.05), and declined mobility risk by 41% (OR = 1.41; 95% CI 1.14–1.75; p ≤ 0.05), mortality risk by 368% (OR = 3.68; 95% CI 3.00–4.52; p ≤ 0.05), and mortality hazard risk by 234% (HR = 2.34; 95% CI 1.91–2.87; p ≤ 0.05) at 1 year. Malnutrition of older patients increases the risk of death and worsens mobility and independence after hip fractures. The results of the present study highlight the importance of nutritional status evaluation of older subjects with hip fractures in order to prevent potential adverse outcomes (Registration No: CRD42023468751).
1. Introduction
Hip fractures in older people is a substantial concern to public health services and society [1,2].
In fact, this type of fracture, in the upper part of the femur, becomes more common with aging. This is because aging-related bone fragility is exacerbated by structural deterioration and bone loss. The combination of both skeletal fragility and a greater propensity to fall leads to a high occurrence of hip fractures with advancing age [1].
It was estimated that hip fracture affected 1.6 million persons globally in 2000, and by 2050, this number is expected to reach 6.3 million [3].
Hip fracture is associated with the development of negative consequences, such as disability, depression, cardiovascular diseases, and, consequently, mortality [2]. Mortality within 1 year ranges from 20% to 40% in older patients [4,5].
Finding the risk factors for functional loss following hip fracture may help treat postoperative problems more effectively and lower the expenses of helping patients who become dependent on assistance due to institutionalization or loss of autonomy [6].
A recent systematic review found a range from 4% to 39.4% of hip fracture patients who were malnourished when they were admitted to hospital [7].
After a fracture, older patients who are undernourished may only partially regain their previous degree of independence in carrying out daily tasks and they have a reduced capacity to return to their pre-fracture functional status [8].
Malnutrition has been identified as a patient’s poor nutritional status and as an important and modifiable prognostic factor for several medical conditions.
Due to its modifiability and the potential for early nutritional intervention to promote hip fracture recovery, malnutrition is a condition of great concern when combined with hip fracture [9].
Malnutrition can be detected by several validated tools (https://guidelines.espen.org/espen-web-app/home/, accessed on 26 September 2023). In particular, the following are the most used for older patients or surgical patients within the hospital setting: the Mini Nutritional Assessment Short Form (MNA-SF), the Mini Nutritional Assessment Long Form (MNA-LF), the Geriatric Nutritional Risk Index (GNRI), the Prognostic Nutritional Index (PNI), and the Controlling Nutritional Status (CONUT) score.
The MNA is a standardized and validated instrument to identify protein–energy malnutrition or the risk of malnutrition in older patients in various care settings and has already been proven as a useful diagnostic tool among older orthopedic patients [10,11].
The GNRI is a tool used for assessing the nutritional status of older individuals considering both body weight and serum albumin levels to evaluate the risk of malnutrition and associated complications in geriatric populations. The GNRI score ranges from 0 to 100, with higher scores indicating better nutritional status [12].
The PNI was developed to investigate the relationship between nutritional status and outcomes in surgical patients. It is based on serum albumin levels and total lymphocyte count. Lower PNI scores indicate poorer nutritional status, while higher scores suggest better nutritional status [13].
The CONUT score is used for nutritional assessment in clinical practice, in particular, for patients with various medical conditions. It considers three parameters: serum albumin levels, total lymphocyte count, and total cholesterol concentration. It was first proposed in 2005 as a means of evaluating the nutritional status and predicting outcomes in hospitalized patients [14].
Two-thirds of older patients are at a particular nutritional risk or are malnourished, with a wide impact on their overall health, physical functioning, and quality of life [15].
Poor nutritional status has been associated with several negative clinical outcomes, such as postoperative complications, pressure ulcers, functional dependence, walking impairment, impaired quality of life, and mortality [9,16].
A recent meta-analysis [17] examined association between nutritional indices and mortality after hip fracture, finding that patients with low GNRI or low MNA-SF scores had a significantly higher risk of mortality compared to those with higher scores, even though it was not assessed for different follow-up periods.
To the best of our knowledge, there are no systematic reviews and meta-analyses that investigate the association between malnutrition and health outcomes in consideration of different follow-up intervals from hip fracture; therefore, the aim of our systematic review is to evaluate the association between malnutrition and selected health outcomes in patients affected by hip fractures, in particular: mortality, mobility, changes in living arrangements, and postoperative complications.
2. Materials and Methods
The present meta-analysis was conducted following the MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines [18] and PRISMA statement [19].
The protocol of this study has been recorded in the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/PROSPERO/ (accessed on 13 October 2023), Registration No: CRD42023468751).
2.1. Search Strategy and Data Source
We carried out a systematic literature search up to 26 September 2023 through the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Web of Science (http://wokinfo.com/), and Scopus (https://www.scopus.com/) databases to identify original articles on the association between malnutrition and selected health outcomes in older individuals with hip fractures.
The literature search included the following medical subject headings (MeSH) and keywords: (“Nutritional Status”[Mesh] OR MNA[Title/Abstract] OR CONUT[Title/Abstract] OR PNI[Title/Abstract] OR GNRI[Title/Abstract]) AND (risk) AND (outcome) AND (((hip[Title/Abstract] OR femoral[Title/Abstract]) AND (injury[Title/Abstract] OR fracture[Title/Abstract])) OR “Proximal Femoral Fractures”[Mesh] OR “Femoral Neck Fractures”[Mesh] OR “Hip Fractures”[Mesh]).
The different associations of keywords combined with Boolean operators used for each database are shown in Supplementary Table S1.
No publication date limitation was applied but due to translation restrictions, only English-language studies were eligible.
We manually examined the reference lists of selected articles and recent relevant reviews to identify possible additional relevant publications.
2.2. Eligibility Criteria
Only the following tools were considered for inclusion: MNA-LF, MNA-SF, GNRI, PNI, and CONUT score.
Articles were included if they met the following criteria: (i) evaluated the relationship between malnutrition, derived by indices such as MNA-LF, MNA-SF, GNRI, PNI, or CONUT score, and health outcome in older patients after hip fractures; (ii) used a case-control, prospective or cross-sectional study design; (iii) reported odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimated with 95% confidence intervals (CIs).
For each potentially included study, two investigators independently carried out the selection, data extraction, and quality assessment. Disagreements were resolved by discussion or in consultation with a third author. Although useful to have background information, reviews and meta-analyses were excluded. No studies were excluded for weakness of design or data quality.
2.3. Data Extraction and Quality Assessment
For each selected study, we extracted the following information: first author’s last name, year of publication, country, study design, sample size, population characteristics (sex, age), duration of follow-up for cohort studies, type of health outcome evaluated (mortality, mobility, changes in living arrangements, and postoperative complications), health outcome assessment method, type of nutritional assessment and categories of nutrition (at risk of malnutrition, malnourished), risk estimates with 95% CIs for the different categories of nutrition, p-value for trend, and confounding factors adjustment. When multiple estimates were reported in the article, we extracted those adjusted for the most confounding factors. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the literature for cohort and case-control studies using a 9-star system, as shown in Supplementary Table S2. The full score was 9 and a total score ≥7 was used to indicate a high-quality study [19].
2.4. Statistical Analysis
The estimated overall effect-size statistic was the average of the logarithm of the observed OR associated with the risk of malnutrition or malnourishing versus the normal state of nutrition. If the reference category was not normal, we reported the results in this form.
Concerning outcome, if it was presented in a positive form such as “survival” or “free walking ability”, the results were inverted or obtained from data extracted from the original text to obtain a negative outcome (“mortality” or “decreased walking ability”).
Change in living arrangements refers to an increase in level of care: living independently at home, living at home with organized home care, living in assisted living accommodation, and living in an institution.
The analysis was performed using the random-effects model to calculate the summary OR and 95% CIs. If the study reported mortality outcome as HR, OR with 95% CI was calculated from data extracted from the original text. The overall analysis of Any Health Outcome was conducted using OR, but for mortality, it was performed using both OR and HR separately.
We restricted this analysis to the following nutritional indexes: MNA-LF, MNA-SF, GNRI, PNI, and CONUT score, and to the following health outcomes: mobility, mortality, living arrangements, and postoperative complications, both overall and for different follow-up periods.
Stratification analysis for at risk of malnutrition and malnutrition groups was performed for observations with MNA and MNA-SF nutritional indexes, while those with GNRI, PNI, and CONUT score were included in the overall analysis.
