Maternal Malnutrition and Elevated Disease Risk in Offspring
Abstract
1. Introduction
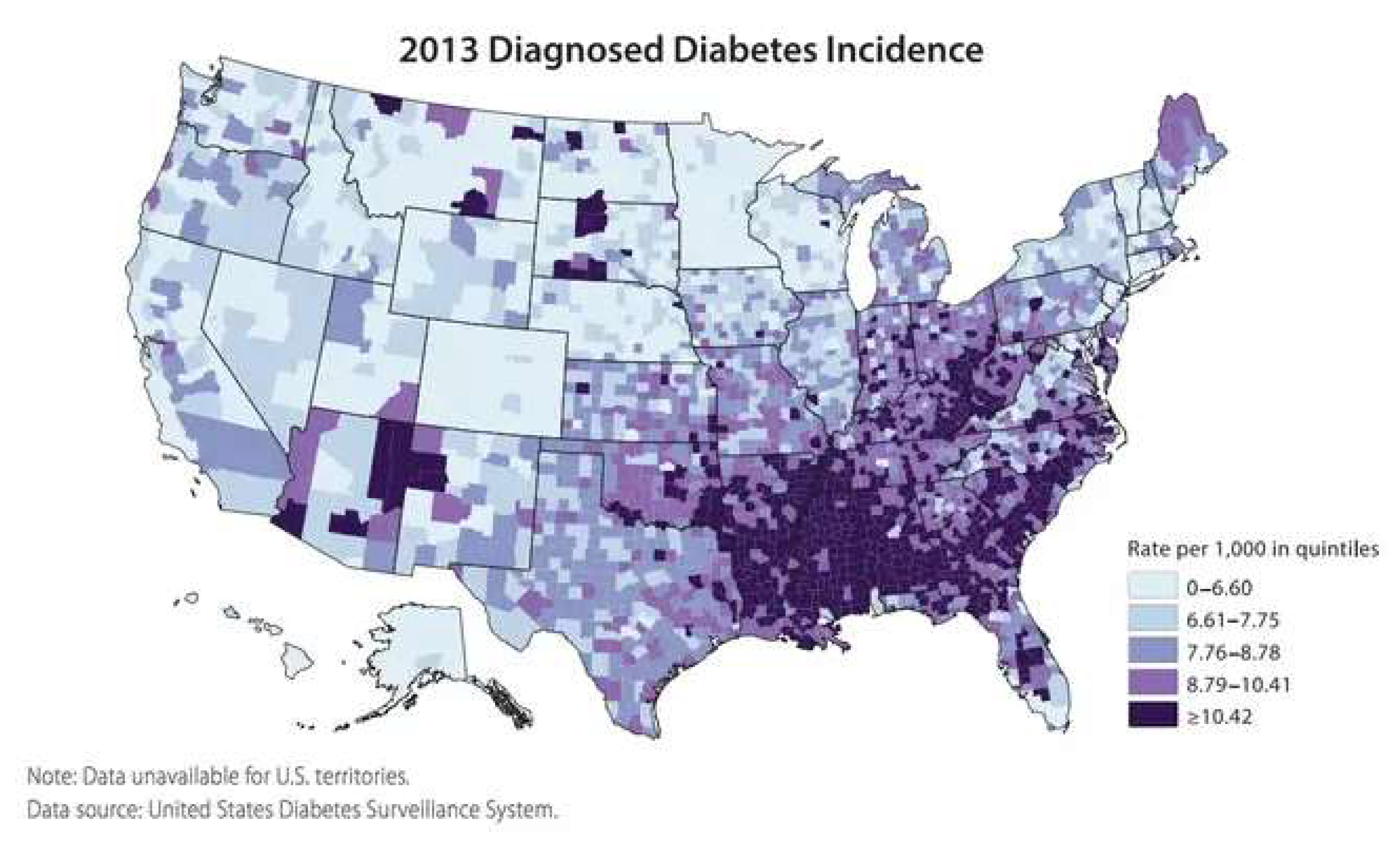
2. Epidemic of Chronic Disease
