The Role of the FODMAP Diet in IBS
Abstract
1. Introduction
2. Low FODMAP Diet
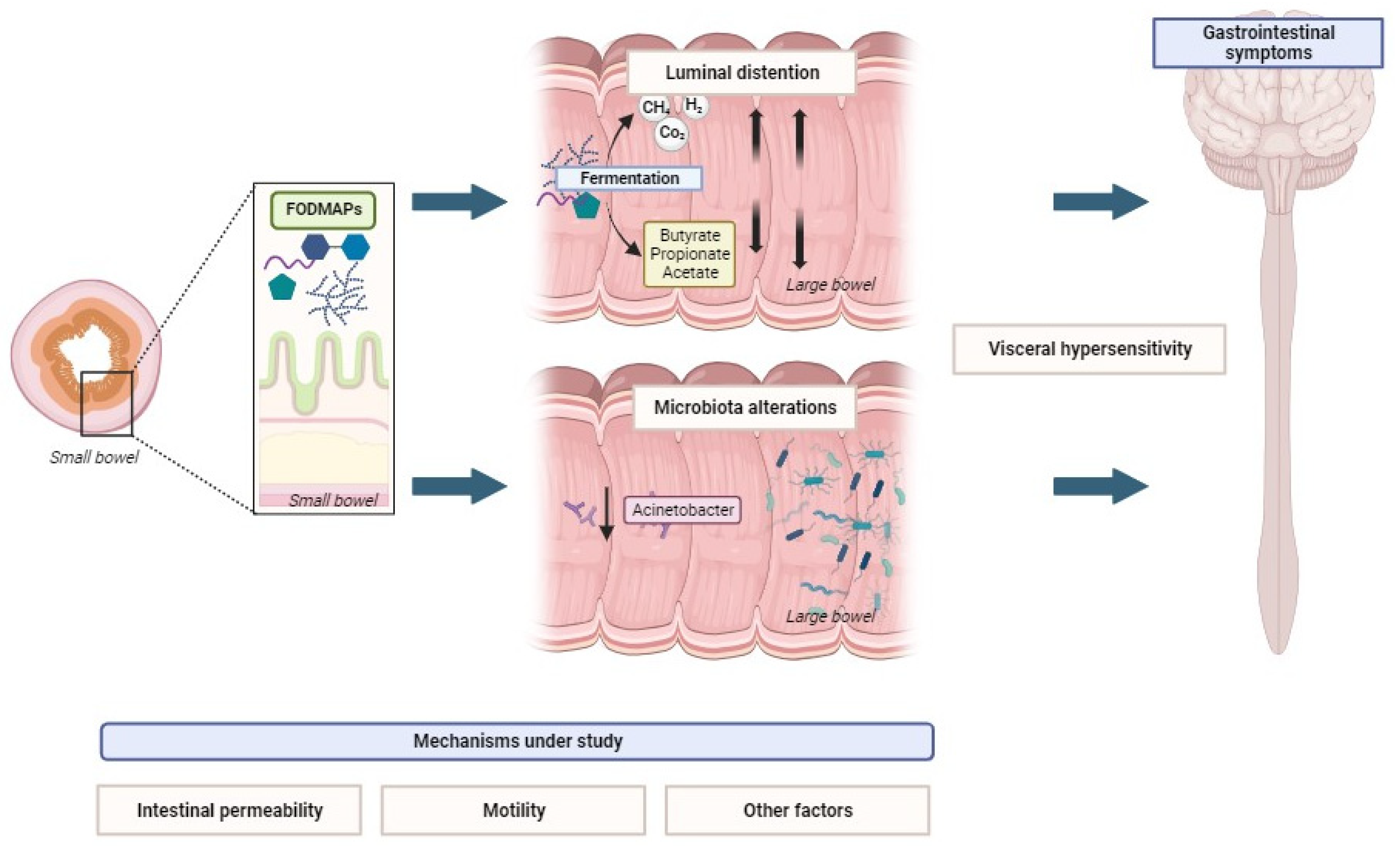
2.1. Luminal Distention by Gas and Water
2.2. Visceral Hypersensitivity
2.3. Increased Intestinal Permeability
2.4. Microbiota Alterations, SCFA Production, and Metabolome
2.5. Motility
3. Symptom Improvement with Low FODMAP Diets
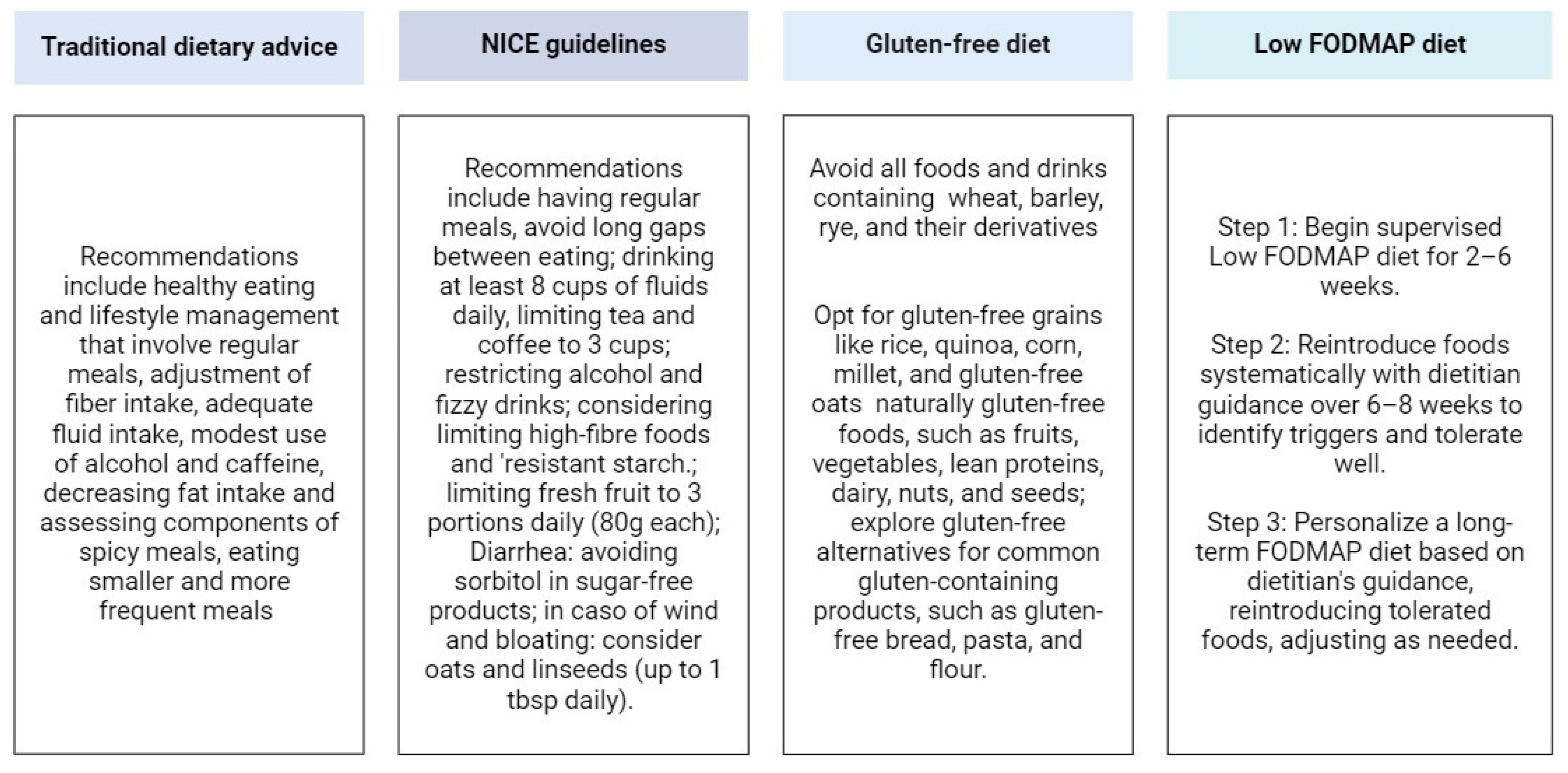
3.1. Low FODMAP Diet Implementation
3.2. Efficacy Compared to Other Diets and Other Treatments for IBS
3.3. Treatment Personalization
4. FODMAP Diet Response Markers
4.1. Breath Test
4.2. Microbiota Analyses
4.3. Fecal and Urinary Metabolites
5. Downsides of the Low FODMAP Diet
5.1. Social and Lifestyle Challenges
5.2. Nutritional Guidance
5.3. Nutritional Deficiencies
5.4. Microbiota Alterations
5.5. Defining the Low and High FODMAP Content of Food
5.6. Constipation
5.7. Eating Disorders and Psychiatric Comorbidities
5.8. Limited Long-Term Data
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Effect of Gender on Prevalence of Irritable Bowel Syndrome in the Community: Systematic Review and Meta-Analysis. Off. J. Am. Coll. Gastroenterol. ACG 2012, 107, 991. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional Bowel Disorders with Diarrhoea: Clinical Guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian Guidelines for the Management of Irritable Bowel Syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and Depression Comorbidities in Irritable Bowel Syndrome (IBS): A Systematic Review and Meta-Analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Cassar, G.E.; Youssef, G.J.; Knowles, S.; Moulding, R.; Austin, D.W. Health-Related Quality of Life in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Gastroenterol. Nurs. 2020, 43, E102. [Google Scholar] [CrossRef]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Bercik, P.; Boeckxstaens, G. Understanding Neuroimmune Interactions in Disorders of Gut-Brain Interaction: From Functional to Immune-Mediated Disorders. Gut 2023, 72, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for Protease Activity in Visceral Pain in Irritable Bowel Syndrome. J. Clin. Investig. 2007, 117, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Lizarraga, J.; Hussein, H.; Boeckxstaens, G.E. Immune Activation in Irritable Bowel Syndrome: What Is the Evidence? Nat. Rev. Immunol. 2022, 22, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of Irritable Bowel Syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Black, C.J.; Teasdale, S.B.; Mikocka-Walus, A.; Keefer, L. Irritable Bowel Syndrome and Mental Health Comorbidity—Approach to Multidisciplinary Management. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 582–596. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Gut Microbiota Signatures and Modulation in Irritable Bowel Syndrome. Microbiome Res. Rep. 2022, 1, 11. [Google Scholar] [CrossRef]
- Black, C.J.; Burr, N.E.; Camilleri, M.; Earnest, D.L.; Quigley, E.M.; Moayyedi, P.; Houghton, L.A.; Ford, A.C. Efficacy of Pharmacological Therapies in Patients with IBS with Diarrhoea or Mixed Stool Pattern: Systematic Review and Network Meta-Analysis. Gut 2020, 69, 74–82. [Google Scholar] [CrossRef]
- Black, C.J.; Burr, N.E.; Ford, A.C. Relative Efficacy of Tegaserod in a Systematic Review and Network Meta-Analysis of Licensed Therapies for Irritable Bowel Syndrome With Constipation. Clin. Gastroenterol. Hepatol. 2020, 18, 1238–1239.e1. [Google Scholar] [CrossRef]