An additional stratification was carried out for the study design (case-control or cohort study) and for age over 75 years.
The chi-square-based Cochran’s Q statistic and the I2 statistic were used to evaluate heterogeneity in results across studies [20].
The I2 statistic yields results ranged from 0% to 100% (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; and I2 = 75–100%, extreme heterogeneity) [21]. The results of the meta-analysis may be biased if the probability of publication is dependent on the study results.
We used the methods of Begg and Mazumdar [22] and Egger et al. [23] to detect publication bias. Both methods tested for funnel plot asymmetry, with the former being based on the rank correlation between the effect estimates and their sampling variances and the latter on a linear regression of a standard normal deviate on its precision.
If a potential bias was detected, we further conducted a sensitivity analysis to assess the strength of combined effect estimates, the possible influence of the bias, and to have the bias corrected. We also conducted a sensitivity analysis to investigate the influence of a single study on the overall risk estimate, by omitting one study in each turn. We considered the funnel plot to be asymmetrical if the intercept of Egger’s regression line deviated from zero, with a p-value < 0.05.
The analyses were performed using the ProMeta 3 statistical program and the calculations on data extracted from the original papers were performed using STATA 13.
3. Results
3.1. Study Selection
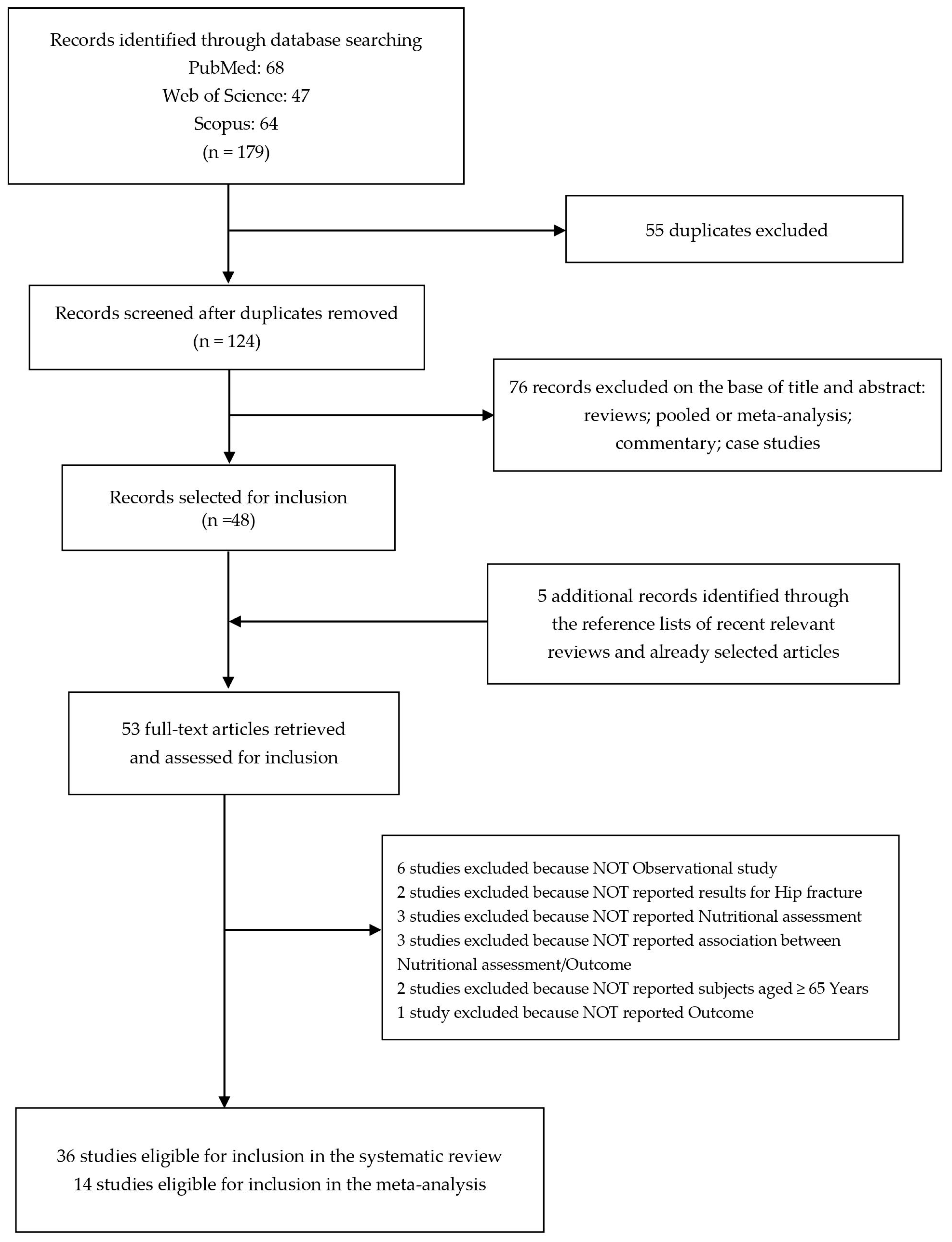
The literature search revealed 68 studies from the PubMed database, 47 from Web of Science, and 64 from Scopus.
After removing duplicates (n = 55), we identified 124 records screened for title and abstract revision (Figure 1). Among these, 76 articles were excluded (reviews, pooled or meta-analysis, commentary, and case studies).
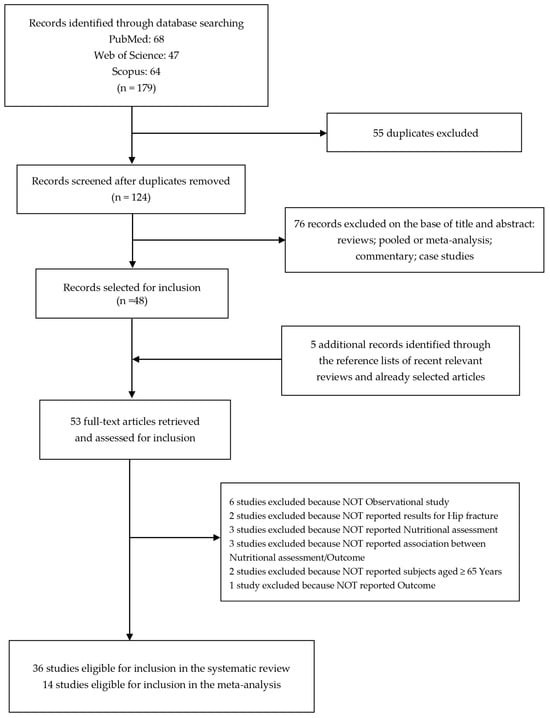
Figure 1.
Flow diagram of the systematic literature search on malnutrition-related health outcomes in older adults with hip fractures.
Therefore, 48 studies were subjected to full-text revision.
We selected 5 additional items through the reference lists of both selected articles and recent relevant reviews [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76].
Subsequently, 17 articles were also excluded because they did not meet the inclusion criteria as follows: 6 studies were not observational studies, 2 studies did not evaluate the impact on hip fracture, 3 studies did not consider the nutritional assessment, 3 studies did not investigate the association between nutritional assessment and outcomes, 2 studies did not report subjects aged ≥ 65 years old, and 1 study did not report the outcome.
At the end of the selection process, 36 studies were eligible for inclusion in the systematic review [25,28,30,31,33,34,36,38,39,40,41,43,44,45,46,48,49,50,51,53,54,56,58,59,60,61,64,67,68,69,70,71,72,73,75,76] and 14 studies were eligible for inclusion in the meta-analysis [34,36,39,43,49,51,53,56,64,67,68,69,70,72].
3.2. Study Characteristics and Quality Assessment
The general characteristics of the 14 studies included in the meta-analysis evaluating the association between malnutrition and health outcome in older adults with hip fractures and are shown in Table 1. Studies were conducted in Finland [34,43,49], the Netherlands [36], Italy [39], China [51,64,68,70], Taiwan [53], United States [56], Japan [67], Austria [69], and Spain [72]. There were 11 cohort studies [34,36,39,43,49,51,56,64,68,70,72] and 3 were case-control studies [53,67,69]. These studies were published between 2015 and 2023. All of these 14 studies were conducted in people aged ≥ 65 years old: women were more involved than men; regarding age, 5 studies [36,56,67,68,69] examined patients > 75 years old.

Table 1.
General characteristics of included studies in the meta-analysis of malnutrition and health outcome.