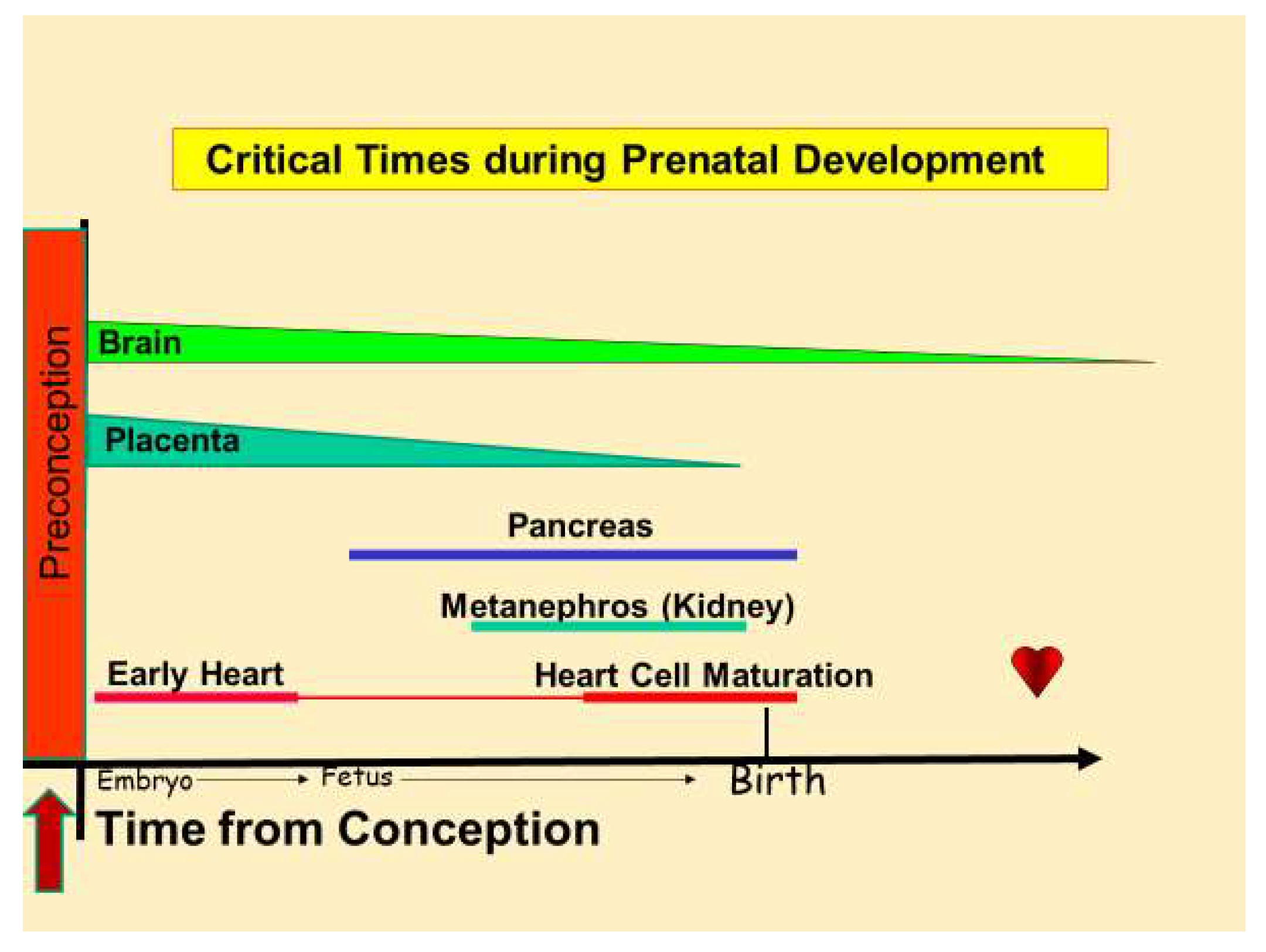
3. Chronic Disease Begins in the Womb
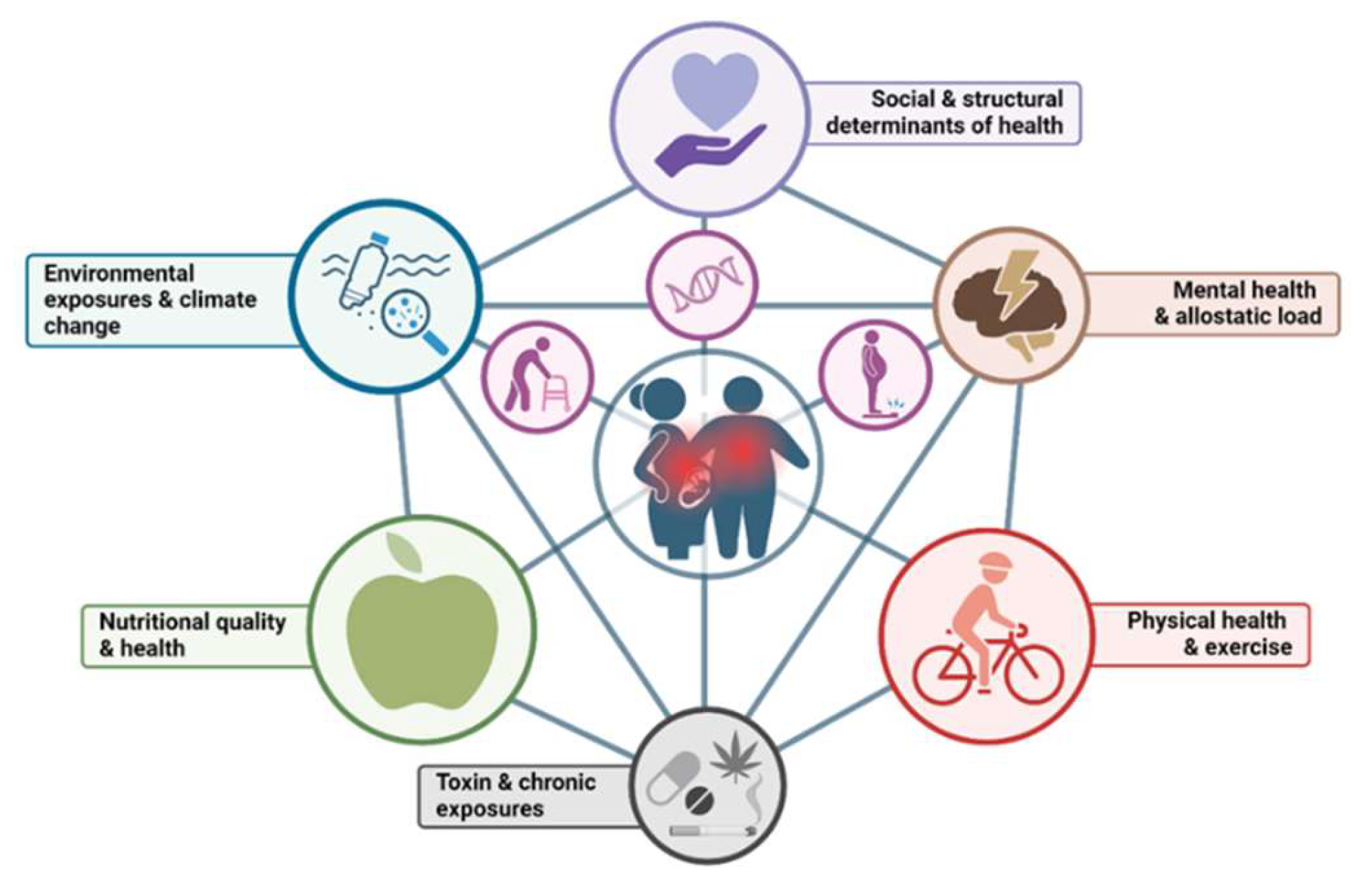
4. Linking Maternal