- Black, C.J.; Burr, N.E.; Quigley, E.M.M.; Moayyedi, P.; Houghton, L.A.; Ford, A.C. Efficacy of Secretagogues in Patients With Irritable Bowel Syndrome With Constipation: Systematic Review and Network Meta-Analysis. Gastroenterology 2018, 155, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Yuan, Y.; Selinger, C.P.; Camilleri, M.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of Soluble Fibre, Antispasmodic Drugs, and Gut–Brain Neuromodulators in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Savarino, E.V.; Black, C.J.; Ford, A.C. Placebo Response Rates in Trials of Licensed Drugs for Irritable Bowel Syndrome With Constipation or Diarrhea: Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e923–e944. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated with More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Dhoble, P.; Desai, D.; Joshi, A.; Gupta, T. Self-Reported Food Intolerances in an Indian Population: Need for Individualization Rather than a Universal Low-FODMAP Diet. JGH Open Open Access J. Gastroenterol. Hepatol. 2023, 7, 772–776. [Google Scholar] [CrossRef]
- Torres, M.J.; Sabate, J.-M.; Bouchoucha, M.; Buscail, C.; Hercberg, S.; Julia, C. Food Consumption and Dietary Intakes in 36,448 Adults and Their Association with Irritable Bowel Syndrome: Nutrinet-Santé Study. Ther. Adv. Gastroenterol. 2018, 11, 1756283X17746625. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Bertin, L.; Maniero, D.; Palo, M.; Lorenzon, G.; Barberio, B.; Ciacci, C.; Savarino, E.V. Myths and Facts about Food Intolerance: A Narrative Review. Nutrients 2023, 15, 4969. [Google Scholar] [CrossRef]
- Chey, W.D.; Keefer, L.; Whelan, K.; Gibson, P.R. Behavioral and Diet Therapies in Integrated Care for Patients With Irritable Bowel Syndrome. Gastroenterology 2021, 160, 47–62. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef]
- Singh, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The Role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a Low FODMAP Diet in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Personal View: Food for Thought—Western Lifestyle and Susceptibility to Crohn’s Disease. The FODMAP Hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Braverman, D.; Stankiewicz, H. Carbohydrate Malabsorption and the Effect of Dietary Restriction on Symptoms of Irritable Bowel Syndrome and Functional Bowel Complaints. Isr. Med. Assoc. J. IMAJ 2000, 2, 583–587. [Google Scholar] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Fructose Malabsorption and Symptoms of Irritable Bowel Syndrome: Guidelines for Effective Dietary Management. J. Am. Diet. Assoc. 2006, 106, 1631–1639. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary Triggers of Abdominal Symptoms in Patients with Irritable Bowel Syndrome: Randomized Placebo-Controlled Evidence. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2008, 6, 765–771. [Google Scholar] [CrossRef]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of Dietary Short Chain Carbohydrates Alters the Pattern of Gas Production and Genesis of Symptoms in Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary Poorly Absorbed, Short-Chain Carbohydrates Increase Delivery of Water and Fermentable Substrates to the Proximal Colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of Fructans, Galacto-Oligosacharides and Other Short-Chain Carbohydrates in Processed Grains and Cereals. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2011, 24, 154–176. [Google Scholar] [CrossRef]
- Tuck, C.J.; Muir, J.G.; Barrett, J.S.; Gibson, P.R. Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols: Role in Irritable Bowel Syndrome. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 819–834. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential Effects of FODMAPs (Fermentable Oligo-, Di-, Mono-Saccharides and Polyols) on Small and Large Intestinal Contents in Healthy Subjects Shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef]