The nutritional status of all the participants was evaluated by different nutritional indexes: in 6 studies [34,39,43,49,69,72] by MNA-SF, 4 studies [53,56,67,68] used GNRI, 3 studies [51,64,68] used PNI, 2 studies [36,43] used MNA-LF, and 1 study [70] used CONUT score.
The health outcomes investigated were mortality in 11 studies [34,36,43,49,53,56,64,67,68,69,72], mobility in 6 studies [34,43,49,56,68,70], living arrangements in 2 studies [34,43], and complications (postoperative delirium) in 2 studies [39,51].
The study design was prospective in 10 studies [34,39,43,49,51,56,64,68,70,72] and retrospective in 4 studies [36,53,67,69].
Mortality assessment was obtained through electronic medical records [34,36,43,53,67,69,72] or telephone interview [49,56,64].
Changes in living arrangements or in mobility level were collected by telephone interview [34,43,49,56], using the Functional Independence Measure-locomotion (FIM-L) Scale in one study [70]. Postoperative delirium was evaluated according to the criteria by the Confusion Assessment Method (CAM) [39,51].
The follow-up considered in this analysis was 1–45 months. In particular, the follow-up for mortality was at ≤1 month in 4 studies, ≤3 months in 6 studies, ≤4 months in 8 studies, ≤6 months in 8 studies, and ≤12 months in all studies, except 1 study [64]; for mobility, follow-up was at ≤1 month in 2 studies, ≤4 months in 5 studies, and ≤12 months in all studies; for living arrangements, follow-up was at ≤1 month in 1 study, ≤4 months in 2 studies, and ≤12 months in all studies; and for complications (postoperative delirium), follow-up was only at ≤1 month.
In one study [64], the follow-up period was up to 45 months, so it was included in the overall analysis of any health outcome and mortality, but not in the follow-up stratification. One article [67] reported OR for “lower GNRI”, with no reference group; therefore, it was included only in the overall analysis of any health outcome and mortality. In one paper [70], the reference group was normal/mild, so it was not included in the stratification analysis, but only in the overall analysis of any health outcome and mobility.
3.3. Meta-Analysis