Nutritional Stress to Chronic Disease
5. The Role of the Placenta in Fetal Nutrition
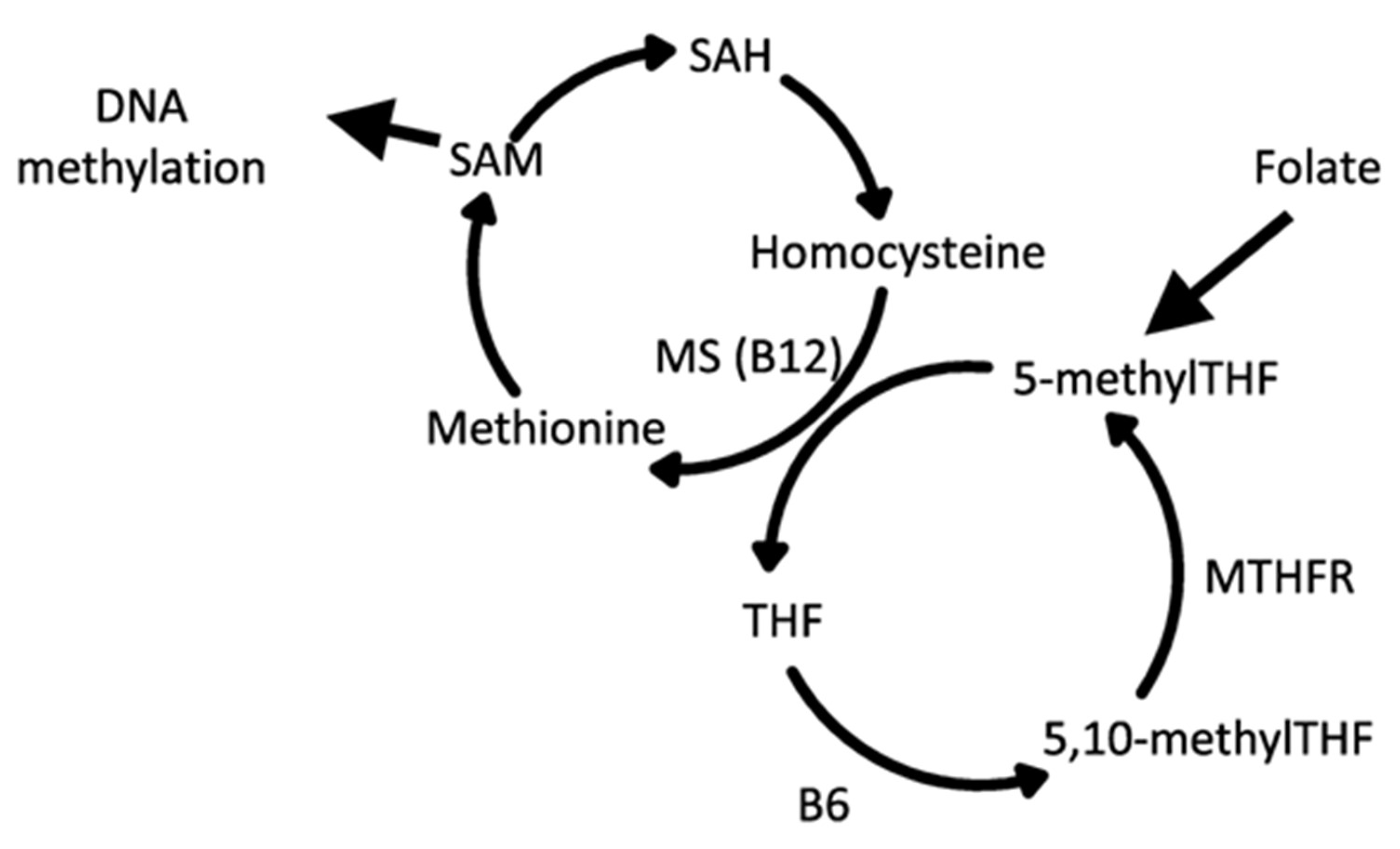
6. Epigenetic Drivers of Chronic Disease
7. Nutrition and the Modern Food Culture
8. Epigenetic Reversal
9. Conclusions
- Providing higher quality food for public and private schools.