- Biesiekierski, J.R. Fructose-Induced Symptoms beyond Malabsorption in FGID. United Eur. Gastroenterol. J. 2014, 2, 10–13. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Lomer, M.C.E.; Gibson, P.R. Short-Chain Carbohydrates and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 707–717. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Olesen, S.S.; Materna, A.; Drewes, A.M. Fermentable Sugar Ingestion, Gas Production, and Gastrointestinal and Central Nervous System Symptoms in Patients with Functional Disorders. Gastroenterology 2018, 155, 1034–1044.e6. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133.e2. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.R.; Jørgensen, J.; Mortensen, P.B. Comparison of Diarrhea Induced by Ingestion of Fructooligosaccharide Idolax and Disaccharide Lactulose: Role of Osmolarity versus Fermentation of Malabsorbed Carbohydrate. Dig. Dis. Sci. 1998, 43, 2696–2707. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-Based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Christopher, N.L.; Bayless, T.M. Role of the Small Bowel and Colon in Lactose-Induced Diarrhea. Gastroenterology 1971, 60, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.C.; Brown, M.L.; Phillips, S.F. Decreased Fluid Tolerance, Accelerated Transit, and Abnormal Motility of the Human Colon Induced by Oleic Acid. Gastroenterology 1986, 91, 100–107. [Google Scholar] [CrossRef]
- Marciani, L.; Cox, E.F.; Hoad, C.L.; Pritchard, S.; Totman, J.J.; Foley, S.; Mistry, A.; Evans, S.; Gowland, P.A.; Spiller, R.C. Postprandial Changes in Small Bowel Water Content in Healthy Subjects and Patients with Irritable Bowel Syndrome. Gastroenterology 2010, 138, 469–477.e1. [Google Scholar] [CrossRef]
- Dellschaft, N.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Small Bowel Water Content Assessed by MRI in Health and Disease: A Collation of Single-Centre Studies. Aliment. Pharmacol. Ther. 2022, 55, 327–338. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Cong, Y.; Chu, H.; Fried, M.; Dai, N.; Fox, M. Bloating and Distention in Irritable Bowel Syndrome: The Role of Gas Production and Visceral Sensation after Lactose Ingestion in a Population with Lactase Deficiency. Am. J. Gastroenterol. 2013, 108, 1516–1525. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The Low FODMAP Diet: Recent Advances in Understanding Its Mechanisms and Efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A Low FODMAP Diet Is Associated with Changes in the Microbiota and Reduction in Breath Hydrogen but Not Colonic Volume in Healthy Subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef]
- Dorn, S.D.; Palsson, O.S.; Thiwan, S.I.M.; Kanazawa, M.; Clark, W.C.; van Tilburg, M.A.L.; Drossman, D.A.; Scarlett, Y.; Levy, R.L.; Ringel, Y.; et al. Increased Colonic Pain Sensitivity in Irritable Bowel Syndrome Is the Result of an Increased Tendency to Report Pain Rather than Increased Neurosensory Sensitivity. Gut 2007, 56, 1202–1209. [Google Scholar] [CrossRef]
- Cibert-Goton, V.; Lam, C.; Lingaya, M.; Falcone, Y.; Wood, J.N.; Bulmer, D.C.; Spiller, R. Pain Severity Correlates With Biopsy-Mediated Colonic Afferent Activation But Not Psychological Scores in Patients With IBS-D. Clin. Transl. Gastroenterol. 2021, 12, e00313. [Google Scholar] [CrossRef]
- Piché, M.; Chen, J.-I.; Roy, M.; Poitras, P.; Bouin, M.; Rainville, P. Thicker Posterior Insula Is Associated with Disease Duration in Women with Irritable Bowel Syndrome (IBS) Whereas Thicker Orbitofrontal Cortex Predicts Reduced Pain Inhibition in Both IBS Patients and Controls. J. Pain 2013, 14, 1217–1226. [Google Scholar] [CrossRef]
- Evans, P.R.; Piesse, C.; Bak, Y.T.; Kellow, J.E. Fructose-Sorbitol Malabsorption and Symptom Provocation in Irritable Bowel Syndrome: Relationship to Enteric Hypersensitivity and Dysmotility. Scand. J. Gastroenterol. 1998, 33, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.B.J.; Guiard, B.; Leveque, M.; Olier, M.; Jouanin, I.; Yvon, S.; Tondereau, V.; Rivière, P.; Guéraud, F.; Chevolleau, S.; et al. Lactose and Fructo-Oligosaccharides Increase Visceral Sensitivity in Mice via Glycation Processes, Increasing Mast Cell Density in Colonic Mucosa. Gastroenterology 2020, 158, 652–663.e6. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Grabauskas, G.; Zhou, S.-Y.; Gao, J.; Zhang, Y.; Owyang, C. High FODMAP Diet Causes Barrier Loss via Lipopolysaccharide-Mediated Mast Cell Activation. JCI Insight 2021, 6, e146529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP Diet Modulates Visceral Nociception by Lipopolysaccharide-Mediated Intestinal Inflammation and Barrier Dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef]