A comprehensive meta-analysis was conducted to investigate the association between nutritional status and various health outcomes among older patients with hip fractures. The analysis included the comparison between malnutrition, at-risk status, and all other nutritional categories, while the health outcomes examined included mortality, living arrangements, mobility, and postoperative delirium, as shown in Table 2. The overall analysis revealed a significant association between being in one of the risk categories of the nutritional tools examined and adverse health outcomes (OR = 2.42; 95% CI 2.07–2.83; p ≤ 0.05).

Table 2.
Results of stratified analysis of the health outcome risk estimates, after hip fracture, according to nutritional status.
The odds ratio showed an increasing trend with longer follow-up periods (OR = 1.70; 95% CI 1.36–2.13; p ≤ 0.05 at ≤1 month, OR = 1.86; 95% CI 1.50–2.32; p ≤ 0.05 at ≤3 months, OR = 2.29; 95% CI 1.90–2.75; p ≤ 0.05 at ≤4 months, OR = 2.40; 95% CI 1.99–2.91; p ≤ 0.05 at ≤6 months, OR = 2.50; 95% CI 2.11–2.97; p ≤ 0.05 at ≤1 year).
While the category malnourished consistently showed significance since the first month of follow-up (OR = 3.01; 95% CI 1.75–5.17; p ≤ 0.05), the at-risk category emerged as significant from the fourth month of follow-up (OR = 1.67; 95% CI 1.37–2.02; p ≤ 0.05) and did so progressively over time (OR = 1.71; 95% CI 1.41–2.09; p ≤ 0.05 at 6 months and OR = 1.80; 95% CI 1.50–2.16; p ≤ 0.05 at 1 year). The heterogeneity was high in both pooled data (I2 = 85.94%) than in different months of follow-up data (I2 = 79.25% at 1 month, I2 = 82.56% at 3 months, I2 = 84.04% at 4 months, I2 = 85.82% at 6 months, and I2 = 86.37% at 1 year).
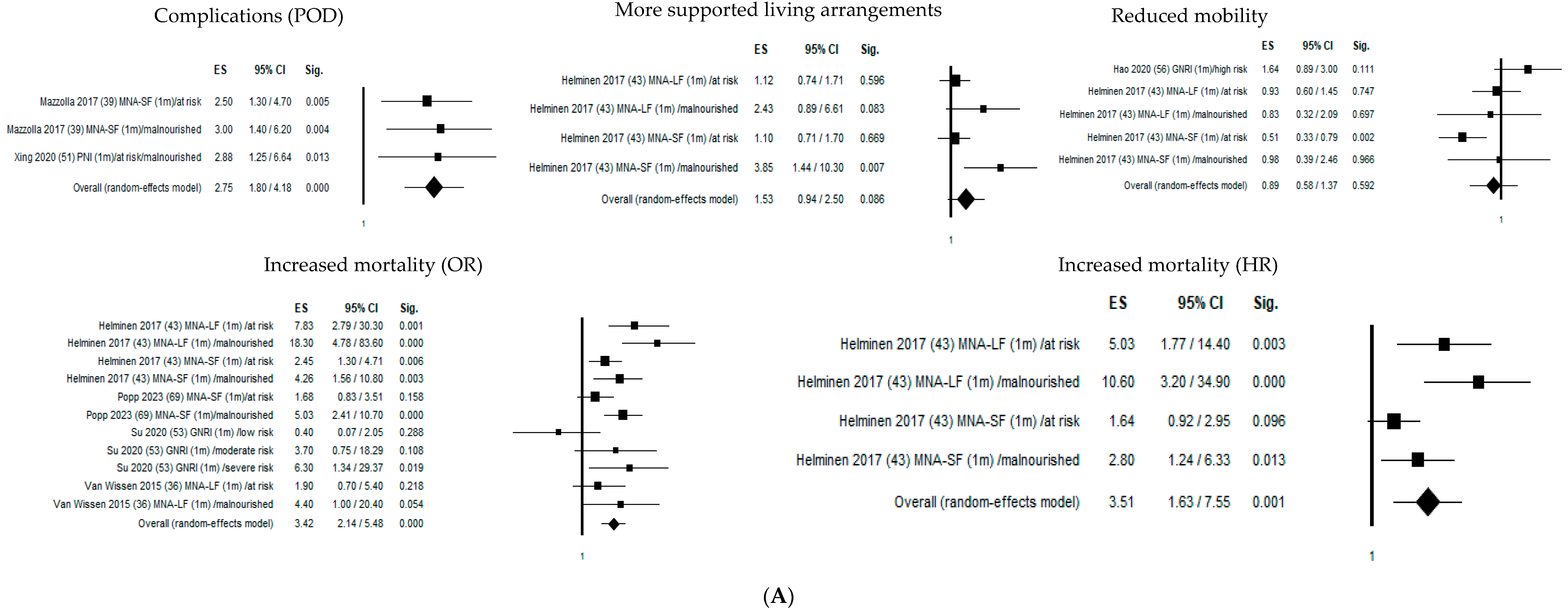
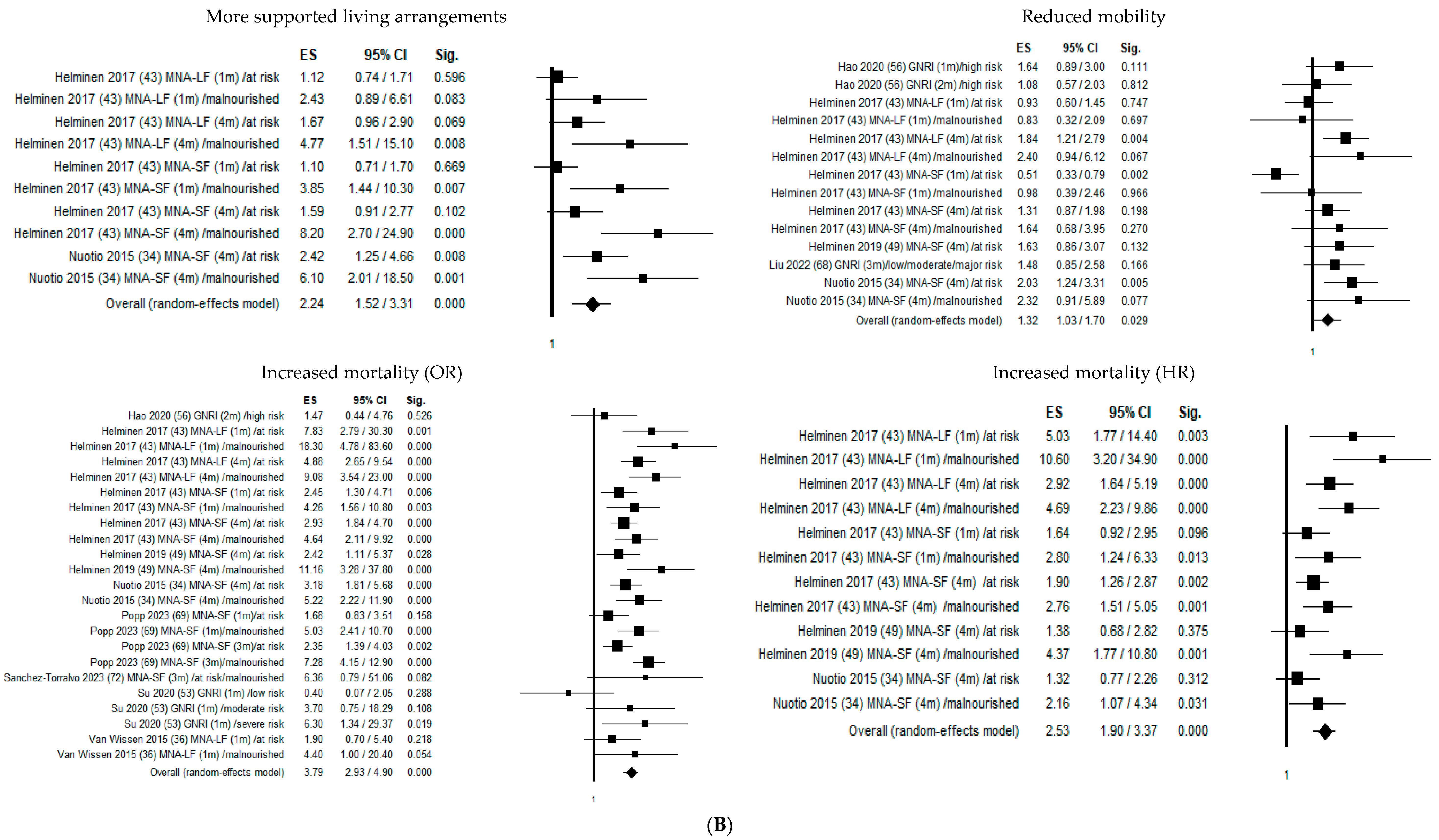
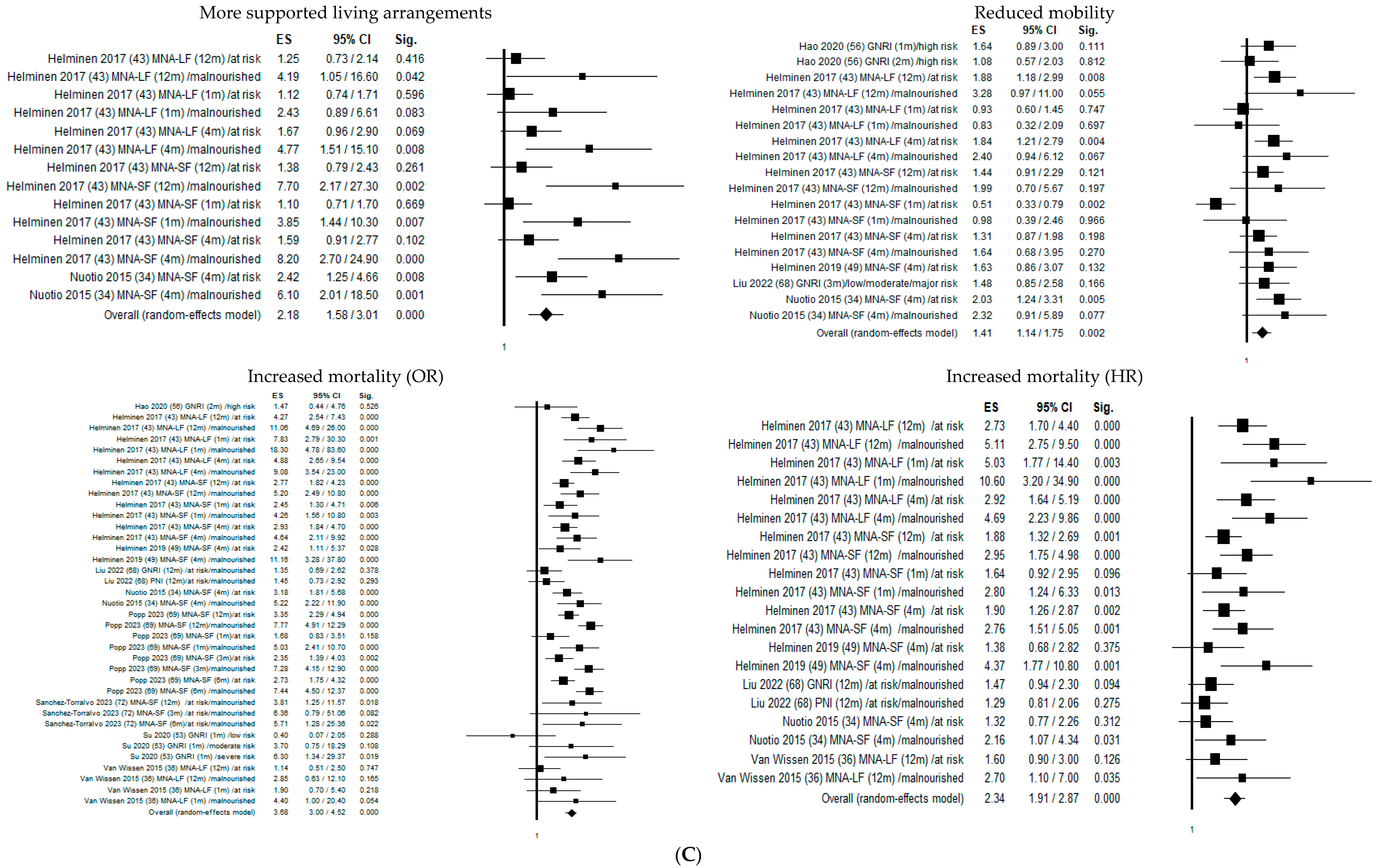
Figure 2 shows the forest plots of the selected studies that examined the associations between nutritional status and outcome at 1 month, 4 months, and 1 year.
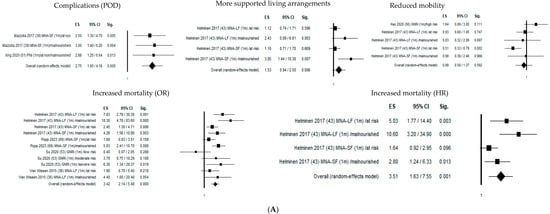
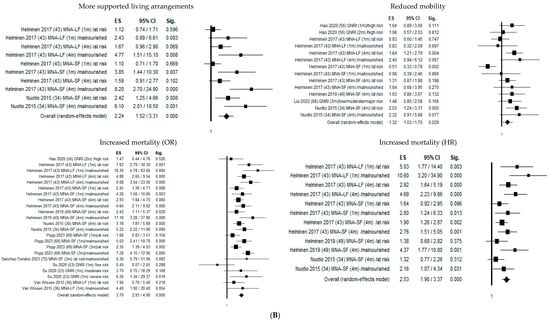
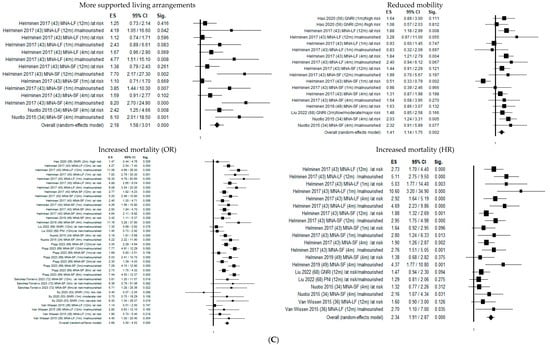
Figure 2.
Forest plots of the selected studies that examined the associations between nutritional status and outcome at 1 month, 4 months, and 1 year. (A) Follow-up 1 month. (B) Follow-up 4 months. (C) Follow-up 12 months.