- Teaching the principles of nutrition in schools and universities.
- Training health professionals regarding nutrition as medicine.
- Working with communities to enable nutrition programs for people of reproductive age.
- Enhancing the nutritional offerings for people served by WIC and SNAP.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Lamb, G.C.; Wu, G. Animal Agriculture: Sustainability, Challenges and Innovations; Academic Press: New York, NY, USA, 2019; Volume 1, p. 558. [Google Scholar]
- Reynolds, L.P.; Ireland, J.J.; Caton, J.S.; Bauman, D.E.; Davis, T.A. Commentary on domestic animals in agricultural and biomedical research: An endangered enterprise. J. Nutr. 2009, 139, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Regnault, T.R. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef]
- Dictionary, C. Cambridge Dictionary: Epidemic. Available online: https://dictionary.cambridge.org/dictionary/english/epidemic (accessed on 10 January 2024).
- WHO. The True Death Toll of COVID-19: Estimating Global Excess Mortality. Available online: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality (accessed on 10 January 2024).
- World Health Organization: Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 8 July 2024).
- Johns Hopkins Medicine Insight. Available online: https://www.hopkinsacg.org/how-can-understanding-common-comorbidities-improve-your-population-health-strategy-and-ultimately-patient-outcomes/ (accessed on 8 July 2024).
- Centers for Medicare & Medicaid Services. National Health Expenditure Data. Available online: https://www.cms.gov/data-research/statistics-trends-and-reports/national-health-expenditure-data (accessed on 7 August 2024).
- Centers for Disease Control and Prevention. Diabetes Report Card 2019. Available online: https://www.cdc.gov/diabetes/pdfs/library/Diabetes-Report-Card-2019-508.pdf (accessed on 2 August 2024).
- Johnson, C.Y. The Lingering Health Effects of the Civil War. Available online: https://www.washingtonpost.com/news/wonk/wp/2016/01/04/the-lingering-health-effects-of-the-civil-war/ (accessed on 10 January 2024).
- Barker, D.J.P. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health 2012, 126, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 2015, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Cadwell, B.L.; Cheng, Y.J.; Cowie, C.C.; Williams, D.E.; Geiss, L.; Engelgau, M.M.; Vinicor, F. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 2004, 27, 2806–2812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 2 August 2024).
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Kim, H.; Hu, E.A.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef]
- Paula, W.O.; Patriota, E.S.O.; Goncalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3242. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.; Bastos, M.P.; Theme-Filha, M.M. Increased consumption of ultra-processed foods during pregnancy is associated with sociodemographic, behavioral, and obstetric factors: A cohort study. Nutr. Res. 2024, 121, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, B.H.; Castro, M.C.; de Morais Sato, P.; Neves, P.A.R.; Vivanco, E.; Lima, D.L.; Cardoso, M.A.; Group, M.I.-B.S. Exposure to ultra-processed foods during pregnancy and ultrasound fetal growth parameters. Br. J. Nutr. 2023, 130, 2136–2145. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Challis, J.R. How to build a healthy heart from scratch. Adv. Exp. Med. Biol. 2014, 814, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A. Fetal Development. Available online: https://www.perinatology.com/Reference/Fetal%20development.htm#TOP (accessed on 1 February 2023).
- Tan, C.M.J.; Lewandowski, A.J. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2020, 47, 373–386. [Google Scholar] [CrossRef]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.I. Metabolic switches during development and regeneration. Development 2023, 150, dev202008. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- McCance, R.A. Critical periods of growth. Proc. Nutr. Soc. 1976, 35, 309–313. [Google Scholar] [CrossRef]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.B.; Scott-Drechsel, D.E.; Chivukula, V.K.; Rugonyi, S.; Thornburg, K.L.; Hinds, M.T. Hyperglycemia Alters the Structure and Hemodynamics of the Developing Embryonic Heart. J. Cardiovasc. Dev. Dis. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.M.; Devine, O.J.; Marcinkevage, J.A.; Gilboa, S.M.; Razzaghi, H.; Bardenheier, B.H.; Sharma, A.J.; Honein, M.A. Diabetes and congenital heart defects: A systematic review, meta-analysis, and modeling project. Am. J. Prev. Med. 2015, 48, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Ventura-Clapier, R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front. Physiol. 2018, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Louey, S.; Thornburg, K.L. Maturation of lipid metabolism in the fetal and newborn sheep heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R809–R819. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Louey, S.; Thornburg, K.L. Intrauterine growth restriction elevates circulating acylcarnitines and suppresses fatty acid metabolism genes in the fetal sheep heart. J. Physiol. 2022, 600, 655–670. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics, National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. Available online: https://stacks.cdc.gov/view/cdc/106273 (accessed on 5 February 2024).