- Ajamian, M.; Rosella, G.; Newnham, E.D.; Biesiekierski, J.R.; Muir, J.G.; Gibson, P.R. Effect of Gluten Ingestion and FODMAP Restriction on Intestinal Epithelial Integrity in Patients with Irritable Bowel Syndrome and Self-Reported Non-Coeliac Gluten Sensitivity. Mol. Nutr. Food Res. 2021, 65, e1901275. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Prospero, L.; Riezzo, G.; Linsalata, M.; Orlando, A.; D’Attoma, B.; Russo, F. Psychological and Gastrointestinal Symptoms of Patients with Irritable Bowel Syndrome Undergoing a Low-FODMAP Diet: The Role of the Intestinal Barrier. Nutrients 2021, 13, 2469. [Google Scholar] [CrossRef] [PubMed]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The Fecal Microbiota of Irritable Bowel Syndrome Patients Differs Significantly From That of Healthy Subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Bonfiglio, F.; Belheouane, M.; Vallier, M.; Sauer, S.; Bang, C.; Bujanda, L.; Andreasson, A.; Agreus, L.; Engstrand, L.; et al. Faecal Microbiota Composition Associates with Abdominal Pain in the General Population. Gut 2018, 67, 778–779. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An Irritable Bowel Syndrome Subtype Defined by Species-Specific Alterations in Faecal Microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.C.; Franke, A.; Heinsen, F.-A.; Völker, U.; Völzke, H.; Aghdassi, A.A.; Mayerle, J.; Weiss, F.U.; et al. Functional Abdominal Pain and Discomfort (IBS) Is Not Associated with Faecal Microbiota Composition in the General Population. Gut 2019, 68, 1131–1133. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Andreasson, A.; Talley, N.J.; Forsberg, A.M.; Kjellström, L.; Schmidt, P.T.; Agreus, L.; Engstrand, L. No Distinct Microbiome Signature of Irritable Bowel Syndrome Found in a Swedish Random Population. Gut 2020, 69, 1076–1084. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Vandeputte, D.; Joossens, M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate Metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention on Gut Microbiota Composition in Healthy Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Choi, C.H.; Temas, D.; Kim, A.; Maier, D.M.; Scott, K.; Galanko, J.A.; Ringel, Y. Altered Colonic Bacterial Fermentation as a Potential Pathophysiological Factor in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2015, 110, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Ezzine, C.; Loison, L.; Montbrion, N.; Bôle-Feysot, C.; Déchelotte, P.; Coëffier, M.; Ribet, D. Fatty Acids Produced by the Gut Microbiota Dampen Host Inflammatory Responses by Modulating Intestinal SUMOylation. Gut Microbes 2022, 14, 2108280. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of Short-Chain Fatty Acids and Their Receptors in Inflammation and Carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Kannampalli, P.; Shaker, R.; Sengupta, J.N. Colonic Butyrate- Algesic or Analgesic? Neurogastroenterol. Motil. 2011, 23, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of Varying Dietary Content of Fermentable Short-Chain Carbohydrates on Symptoms, Fecal Microenvironment, and Cytokine Profiles in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- So, D.; Loughman, A.; Staudacher, H.M. Effects of a Low FODMAP Diet on the Colonic Microbiome in Irritable Bowel Syndrome: A Systematic Review with Meta-Analysis. Am. J. Clin. Nutr. 2022, 116, 943–952. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets That Differ in Their FODMAP Content Alter the Colonic Luminal Microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Valeur, J.; Røseth, A.G.; Knudsen, T.; Malmstrøm, G.H.; Fiennes, J.T.; Midtvedt, T.; Berstad, A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion 2016, 94, 50–56. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut Microbiota Bridges Dietary Nutrients and Host Immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The Food Metabolome: A Window over Dietary Exposure123. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef]