One month after hip fracture, malnutritional status significantly increased delirium risk by 275% (OR = 2.75; 95% CI 1.80–4.18; p ≤ 0.05), mortality risk by 342% (OR = 3.42; 95% CI 2.14–5.48; p ≤ 0.05), and mortality hazard risk by 351% (HR = 3.51; 95% CI 1.63–7.55; p ≤ 0.05).
Four months from hip fracture, malnutrition significantly increased the probability of transfer to a higher level of care by 224% (OR = 2.24; 95% CI 1.52–3.31; p ≤ 0.05) and declined mobility risk by 32% (OR = 1.32; 95% CI 1.03–1.70; p ≤ 0.05), mortality risk by 379% (OR = 3.79; 95% CI 2.93–4.90; p ≤ 0.05), and mortality hazard risk by 253% (OR = 2.53; 95% CI 1.90–3.37; p ≤ 0.05).
One year after hip fracture, malnutrition significantly increased the probability of transfer to more supported living arrangements by 218% (OR = 2.18; 95% CI 1.58–3.01; p ≤ 0.05) and declined mobility risk by 41% (OR = 1.41; 95% CI 1.14–1.75; p ≤ 0.05), mortality risk by 368% (OR = 3.68; 95% CI 3.00–4.52; p ≤ 0.05), and mortality hazard risk by 234% (HR = 2.34; 95% CI 1.91–2.87; p ≤ 0.05). The heterogeneity was rather high in both pooled data (I2 = 85.94%), mobility (I2 = 51.31%), living arrangements (I2 = 64.54%), and mortality (I2 = 82.85% and I2 = 54.20% for OR and HR, respectively), while it was not present in delirium (I2 = 00.00%) (Table 2).
Furthermore, stratification by age group revealed significant findings in both any health negative outcome and mortality throughout the intervals of follow-up, while stratification by study type revealed significant findings in both case-control and cohort studies throughout the intervals of follow-up. Despite variations in the number of studies included, with cohort studies outnumbering case-control studies, the significance of the association remained consistent across study designs.
Because of the small amount of data, no further stratification according to gender, nutritional assessment, and different types of management of hip fractures were possible.
3.4. Sensitivity Analysis and Publication Bias
Table 2 reports results of both heterogeneity and publication bias tests. Considering the pooled data, on the basis of funnel plot symmetry for all outcomes), evidence of publication bias was detected at 1 month, 3 months, 4 months, 6 months, and 1 year. Accordingly, the corresponding statistical publication bias evaluation resulted in the p-value being significant for Begg/Egger’s test.
When results were stratified according to the specific outcome, a significant publication bias was observed only in mortality HR (Overall: Egger 0.005, Begg 0.022; 1-month All: Egger 0.12, Begg 0.042; 4-month All: Egger 0.011, Begg 0.020; 12-month All: Egger 0.007, Begg 0.023) and living arrangements (1-month All: Egger 0.035; 4-month All: Egger 0.00, Begg 0.002; 12-month All: Egger 0.00, Begg 0.01). No publication bias was detected in complication and mobility outcomes and in subgroup population aged >75 years. In addition, when results were stratified according to the type of outcome, a significant publication bias was observed only in the cohort studies on mortality HR (1, 4, and 12 months) and mortality OR (1 month).
A sensitivity analysis excluding Helminen 2017 [43], which caused asymmetry of the funnel plot, produced a combined risk estimate of:
- -
- 2.32 (95% CI 1.65–3.28; p ≤ 0.05) with I2 = 0.00%, p = 0.568, and p = 0.212 and p = 0.174 for publication bias by the Egger and Begg methods, respectively, for any health outcome after hip fracture (OR) at 1 month in cohort study;
- -
- 1.87 (95% CI 1.40–2.51; p ≤ 0.05) with I2 = 18.63%, p = 0.282, and p = 0.224 and p = 0.083 for publication bias by the Egger and Begg methods, respectively, for any health outcome after hip fracture (OR) at 3 months in cohort study;
- -
- 1.88 (95% CI 1.16–3.06; p ≤ 0.05) with I2 = 47.91%, p = 0.124, and p = 0.137 and p = 0.174 for publication bias by the Egger and Begg methods, respectively, for mortality HR after hip fracture at 4 months in cohort study;
- -
- 1.60 (95% CI 1.20–2.12; p ≤ 0.05) with I2 = 28.45%, p = 0.222, and p = 0.06 and p = 0.188 for publication bias by the Egger and Begg methods, respectively, for mortality HR after hip fracture at 1 year in cohort study.
4. Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to summarize the relationships between malnutrition and various health outcomes in older adults with hip fractures at different follow-up intervals. We considered data regarding all health outcomes and mortality, mobility, living arrangements, and complications, and analyzed them both together and separately for different follow-up intervals. Comparable results were observed in an earlier systematic review regarding the correlation between malnutrition and various clinical outcomes among hip fracture patients, including mortality rates, functional status, increased need for assisted living arrangements, and mobility levels [7,8]. We found a statistically significant association between malnutrition and any-health-negative-outcome risk in older subjects with hip fractures for all follow-up periods, with an increasing risk for longer follow-up periods. Malnutrition significantly increased any negative health outcome risk by 70% at 1 month to 250% at 1 year. While the malnourished category consistently showed significance since the first month of follow-up (OR = 3.01; 95% CI 1.75–5.17; p ≤ 0.05), the at-risk category emerged as significant from the fourth month of follow-up and did so progressively over time, suggesting a potential window for nutritional interventions to mitigate adverse outcomes following hip fracture. These findings underscore the critical role of nutritional status in influencing health outcomes among older individuals with hip fractures and suggest the importance of targeted interventions to improve nutritional status. With widespread rising life expectancy, there will be even more public health concern and an increasing need for initiatives in order to reduce this alarm.
The integration of both rehabilitation and nutritional therapy in older patients with hip fractures is a key strategy, resulting in decreased mortality and fewer postoperative complications, while also improving grip strength [77].
Therefore, it is advisable to endorse enhanced nutritional support alongside rehabilitation for individuals aged 65 years and older who have experienced hip fractures, aiming to mitigate mortality rates, minimize complications, and enhance ADL functionality [78].
We primarily focused on the association between hip fracture and malnutrition, overlooking the primary causes of hip fractures in older people: the role of diet and of osteoporosis. In fact, it is noted that diet is linked to the risk of osteoporotic fractures and plays a role in osteoporotic fracture healing and retaining their independence during osteoporosis prophylaxis [8,79,80].
4.1. Stratification Based on the Type of Outcome
Examining the mortality outcome, we observe a decline in the hazard ratio as the follow-up duration extends, with significance in the risk emerging noticeably from the fourth month onward, while the odds ratio concerning mortality rises with prolonged follow-up periods. The significance of living arrangements as an outcome becomes apparent from the fourth month, although it is important to underline the limited observations in the first month and the likelihood of patients still being hospitalized during this period. Similarly, the risk of declined mobility is significant from the fourth month, taking into account the probability of patients remaining hospitalized within the initial month. Lastly, postoperative delirium risk is significant in the only follow-up period available.
4.1.1. Mortality
Our results are consistent with those of two prior meta-analyses, which found that being at risk of malnutrition and malnourished nutritional status according to MNA were both significantly associated with higher total mortality [17,81], and that patients with low GNRI or low MNA-SF scores had a significantly higher risk of mortality compared to those with higher scores. Other studies included in the systematic review found a significant correlation between nutritional status and mortality from a qualitative point of view: at risk of malnutrition/malnourished status is negatively correlated with 3, 6, and 12 months mortality according to MNA [48], and with 6 months mortality according to CONUT score [50] and GNRI [59,71].
4.1.2. Postoperative Complications
A recent meta-analysis investigating the association between preoperative PNI and the incidence of POD in adult patients receiving surgery under general anesthesia demonstrated that a lower PNI was correlated with a higher risk of POD [82].
Other studies included in our systematic review evaluating postoperative complications such as urinary tract infection, heart failure, surgical site infection, refracture, pneumonia, arrhythmia, enteritis, liver dysfunction, implant failure, acute myocardial infarction, venous thromboembolism, confusion, delirium and dementia, decubitus, and the risk of falls found a significant correlation with GNRI and CONUT score [54,59,73].
4.1.3. Mobility
A previous systematic review highlighted that interventions aimed at enhancing mobility post-hip fracture may result in clinically significant improvements in mobility and walking speed, both during hospitalization and in post-hospital settings, compared to standard care practices [83].