- Barker, D.J.; Thornburg, K.L. The obstetric origins of health for a lifetime. Clin. Obstet. Gynecol. 2013, 56, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Thornburg, K.L. Placental programming of chronic diseases, cancer and lifespan: A review. Placenta 2013, 34, 841–845. [Google Scholar] [CrossRef]
- Fall, C.H. Fetal programming and the risk of noncommunicable disease. Indian. J. Pediatr. 2013, 80 (Suppl. 1), S13–S20. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans, 2020–2025. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 2 August 2024).
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Zampelas, A.; Tsigos, C. Nutrition and inflammatory load. Ann. N. Y. Acad. Sci. 2006, 1083, 214–238. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 2021, 13, 9419–9432. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Ross, M.G. Maternal-infant nutrition and development programming of offspring appetite and obesity. Nutr. Rev. 2020, 78, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Aurich, S.; Muller, L.; Kovacs, P.; Keller, M. Implication of DNA methylation during lifestyle mediated weight loss. Front Endocrinol 2023, 14, 1181002. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef]
- Kim, J.; Song, G.; Wu, G.; Bazer, F.W. Functional roles of fructose. Proc. Natl. Acad. Sci. USA 2012, 109, E1619–E1628. [Google Scholar] [CrossRef]
- Thompson, M.D.; DeBosch, B.J. Maternal Fructose Diet-Induced Developmental Programming. Nutrients 2021, 13, 3278. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ana, A.-H.; Kosugi, T.; Sanchez-Lozada, L.G.; Stenvinkel, P.; Kublickiene, K.; Ananth Karumanchi, S.; Kang, D.H.; Kojima, H.; Rodriguez-Iturbe, B.; et al. Fructose might be a clue to the origin of preeclampsia insights from nature and evolution. Hypertens. Res. 2023, 46, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Asghar, Z.A.; Thompson, A.; Chi, M.; Cusumano, A.; Scheaffer, S.; Al-Hammadi, N.; Saben, J.L.; Moley, K.H. Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Sci. Rep. 2016, 6, 25091. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H. Fetal malnutrition and long-term outcomes. Nestle Nutr. Inst. Workshop Ser. 2013, 74, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.E. The nutritional basis of the fetal origins of adult disease. Int. J. Epidemiol. 2001, 30, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.S. Researches on Pre-Natal Life; Blackwell Scientific Publications: Oxford, UK, 1946; Volume 1, p. 292. [Google Scholar]
- Kolahi, K.S.; Valent, A.M.; Thornburg, K.L. Cytotrophoblast, Not Syncytiotrophoblast, Dominates Glycolysis and Oxidative Phosphorylation in Human Term Placenta. Sci. Rep. 2017, 7, 42941. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T.L. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Marshall, N. The placenta is the center of the chronic disease universe. Am. J. Obstet. Gynecol. 2015, 213, S14–S20. [Google Scholar] [CrossRef]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef] [PubMed]
- Trivett, C.; Lees, Z.J.; Freeman, D.J. Adipose tissue function in healthy pregnancy, gestational diabetes mellitus and pre-eclampsia. Eur. J. Clin. Nutr. 2021, 75, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.N.; Halliday, D. Protein turnover in pregnancy. Eur. J. Clin. Nutr. 1992, 46, 411–417. [Google Scholar] [PubMed]
- Duggleby, S.L.; Jackson, A.A. Relationship of maternal protein turnover and lean body mass during pregnancy and birth length. Clin. Sci. 2001, 101, 65–72. [Google Scholar] [CrossRef]
- Jones, H.N.; Powell, T.L.; Jansson, T. Regulation of placental nutrient transport—A review. Placenta 2007, 28, 763–774. [Google Scholar] [CrossRef]
- Lager, S.; Powell, T.L. Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef]
- Myatt, L.; Thornburg, K.L. Effects of Prenatal Nutrition and the Role of the Placenta in Health and Disease. Methods Mol. Biol. 2018, 1735, 19–46. [Google Scholar] [CrossRef]