- Dekkers, K.F.; Sayols-Baixeras, S.; Baldanzi, G.; Nowak, C.; Hammar, U.; Nguyen, D.; Varotsis, G.; Brunkwall, L.; Nielsen, N.; Eklund, A.C.; et al. An Online Atlas of Human Plasma Metabolite Signatures of Gut Microbiome Composition. Nat. Commun. 2022, 13, 5370. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The Fecal Metabolome as a Functional Readout of the Gut Microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Nordin, E.; Hellström, P.M.; Vuong, E.; Ribbenstedt, A.; Brunius, C.; Landberg, R. IBS Randomized Study: FODMAPs Alter Bile Acids, Phenolic- and Tryptophan Metabolites, While Gluten Modifies Lipids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R248–R259. [Google Scholar] [CrossRef]
- Lin, H.C.; Elashoff, J.D.; Kwok, G.M.; Gu, Y.G.; Meyer, J.H. Stimulation of Duodenal Motility by Hyperosmolar Mannitol Depends on Local Osmoreceptor Control. Am. J. Physiol. 1994, 266, G940–G943. [Google Scholar] [CrossRef]
- Thor, P.; Laskiewicz, J.; Konturek, J.W.; Konturek, S.J.; Creutzfeldt, W. Role of GIP and Insulin in Glucose-Induced Changes in Intestinal Motility Patterns. Am. J. Physiol. 1987, 252, G8–G12. [Google Scholar] [CrossRef]
- Masuy, I.; Van Oudenhove, L.; Tack, J.; Biesiekierski, J.R. Effect of Intragastric FODMAP Infusion on Upper Gastrointestinal Motility, Gastrointestinal, and Psychological Symptoms in Irritable Bowel Syndrome vs Healthy Controls. Neurogastroenterol. Motil. 2018, 30, e13167. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Masuy, I.; Biesiekierski, J.R.; Fitzke, H.E.; Parikh, C.; Schofield, L.; Shaikh, H.; Bhagwanani, A.; Aziz, Q.; Taylor, S.A.; et al. Gut-Brain Axis Dysfunction Underlies FODMAP-Induced Symptom Generation in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2022, 55, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Moshiree, B.; Drossman, D.; Shaukat, A. AGA Clinical Practice Update on Evaluation and Management of Belching, Abdominal Bloating, and Distention: Expert Review. Gastroenterology 2023, 165, 791–800.e3. [Google Scholar] [CrossRef]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review Article: Implementation of a Diet Low in FODMAPs for Patients with Irritable Bowel Syndrome—Directions for Future Research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Gwee, K.A.; Gonlachanvit, S.; Ghoshal, U.C.; Chua, A.S.B.; Miwa, H.; Wu, J.; Bak, Y.-T.; Lee, O.Y.; Lu, C.-L.; Park, H.; et al. Second Asian Consensus on Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2019, 25, 343–362. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Tornkvist, N.T.; Törnblom, H. European Guidelines on Functional Bowel Disorders with Diarrhoea: United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) Statements and Recommendations. United Eur. Gastroenterol. J. 2022, 10, 615–616. [Google Scholar] [CrossRef]
- Chey, W.D.; Hashash, J.G.; Manning, L.; Chang, L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology 2022, 162, 1737–1745.e5. [Google Scholar] [CrossRef] [PubMed]
- British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Available online: https://www.bsg.org.uk/clinical-resource/british-society-of-gastroenterology-guidelines-on-the-management-of-irritable-bowel-syndrome/ (accessed on 10 December 2023).
- Dimidi, E.; Belogianni, K.; Whelan, K.; Lomer, M.C.E. Gut Symptoms during FODMAP Restriction and Symptom Response to Food Challenges during FODMAP Reintroduction: A Real-World Evaluation in 21,462 Participants Using a Mobile Application. Nutrients 2023, 15, 2683. [Google Scholar] [CrossRef]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 1824. [Google Scholar] [CrossRef]