Studies included in the systematic review evidenced how decreased mobility level at discharge is significantly associated with MNA-SF, GNRI [45], and PNI [76].
Walking speed (m/sec) 14 days postoperative or at discharge is significantly lower in malnourished (MNA-SF) and major-risk and mild-risk patients (GNRI).
Also, rehabilitation effectiveness calculated using the FIM Scale is significantly lower in malnourished patients according to MNA-SF and GNRI [45,46], and they were more likely to be unable to walk 6 months postoperatively [58,59].
4.1.4. Living Arrangements
Two studies included in the systematic review discovered that those who were malnourished according to MNA [33] and GNRI [58] exhibited notably lower scores in activities of daily living (ADL) within 48 h post-operation, upon discharge, and even at the 6-month mark post-discharge, but no significant relationship was found with discharge level of care [46].
4.2. Strengths and Limitations
The strength of this study lies in its approach of including several follow-up intervals for each outcome considered, which has not been seen before in a meta-analysis investigating health outcomes following hip fracture, as far as we are aware. Additionally, all included studies were of a high quality, scoring above 7 on the Newcastle–Ottawa Scale (NOS), ensuring reliability, even if we are aware of their possible limitations, especially in inter-rater agreement between observers of the NOS Scale [84,85,86].
Furthermore, this study stands out for evaluating various nutritional assessment tools to disclose associations with the outcomes examined.
Moreover, all articles included in this analysis were published within the last decade, reflecting the most current research in this field. However, several limitations should be acknowledged. The considerable heterogeneity observed across studies can be attributed to the diverse range of observations, nutritional assessments, and outcomes evaluated. Additionally, due to limited data availability, further stratification according to gender, nutritional assessment methods, or different types of hip fracture management was not possible.
Furthermore, while improvements in pre-surgery hospital stay and complications management have been noted, functional deterioration among hip fracture patients remains a concern in the literature. Specifically, although early surgery within 24 h or 48 h of admission has been associated with fewer postoperative complications, functional decline persists in many cases [87,88].
5. Conclusions
Older adult hip fracture patients exhibit a significant prevalence of malnutrition.
The present study underscores that malnutrition among older patients with hip fractures leads to an increased risk of mortality, impaired mobility, and loss of independence. Therefore, it is imperative to assess the nutritional status of older individuals with hip fractures and take proactive measures. Detecting malnutrition can aid in evaluating risks, initiating discussions about care goals, and making treatment decisions.
Our findings highlight the correlation between malnutrition and prognosis in older adults undergoing hip fracture surgery, supporting the utilization of nutritional assessment tools to furnish valuable prognostic insights. Incorporating these indicators into routine hospital admission procedures could prove beneficial in the management of hip fracture patients.
It is important to underline that all older patients can benefit from nutritional assessment and eventual appropriate interventions.
Nutritional rehabilitation stands out as a crucial strategy in preventing malnutrition, and the incorporation of nutritional substitution therapy presents a cost-effective and practicable solution. While there may be associated expenses with intensified nutritional interventions, the potential benefits are notable, including a potential reduction in the necessity for long-term care, thus alleviating the social burden.
Further research into nutritional interventions for older individuals with hip fractures is recommended and targeted interventions aimed at enhancing nutritional status during hip fracture management can be implemented based on these assessments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16071069/s1: Supplementary Table S1. Search strategy. Supplementary Table S2A. Newcastle–Ottawa Scale of the studies selected from the Cohort studies. Supplementary Table S2B. Newcastle–Ottawa Scale of the studies selected from the Case-Control studies. Supplementary Table S3. PRISMA Checklist (2020).
Author Contributions
Conceptualization—initiation, M.C. and P.B.; Conceptualization—agreement, A.G., G.M.R., M.I.F., A.R., O.D.T. and M.M.D.; Methodology—from literature search to meta-analysis, M.C., A.G., G.M.R., M.I.F., A.R., O.D.T., M.M.D. and P.B.; Formal analysis—meta-analysis, M.C., A.G., G.M.R. and P.B.; Data curation, A.G., G.M.R. and M.I.F.; Writing—original draft preparation, M.C., A.G., G.M.R. and P.B.; Writing—review and editing, M.C., A.G., G.M.R. and P.B.; Supervision, P.B. and M.M.D.; Project administration, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ensrud, K.E. Epidemiology of fracture risk with advancing age. J. Gerontol. Ser. A 2013, 68, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Maggi, S. Epidemiology and social costs of hip fracture. Injury 2018, 49, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef]
- Braithwaite, R.S.; Col, N.F.; Wong, J.B. Estimating hip fracture morbidity, mortality and costs. J. Am. Geriatr. Soc. 2003, 51, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Guzon-Illescas, O.; Perez Fernandez, E.; Crespí Villarias, N.; Quirós Donate, F.J.; Peña, M.; Alonso-Blas, C.; García-Vadillo, A.; Mazzucchelli, R. Mortality after osteoporotic hip fracture: Incidence, trends, and associated factors. J. Orthop. Surg. Res. 2019, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Gray, A.M.; Prieto-Alhambra, D.; Arden, N.K.; Cooper, C.; Javaid, M.K.; Judge, A.; REFReSH study group. Impact of hip fracture on hospital care costs: A population-based study. Osteoporos. Int. 2016, 27, 549–558. [Google Scholar] [CrossRef]
- Foo, M.X.E.; Wong, G.J.Y.; Lew, C.C.H. A systematic review of the malnutrition prevalence in hospitalized hip fracture patients and its associated outcomes. JPEN J. Parenter. Enteral. Nutr. 2021, 45, 1141–1152. [Google Scholar] [CrossRef]
- Millrose, M.; Schmidt, W.; Krickl, J.; Ittermann, T.; Ruether, J.; Bail, H.J.; Gesslein, M. Influence of Malnutrition on Outcome after Hip Fractures in Older Patients. J. Pers. Med. 2023, 13, 109. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485; discussion 485–487. [Google Scholar]
- Murphy, M.C.; Brooks, C.N.; New, S.A.; Lumbers, M.L. The use of the Mini-Nutritional Assessment (MNA) tool in elderly orthopaedic patients. Eur. J. Clin. Nutr. 2000, 54, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. (In Japanese) [Google Scholar]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Corish, C.A.; Bardon, L.A. Malnutrition in older adults: Screening and determinants. Proc. Nutr. Soc. 2019, 78, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, M.; Benetou, V.; Karapetyan, T.; Feskanich, D.; Grodstein, F.; Pettersson-Kymmer, U.; Eriksson, S.; Wilsgaard, T.; Jørgensen, L.; Ahmed, L.A.; et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: The CHANCES project. J. Intern. Med. 2017, 281, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lv, L.; Jiao, J.; Zhang, Y.; Zuo, X.L. Association between nutritional indices and mortality after hip fracture: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Koval, K.J.; Maurer, S.G.; Su, E.T.; Aharonoff, G.B.; Zuckerman, J.D. The Effects of Nutritional Status on Outcome After Hip Fracture. J. Orthop. Trauma 1999, 13, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Chivite, D.; Mascaró, J.; Ramón, J.M.; Pujol, R. No correlation between mininutritional assessment (short form) scale and clinical outcomes in 73 elderly patients admitted for hip fracture. Aging Clin. Exp. Res. 2005, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.G.; Beck, S.J.; Hood, K.; Johansen, A. Using Dietetic Assistants to Improve the Outcome of Hip Fracture: A Randomised Controlled Trial of Nutritional Support in an Acute Trauma Ward. Age Ageing 2006, 35, 148–153. [Google Scholar] [CrossRef]