- Gete, D.G.; Waller, M.; Mishra, G.D. Effects of maternal diets on preterm birth and low birth weight: A systematic review. Br. J. Nutr. 2020, 123, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L. The Agouti mouse: A biosensor for environmental epigenomics studies investigating the developmental origins of health and disease. Epigenomics 2014, 6, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Zaidi, R.; Shah, S.; Oakley, M.E.; Pavlatos, C.; El Idrissi, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE 2018, 13, e0192606. [Google Scholar] [CrossRef] [PubMed]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Hornstra, J.M.; Kok, R.M.; Blom, H.J.; Smulders, Y.M. Folic acid supplementation does not reduce intracellular homocysteine, and may disturb intracellular one-carbon metabolism. Clin. Chem. Lab. Med. 2013, 51, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, K.L.; Shannon, J.; Thuillier, P.; Turker, M.S. In utero life and epigenetic predisposition for disease. Adv. Genet. 2010, 71, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Perrier, F.; Viallon, V.; Ambatipudi, S.; Ghantous, A.; Cuenin, C.; Hernandez-Vargas, H.; Chajes, V.; Baglietto, L.; Matejcic, M.; Moreno-Macias, H.; et al. Association of leukocyte DNA methylation changes with dietary folate and alcohol intake in the EPIC study. Clin. Epigenetics 2019, 11, 57. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Liberman, N.; Wang, S.Y.; Greer, E.L. Transgenerational epigenetic inheritance: From phenomena to molecular mechanisms. Curr. Opin. Neurobiol. 2019, 59, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Tarry-Adkins, J.L.; Ozanne, S.E. Transgenerational effects of maternal diet on metabolic and reproductive ageing. Mamm. Genome 2016, 27, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Ross, M.G. Epigenomics, gestational programming and risk of metabolic syndrome. Int. J. Obes. 2015, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Serpeloni, F.; Radtke, K.; de Assis, S.G.; Henning, F.; Natt, D.; Elbert, T. Grandmaternal stress during pregnancy and DNA methylation of the third generation: An epigenome-wide association study. Transl. Psychiatry 2017, 7, e1202. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J. Epidemiological evidence for the developmental origins of health and disease: Effects of prenatal undernutrition in humans. J. Endocrinol. 2019, 242, T135–T144. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D.; Slagboom, P.E.; Heijmans, B.T. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Burdge, G.C. Maternal diet as a modifier of offspring epigenetics. J. Dev. Orig. Health Dis. 2015, 6, 88–95. [Google Scholar] [CrossRef]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA-mRNA networks: Examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus. Biol. Sex. Differ. 2017, 8, 27. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Yeung, E.H.; Granstrom, C.; Bjerregaard, A.A.; Wu, J.; Rawal, S.; Chavarro, J.E.; et al. Maternal dietary intakes of refined grains during pregnancy and growth through the first 7 y of life among children born to women with gestational diabetes. Am. J. Clin. Nutr. 2017, 106, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Sheppard, A.; Gluckman, P.D.; Lillycrop, K.A.; Burdge, G.C.; McLean, C.; Rodford, J.; Slater-Jefferies, J.L.; Garratt, E.; Crozier, S.R.; et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 2011, 60, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Park, L.K.; Friso, S.; Choi, S.W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Economic Research Service U.S. Department of Agriculture; Agriculture and Food Statistics. Charting the Essentials. Available online: https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials (accessed on 10 January 2024).