- Goyal, O.; Batta, S.; Nohria, S.; Kishore, H.; Goyal, P.; Sehgal, R.; Sood, A. Low Fermentable Oligosaccharide, Disaccharide, Monosaccharide, and Polyol Diet in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Prospective, Randomized Trial. J. Gastroenterol. Hepatol. 2021, 36, 2107–2115. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-Free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S.; Shaw, C.C.; Buckle, R.; Trott, N.; Agrawal, A.; Aziz, I. Efficacy and Acceptability of Dietary Therapies in Non-Constipated Irritable Bowel Syndrome: A Randomized Trial of Traditional Dietary Advice, the Low FODMAP Diet, and the Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2022, 20, 2876–2887.e15. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Gibson, P.R. Traditional Dietary Advice, Low FODMAP Diet, or Gluten-Free Diet for IBS: Growing Understanding but Uncertainties Remain. Clin. Gastroenterol. Hepatol. 2023, 21, 1119–1120. [Google Scholar] [CrossRef] [PubMed]
- Natalia Pedersen, N.N.A.; Gh, L.J. Ehealth: Low FODMAP Diet vs Lactobacillus Rhamnosus GG in Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 16215–16226. [Google Scholar] [CrossRef]
- Menees, S.B.; Jackson, K.; Baker, J.R.; Fenner, D.E.; Eswaran, S.; Nojkov, B.; Saad, R.; Lee, A.A.; Chey, W.D. A Randomized Pilot Study to Compare the Effectiveness of a Low FODMAP Diet vs Psyllium in Patients With Fecal Incontinence and Loose Stools. Clin. Transl. Gastroenterol. 2022, 13, e00454. [Google Scholar] [CrossRef]
- Carbone, F.; Van den Houte, K.; Besard, L.; Tack, C.; Arts, J.; Caenepeel, P.; Piessevaux, H.; Vandenberghe, A.; Matthys, C.; Biesiekierski, J.; et al. Diet or Medication in Primary Care Patients with IBS: The DOMINO Study—A Randomised Trial Supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute. Gut 2022, 71, 2226–2232. [Google Scholar] [CrossRef]
- Peters, S.L.; Yao, C.K.; Philpott, H.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised Clinical Trial: The Efficacy of Gut-Directed Hypnotherapy Is Similar to that of the Low FODMAP Diet for the Treatment of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2016, 44, 447–459. [Google Scholar] [CrossRef]
- Schumann, D.; Langhorst, J.; Dobos, G.; Cramer, H. Randomised Clinical Trial: Yoga vs a Low-FODMAP Diet in Patients with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2018, 47, 203–211. [Google Scholar] [CrossRef]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet Into Gastroenterological Practice. J. Neurogastroenterol. Motil. 2022, 28, 343–356. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and Reality of the FODMAP Diet for Patients with Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Cuff, C.; Lin, L.D.; Mahurkar-Joshi, S.; Jacobs, J.P.; Lagishetty, V.; Jaffe, N.; Smith, J.; Dong, T.; Sohn, J.; Chang, L. Randomized Controlled Pilot Study Assessing Fructose Tolerance during Fructose Reintroduction in Non-Constipated Irritable Bowel Syndrome Patients Successfully Treated with a Low FODMAP Diet. Neurogastroenterol. Motil. 2023, 35, e14575. [Google Scholar] [CrossRef]
- Chojnacki, C.; Poplawski, T.; Blonska, A.; Konrad, P.; Chojnacki, J.; Blasiak, J. The Usefulness of the Low-FODMAP Diet with Limited Tryptophan Intake in the Treatment of Diarrhea-Predominant Irritable Bowel Syndrome. Nutrients 2023, 15, 1837. [Google Scholar] [CrossRef]
- Algera, J.P.; Demir, D.; Törnblom, H.; Nybacka, S.; Simrén, M.; Störsrud, S. Low FODMAP Diet Reduces Gastrointestinal Symptoms in Irritable Bowel Syndrome and Clinical Response Could Be Predicted by Symptom Severity: A Randomized Crossover Trial. Clin. Nutr. Edinb. Scotl. 2022, 41, 2792–2800. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Mustafa, U.; Mukhopadhyay, S.K. FODMAP Meal Challenge Test: A Novel Investigation to Predict Response to Low-FODMAP Diet in Non-Constipating Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate Modelling of Faecal Bacterial Profiles of Patients with IBS Predicts Responsiveness to a Diet Low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kaser, A.; Kiely, C.J.; Neville, B.A.; Powell, N.; et al. Two Microbiota Subtypes Identified in Irritable Bowel Syndrome with Distinct Responses to the Low FODMAP Diet. Gut 2022, 71, 1821–1830. [Google Scholar] [CrossRef]