- Olofsson, B.; Stenvall, M.; Lundström, M.; Svensson, O.; Gustafson, Y. Malnutrition in hip fracture patients: An intervention study. J. Clin. Nurs. 2007, 16, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Yang, H.; Qian, H.; Huang, L.; Guo, Z.; Tang, T. The Effects of Different Nutritional Measurements on Delayed Wound Healing After Hip Fracture in the Elderly. J. Surg. Res. 2010, 159, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.A.; Dowrick, A.S.; Liew, S.M. Nutritional status and short-term outcome of hip arthroplasty. J. Orthop. Surg. 2012, 20, 331–335. [Google Scholar] [CrossRef]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Grosman, B.; Frishman, S.; Salai, M.; Beloosesky, Y.-Y. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin. Nutr. 2012, 31, 917–921. [Google Scholar] [CrossRef]
- Gumieiro, D.N.; Rafacho, B.P.; Gonçalves, A.F.; Tanni, S.E.; Azevedo, P.S.; Sakane, D.T.; Carneiro, C.A.; Gaspardo, D.; Zornoff, L.A.; Pereira, G.J.; et al. Mini Nutritional Assessment predicts gait status and mortality 6 months after hip fracture. Br. J. Nutr. 2013, 109, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Cheng, H.S.; Liang, J.; Wu, C.C.; Shyu, Y.I.L. Functional recovery of older people with hip fracture: Does malnutrition make a difference? J. Adv. Nurs. 2013, 69, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Goisser, S.; Schrader, E.; Singler, K.; Bertsch, T.; Gefeller, O.; Biber, R.; Bail, H.J.; Sieber, C.C.; Volkert, D. Malnutrition According to Mini Nutritional Assessment Is Associated with Severe Functional Impairment in Geriatric Patients Before and up to 6 Months After Hip Fracture. J. Am. Med. Dir. Assoc. 2015, 16, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Nuotio, M.; Tuominen, P.; Luukkaala, T. Association of nutritional status as measured by the Mini-Nutritional Assessment Short Form with changes in mobility, institutionalization and death after hip fracture. Eur. J. Clin. Nutr. 2016, 70, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Anbar, R.; Gross Nevo, R.F.; Schlesinger, A.; Frishman, S.; Salai, M.; Beloosesky, Y. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin. Nutr. 2016, 35, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Van Wissen, J.; van Stijn, M.F.; Doodeman, H.J.; Houdijk, A.P. Mini Nutritional Assessment and Mortality after Hip Fracture Surgery in the Elderly. J. Nutr. Health Aging 2016, 20, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, F.; Parchi, P.D.; Vigorito, A.; Pasqualetti, G.; Monzani, F.; Lisanti, M. The Correlation between Preoperative Levels of Albumin and Tlc and Mortality in Patients with Femoral Neck Fracture. J. Biol. Regul. Homeost. Agents 2016, 30, 187–191. [Google Scholar]
- Morice, A.; Reina, N.; Gracia, G.; Bonnevialle, P.; Laffosse, J.-M.; Wytrykowski, K.; Cavaignac, E.; Bonnevialle, N. Proximal Femoral Fractures in Centenarians. A Retrospective Analysis of 39 Patients. Orthop. Traumatol. Surg. Res. OTSR 2017, 103, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.; Ward, L.; Zazzetta, S.; Broggini, V.; Anzuini, A.; Valcarcel, B.; Brathwaite, J.S.; Pasinetti, G.M.; Bellelli, G.; Annoni, G. Association Between Preoperative Malnutrition and Postoperative Delirium After Hip Fracture Surgery in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 1222–1228. [Google Scholar] [CrossRef]
- Miu, K.Y.D.; Lam, P.S. Effects of Nutritional Status on 6-Month Outcome of Hip Fractures in Elderly Patients. Ann. Rehabil. Med. 2017, 41, 1005–1012. [Google Scholar] [CrossRef]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Pre-fracture nutritional status is predictive of functional status at discharge during the acute phase with hip fracture patients: A multicenter prospective cohort study. Clin. Nutr. 2017, 36, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M.S. Changes in nutritional status and associated factors in a geriatric post-hip fracture assessment. Eur. Geriatr. Med. 2017, 8, 134–139. [Google Scholar] [CrossRef]
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M. Comparison of the Mini-Nutritional Assessment short and long form and serum albumin as prognostic indicators of hip fracture outcomes. Injury 2017, 48, 903–908. [Google Scholar] [CrossRef]
- Ren, H.; Wu, L.; Hu, W.; Ye, X.; Yu, B. Prognostic value of the c-reactive protein/prognostic nutritional index ratio after hip fracture surgery in the elderly population. Oncotarget 2017, 8, 61365–61372. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Misu, S.; Tanaka, T.; Kakehi, T.; Ono, R. Acute phase nutritional screening tool associated with functional outcomes of hip fracture patients: A longitudinal study to compare MNA-SF, MUST, NRS-2002 and GNRI. Clin. Nutr. 2019, 38, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Momosaki, R. Nutritional Status Changes and Activities of Daily Living after Hip Fracture in Con-valescent Rehabilitation Units: A Retrospective Observational Cohort Study from the Japan Rehabilitation Nutrition Database. J. Acad. Nutr. Diet. 2018, 118, 1270–1276. [Google Scholar] [CrossRef]
- Kramer, I.F.; Blokhuis, T.J.; Verdijk, L.B.; van Loon, L.J.C.; Poeze, M. Perioperative nutritional supplementation and skeletal muscle mass in older hip-fracture patients. Nutr. Rev. 2019, 77, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gortan Cappellari, G.; Ratti, C.; Ceschia, G.; Murena, L.; De Colle, P.; Barazzoni, R. Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip-fracture. Clin. Nutr. 2019, 38, 1607–1612. [Google Scholar] [CrossRef]
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M.S. Predictive Value of the Mini-Nutritional Assessment Short Form (MNA-SF) and Nutritional Risk Screening (NRS2002) in Hip Fracture. Eur. J. Clin. Nutr. 2019, 73, 112–120. [Google Scholar] [CrossRef]
- Kotera, A. Geriatric Nutritional Risk Index and Controlling Nutritional Status Score can predict postoperative 180-day mortality in hip fracture surgeries. JA Clin. Rep. 2019, 5, 62. [Google Scholar] [CrossRef]
- Xing, H.; Xiang, D.; Li, Y.; Ji, X.; Xie, G. Preoperative prognostic nutritional index predicts postoperative delirium in elderly patients after hip fracture surgery. Psychogeriatrics 2020, 20, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hill-Mündel, K.; Schlegl, J.; Biesalski, H.K.; Ehnert, S.; Schröter, S.; Bahrs, C.; Nohr, D.; Nüssler, A.K.; Ihle, C. Preoperative Ascorbic Acid Levels in Proximal Femur Fracture Patients Have No Postoperative Clinical Impact, While Ascorbic Acid Levels upon Discharge Have a Major Effect on Postoperative Outcome. J. Clin. Med. 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Wu, S.C.; Huang, C.Y.; Chou, S.E.; Tsai, C.H.; Li, C.; Hsu, S.Y.; Hsieh, C.H. Geriatric Nutritional Risk Index as a Screening Tool to Identify Patients with Malnutrition at a High Risk of In Hospital Mortality among Elderly Patients with Femoral Fractures-A Ret-rospective Study in a Level I Trauma Center. Int. J. Environ. Res. Public Health 2020, 17, 8920. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Oshita, Y.; Okano, I.; Kuroda, T.; Ishikawa, K.; Nagai, T.; Inagaki, K. Controlling nutritional status score predicts postoperative complications after hip fracture surgery. BMC Geriatr. 2020, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Sezen, Ö.; Cevik, B. The Impact of Preoperative Nutritional Status of Elderly Patients on the Postoperative Outcome: Comparison of Two Nutritional Assessment Tests and Biochemical Tools. Cyprus J. Med. Sci. 2020, 5, 333–338. [Google Scholar] [CrossRef]
- Hao, L.; Carson, J.L.; Schlussel, Y.; Noveck, H.; Shapses, S.A. Vitamin D Deficiency Is Associated with Reduced Mobility after Hip Fracture Surgery: A Prospective Study. Am. J. Clin. Nutr. 2020, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Fu, S.; Yao, Y.; Yuan, W.; Zhao, Y. Age, Prognostic Nutritional Index, and Charlson Comorbidity Index Were Independent Risk Factors for Postoperative Long-Term Mortality in Chinese Geriatric Patients Who Sustain Hip Fracture. J. Am. Med. Dir. Assoc. 2021, 22, 2602–2603. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, Y.; Matsuura, M.; Shiba, S.; Nishikimi, T. Effect of heart failure and malnutrition, alone and in combination, on rehabilitation effectiveness in patients with hip fracture. Clin. Nutr. ESPEN 2021, 44, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ukai, T.; Watanabe, M. Effect of nutritional status before femoral neck fracture surgery on postoperative outcomes: A retrospective study. BMC Musculoskelet. Disord. 2021, 22, 1027. [Google Scholar] [CrossRef]