- Moss, M. Salt Sugar Fat: How the Food Giants Hookes Us, 1st ed.; Random House: New York, NY, USA, 2013; Volume 1, p. 449. [Google Scholar]
- Fuhrman, J. The Hidden Dangers of Fast and Processed Food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bayol, S.A.; Macharia, R.; Farrington, S.J.; Simbi, B.H.; Stickland, N.C. Evidence that a maternal “junk food” diet during pregnancy and lactation can reduce muscle force in offspring. Eur. J. Nutr. 2009, 48, 62–65. [Google Scholar] [CrossRef]
- Bayol, S.A.; Simbi, B.H.; Fowkes, R.C.; Stickland, N.C. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology 2010, 151, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Reyes, T.M. Offspring neuroimmune consequences of maternal malnutrition: Potential mechanism for behavioral impairments that underlie metabolic and neurodevelopmental disorders. Front. Neuroendocrinol. 2017, 47, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Yang, W. Women’s periconceptional lowered carbohydrate intake and NTD-affected pregnancy risk in the era of prefortification with folic acid. Birth Defects Res. 2019, 111, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J.; et al. Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement From the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef]
- Gohir, W.; Kennedy, K.M.; Wallace, J.G.; Saoi, M.; Bellissimo, C.J.; Britz-McKibbin, P.; Petrik, J.J.; Surette, M.G.; Sloboda, D.M. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J. Physiol. 2019, 597, 3029–3051. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.E.; Miller, E.E.; Calderwood, L.E.; Shivappa, N.; Steck, S.E.; Forman, M.R.; M, A.M.; Maguire, R.; Fuemmeler, B.F.; Kollins, S.H.; et al. Maternal inflammatory diet and adverse pregnancy outcomes: Circulating cytokines and genomic imprinting as potential regulators? Epigenetics 2017, 12, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Zavalza-Gomez, A.B.; Anaya-Prado, R.; Rincon-Sanchez, A.R.; Mora-Martinez, J.M. Adipokines and insulin resistance during pregnancy. Diabetes Res. Clin. Pract. 2008, 80, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Powell, T.L.; Jansson, T. Review: Adiponectin—The missing link between maternal adiposity, placental transport and fetal growth? Placenta 2013, 34, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Champagne, F.A.; Brown, S.E.; Dymov, S.; Sharma, S.; Meaney, M.J.; Szyf, M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Soliman, K.F. Epigenetics and nutritional environmental signals. Integr. Comp. Biol. 2014, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, F.; Chavarro, J.E.; Ley, S.H.; Tobias, D.K.; Yeung, E.; Hinkle, S.N.; Bao, W.; Li, M.; Liu, A.; et al. Modifiable risk factors and long term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: Prospective cohort study. BMJ 2022, 378, e070312. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Rainford, M.; Barbour, L.A.; Birch, D.; Catalano, P.; Daniels, E.; Gremont, C.; Marshall, N.E.; Wharton, K.; Thornburg, K. Barriers to implementing good nutrition in pregnancy and early childhood: Creating equitable national solutions. Ann. N. Y. Acad. Sci. 2024, 1534, 94–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornburg, K.L.; Valent, A.M. Maternal Malnutrition and Elevated Disease Risk in Offspring. Nutrients 2024, 16, 2614. https://doi.org/10.3390/nu16162614
Thornburg KL, Valent AM. Maternal Malnutrition and Elevated Disease Risk in Offspring. Nutrients. 2024; 16(16):2614. https://doi.org/10.3390/nu16162614
Chicago/Turabian StyleThornburg, Kent L., and Amy M. Valent. 2024. "Maternal Malnutrition and Elevated Disease Risk in Offspring" Nutrients 16, no. 16: 2614. https://doi.org/10.3390/nu16162614
APA StyleThornburg, K. L., & Valent, A. M. (2024). Maternal Malnutrition and Elevated Disease Risk in Offspring. Nutrients, 16(16), 2614. https://doi.org/10.3390/nu16162614