- Colomier, E.; Van Oudenhove, L.; Tack, J.; Böhn, L.; Bennet, S.; Nybacka, S.; Störsrud, S.; Öhman, L.; Törnblom, H.; Simrén, M. Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients 2022, 14, 397. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised Clinical Trial: Gut Microbiome Biomarkers Are Associated with Clinical Response to a Low FODMAP Diet in Children with the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef]
- Eetemadi, A.; Tagkopoulos, I. Methane and Fatty Acid Metabolism Pathways Are Predictive of Low-FODMAP Diet Efficacy for Patients with Irritable Bowel Syndrome. Clin. Nutr. 2021, 40, 4414–4421. [Google Scholar] [CrossRef] [PubMed]
- Valeur, J.; Småstuen, M.C.; Knudsen, T.; Lied, G.A.; Røseth, A.G. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig. Dis. Sci. 2018, 63, 429–436. [Google Scholar] [CrossRef]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef]
- Wilson, B.; Kanno, T.; Slater, R.; Rossi, M.; Irving, P.M.; Lomer, M.C.; Probert, C.; Mason, A.J.; Whelan, K. Faecal and Urine Metabolites, but Not Gut Microbiota, May Predict Response to Low FODMAP Diet in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2023, 58, 404–416. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Végh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of Patients with Functional Bowel Symptoms Treated with a Low FODMAP Diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-Term Impact of the Low-FODMAP Diet on Gastrointestinal Symptoms, Dietary Intake, Patient Acceptability, and Healthcare Utilization in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef]
- Dimidi, E.; McArthur, A.J.; White, R.; Whelan, K.; Lomer, M.C.E. Optimizing Educational Methods for the Low FODMAP Diet in Disorders of Gut-Brain Interaction: A Feasibility Randomized Controlled Trial. Neurogastroenterol. Motil. 2023, 35, e14640. [Google Scholar] [CrossRef]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the Low FODMAP Diet in Functional Gastrointestinal Symptoms: A Real-World Experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The Low FODMAP Diet in the Management of Irritable Bowel Syndrome: An Evidence-Based Review of FODMAP Restriction, Reintroduction and Personalisation in Clinical Practice. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2018, 31, 239–255. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Bek, S.; Teo, Y.N.; Tan, X.-H.; Fan, K.H.R.; Siah, K.T.H. Association between Irritable Bowel Syndrome and Micronutrients: A Systematic Review. J. Gastroenterol. Hepatol. 2022, 37, 1485–1497. [Google Scholar] [CrossRef]
- Eswaran, S.; Dolan, R.D.; Ball, S.C.; Jackson, K.; Chey, W. The Impact of a 4-Week Low-FODMAP and mNICE Diet on Nutrient Intake in a Sample of US Adults with Irritable Bowel Syndrome with Diarrhea. J. Acad. Nutr. Diet. 2020, 120, 641–649. [Google Scholar] [CrossRef]
- Morariu, I.-D.; Avasilcai, L.; Vieriu, M.; Lupu, V.V.; Morariu, B.-A.; Lupu, A.; Morariu, P.-C.; Pop, O.-L.; Starcea, I.M.; Trandafir, L. Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults-A Narrative Review. Nutrients 2023, 15, 2295. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Rej, A.; Shaw, C.C.; Buckle, R.L.; Trott, N.; Agrawal, A.; Mosey, K.; Sanders, K.; Allen, R.; Martin, S.; Newton, A.; et al. The Low FODMAP Diet for IBS; A Multicentre UK Study Assessing Long Term Follow Up. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Danneskiold-Samsøe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Maróstica Júnior, M.R. Interplay between Food and Gut Microbiota in Health and Disease. Food Res. Int. Ott. Ont 2019, 115, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of Fructans, Prebiotics and Fibres on the Human Gut Microbiome Assessed by 16S rRNA-Based Approaches: A Review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-N.; Wu, H.; Chen, Y.-Z.; Chen, Y.-J.; Shen, X.-Z.; Liu, T.-T. Altered Molecular Signature of Intestinal Microbiota in Irritable Bowel Syndrome Patients Compared with Healthy Controls: A Systematic Review and Meta-Analysis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2017, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and Deep Molecular Analysis of Microbiota Signatures in Fecal Samples from Patients with Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-Term Personalized Low FODMAP Diet Improves Symptoms and Maintains Luminal Bifidobacteria Abundance in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in Conjunction With Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef]