- O’Leary, L.; Jayatilaka, L.; Leader, R.; Fountain, J. Poor nutritional status correlates with mortality and worse postoperative outcomes in patients with femoral neck fractures. Bone Jt. J. 2021, 103-B, 164–169. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Kayashita, J.; Taketani, Y.; Momosaki, R. Predictive validity of the Mini Nutritional Assessment Short-Form for rehabilitation patients: A retrospective analysis of the Japan Rehabilitation Nutrition Database. J. Hum. Nutr. Diet. 2021, 34, 881–889. [Google Scholar] [CrossRef]
- Han, T.S.; Yeong, K.; Lisk, R.; Fluck, D.; Fry, C.H. Prevalence and consequences of malnutrition and malnourishment in older individuals admitted to hospital with a hip fracture. Eur. J. Clin. Nutr. 2021, 75, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Cao, A.; Luo, W.; Xu, Z.; Sheng, Z.; Wang, J.; Zhu, B. Modified Frailty Index Combined with a Prognostic Nutritional Index for Predicting Postoperative Complications of Hip Fracture Surgery in Elderly. J. Investig. Surg. 2022, 35, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, W.; Ping, P.; Ma, T.; Li, Y.; Xu, L.; Feng, Z.; Zhao, Y.; Fu, S. Preoperative malnutrition as an independent risk factor for the postoperative mortality in elderly Chinese individuals undergoing hip surgery: A singlecenter observational study. Ther. Adv. Chronic Dis. 2022, 13, 20406223221102739. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, O.; Özkalkanlı, M.Y.; Yılmaz, F.; Altay, T. Comparison of malnutrition screening tests in predicting postoperative complications in elderly patients with femur fracture. Ain-Shams J. Anesthesiol. 2022, 14, 83. [Google Scholar] [CrossRef]
- Tseng, M.Y.; Liang, J.; Wu, C.C.; Cheng, H.S.; Chen, C.Y.; Lin, Y.E.; Weng, C.J.; Yu, Y.H.; Shyu, Y.L. Influence of Nutritional Status on a Family-Centered Care Intervention for Older Adults with Cognitive Impairment following Hip-Fracture Surgery: Secondary Data Analysis of a Randomized Controlled Trial. J. Nutr. Health Aging 2022, 26, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Setoguchi, T.; Ishidou, Y.; Taniguchi, N. Low geriatric nutritional risk index is a risk f actor for death within 1 year following hip fracture. J. Orthop. Surg. 2022, 30, 10225536221103360. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, S.; Yang, C.; Zhang, T.; Han, N.; Pan, Y.; Xu, X.; Lin, J.; Sun, G. Prealbumin as a nutrition status indicator may be associated with outcomes of geriatric hip fractures: A propensity score matching and 1-year follow-up study. Aging Clin. Exp. Res. 2022, 34, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Popp, D.; Nia, A.; Biedermann, G.; Schmoelz, L.; Silvaieh, S.; Tiefenboeck, T.M.; Hajdu, S.; Widhalm, H.K. Predictive Validity of Mortality after Surgically Treated Proximal Femur Fractures Based on Four Nutrition Scores-A Retrospective Data Analysis. Nutrients 2023, 15, 3357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, W.; Yan, J.; Yang, Z.; Li, C.; Wu, D.; Wang, T.; Zhang, Y.; Zhu, Y. Association of preoperative nutritional status evaluated by the controlling nutritional status score with walking independence at 180 days postoperatively: A prospective cohort study in Chinese older patients with hip fracture. Int. J. Surg. 2023, 109, 2660–2671. [Google Scholar] [CrossRef]
- Tsutsui, T.; Fujiwara, T.; Matsumoto, Y.; Kimura, A.; Kanahori, M.; Arisumi, S.; Oyamada, A.; Ohishi, M.; Ikuta, K.; Tsuchiya, K.; et al. Geriatric nutritional risk index as the prognostic factor in older patients with fragility hip fractures. Osteoporos. Int. 2023, 34, 1207–1221. [Google Scholar] [CrossRef]
- Sánchez-Torralvo, F.J.; Pérez-Del-Río, V.; García-Olivares, M.; Porras, N.; Abuín-Fernández, J.; Bravo-Bardají, M.F.; García-de-Quevedo, D.; Olveira, G. Global Subjective Assessment and Mini Nutritional Assessment Short Form Better Predict Mortality Than GLIM Malnutrition Criteria in Elderly Patients with Hip Fracture. Nutrients 2023, 15, 1828. [Google Scholar] [CrossRef]
- Berk, T.; Thalmann, M.; Jensen, K.O.; Schwarzenberg, P.; Jukema, G.N.; Pape, H.C.; Halvachizadeh, S. Implementation of a novel nursing assessment tool in geriatric trauma patients with proximal femur fractures. PLoS ONE 2023, 18, e0284320. [Google Scholar] [CrossRef] [PubMed]
- Fernández Miró, M.; Cabrejo Gavidia, V.; Carrascosa Piquer, O.; Valero Lanau, J.; Toapanta Valencia, M.; Aguado Jodar, A. Malnutrition is associated with postoperative complications in elderly patients undergoing total hip arthroplasty. Endocrinol. Diabetes Nutr. Engl. Ed. 2023, 70 (Suppl. S3), 59–66. [Google Scholar] [CrossRef]
- Rutenberg, T.F.; Gabarin, R.; Kilimnik, V.; Daglan, E.; Iflah, M.; Zach, S.; Shemesh, S. Nutritional and Inflammatory Indices and the Risk of Surgical Site Infection After Fragility Hip Fractures: Can Routine Blood Test Point to Patients at Risk? Surg. Infect. 2023, 24, 645–650. [Google Scholar] [CrossRef]
- Faust, L.M.; Lerchenberger, M.; Gleich, J.; Linhart, C.; Keppler, A.M.; Schmidmaier, R.; Böcker, W.; Neuerburg, C.; Zhang, Y. Predictive Value of Prognostic Nutritional Index for Early Postoperative Mobility in Elderly Patients with Pertrochanteric Fracture Treated with Intramedullary Nail Osteosynthesis. J. Clin. Med. 2023, 12, 1792. [Google Scholar] [CrossRef]
- Takahashi, K.; Momosaki, R.; Yasufuku, Y.; Nakamura, N.; Maeda, K. Nutritional Therapy in Older Patients with Hip Fractures Undergoing Rehabilitation: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1364–1364.e6. [Google Scholar] [CrossRef]
- Nishioka, S.; Aragane, H.; Suzuki, N.; Yoshimura, Y.; Fujiwara, D.; Mori, T.; Kanehisa, Y.; Iida, Y.; Higashi, K.; Yoshimura-Yokoi, Y.; et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin. Nutr. ESPEN 2021, 43, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns in Relation to Low Bone Mineral Density and Fracture Risk: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Chiavarini, M.; Naldini, G.; Fabiani, R. The Role of Diet in Osteoporotic Fracture Healing: A Systematic Review. Curr. Osteoporos. Rep. 2020, 18, 138–147. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Zheng, H.; Wang, X.; Liu, Z.; Sun, T. Prognostic Role of Serum Albumin, Total Lymphocyte Count, and Mini Nutritional Assessment on Outcomes After Geriatric Hip Fracture Surgery: A Meta-Analysis and Systematic Review. J. Arthroplast. 2019, 34, 1287–1296. [Google Scholar] [CrossRef]
- Hung, K.C.; Chiu, C.C.; Hsu, C.W.; Ho, C.N.; Ko, C.C.; Chen, I.W.; Sun, C.K. Association of preoperative prognostic nutritional index with risk of postoperative delirium: A systematic review and meta-analysis. Front. Med. 2023, 9, 1017000. [Google Scholar] [CrossRef] [PubMed]
- Fairhall, N.J.; Dyer, S.M.; Mak, J.C.; Diong, J.; Kwok, W.S.; Sherrington, C. Interventions for improving mobility after hip fracture surgery in adults. Cochrane Database Syst. Rev. 2022, 9, CD001704. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Oremus, C.; Hall, G.B.; McKinnon, M.C.; ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ Open 2012, 2, e001368. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Dryden, D.M. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Bellelli, G.; Mazzola, P.; Corsi, M.; Zambon, A.; Corrao, G.; Castoldi, G.; Zatti, G.; Annoni, G. The combined effect of ADL impairment and delay in time from fracture to surgery on 12-month mortality: An observational study in orthogeriatric patients. J. Am. Med. Dir. Assoc. 2012, 13, 664.e9–664.e14. [Google Scholar] [CrossRef]
- Hip Fracture: Management; National Institute for Health and Care Excellence (NICE): London, UK, 2023; Available online: https://www.nice.org.uk/guidance/cg124 (accessed on 26 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).