- Muir, J.G.; Shepherd, S.J.; Rosella, O.; Rose, R.; Barrett, J.S.; Gibson, P.R. Fructan and Free Fructose Content of Common Australian Vegetables and Fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R. Measurement of Short-Chain Carbohydrates in Common Australian Vegetables and Fruits by High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Zanzer, Y.C.; Theis, S. Systematic Review and Meta-Analysis of Habitual Intake of Fermentable Oligo-, Di-, Mono- Saccharides and Polyols in the General Population and Revisiting the Low FODMAP Diet Concept. J. Funct. Foods 2024, 112, 105914. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S.; Buckle, R.L.; Trott, N.; Aziz, I.; Shaw, C.C. What Is the Optimal FODMAP Threshold in IBS? J. Gastroenterol. Hepatol. 2021, 36, 1723–1725. [Google Scholar] [CrossRef]
- McMeans, A.R.; King, K.L.; Chumpitazi, B.P. Low FODMAP Dietary Food Lists Are Often Discordant. Am. J. Gastroenterol. 2017, 112, 655–656. [Google Scholar] [CrossRef]
- San Mauro Martín, I.; Garicano Vilar, E.; López Oliva, S.; Sanz Rojo, S. Existing Differences between Available Lists of FODMAP-Containing Foods. Rev. Esp. Enferm. Dig. 2023, 115, 374–384. [Google Scholar] [CrossRef]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Review Article: FODMAPS, Prebiotics and Gut Health-the FODMAP Hypothesis Revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Yao, C.K.; Ardalan, Z.S.; Thwaites, P.A.; Kalantar-Zadeh, K.; Gibson, P.R.; Muir, J.G. Supplementing Dietary Fibers with a Low FODMAP Diet in Irritable Bowel Syndrome: A Randomized Controlled Crossover Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 2112–2120.e7. [Google Scholar] [CrossRef] [PubMed]
- Gwioździk, W.; Krupa-Kotara, K.; Całyniuk, B.; Helisz, P.; Grajek, M.; Głogowska-Ligus, J. Traditional, Vegetarian, or Low FODMAP Diets and Their Relation to Symptoms of Eating Disorders: A Cross-Sectional Study among Young Women in Poland. Nutrients 2022, 14, 4125. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Dallio, M.; Romeo, M.; Di Somma, A.; Cotticelli, G.; Loguercio, C.; Federico, A. Adherence and Effects Derived from FODMAP Diet on Irritable Bowel Syndrome: A Real Life Evaluation of a Large Follow-Up Observation. Nutrients 2020, 12, 928. [Google Scholar] [CrossRef]
- de Roest, R.H.; Dobbs, B.R.; Chapman, B.A.; Batman, B.; O’Brien, L.A.; Leeper, J.A.; Hebblethwaite, C.R.; Gearry, R.B. The Low FODMAP Diet Improves Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome: A Prospective Study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, L.; Zanconato, M.; Crepaldi, M.; Marasco, G.; Cremon, C.; Barbara, G.; Barberio, B.; Zingone, F.; Savarino, E.V. The Role of the FODMAP Diet in IBS. Nutrients 2024, 16, 370. https://doi.org/10.3390/nu16030370
Bertin L, Zanconato M, Crepaldi M, Marasco G, Cremon C, Barbara G, Barberio B, Zingone F, Savarino EV. The Role of the FODMAP Diet in IBS. Nutrients. 2024; 16(3):370. https://doi.org/10.3390/nu16030370
Chicago/Turabian StyleBertin, Luisa, Miriana Zanconato, Martina Crepaldi, Giovanni Marasco, Cesare Cremon, Giovanni Barbara, Brigida Barberio, Fabiana Zingone, and Edoardo Vincenzo Savarino. 2024. "The Role of the FODMAP Diet in IBS" Nutrients 16, no. 3: 370. https://doi.org/10.3390/nu16030370
APA StyleBertin, L., Zanconato, M., Crepaldi, M., Marasco, G., Cremon, C., Barbara, G., Barberio, B., Zingone, F., & Savarino, E. V. (2024). The Role of the FODMAP Diet in IBS. Nutrients, 16(3), 370. https://doi.org/10.3390/nu16030370






