Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans
Abstract
1. Introduction
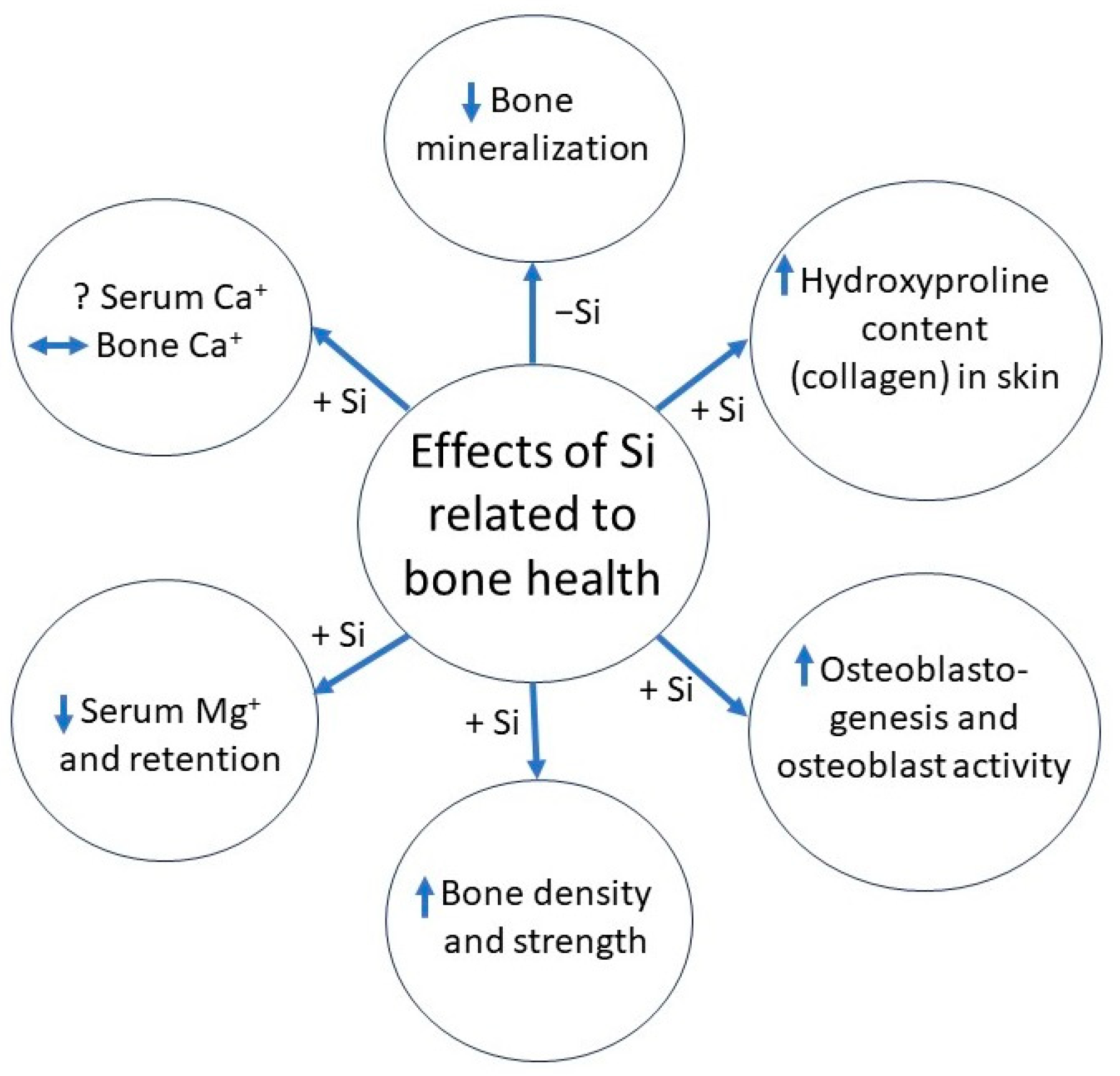
1.1. Role of Silicon in Bone Development
1.2. Effects of Silicon Supplementation
1.3. Clinical Relevance
2. Methods
2.1. Inclusion/Exclusion Criteria
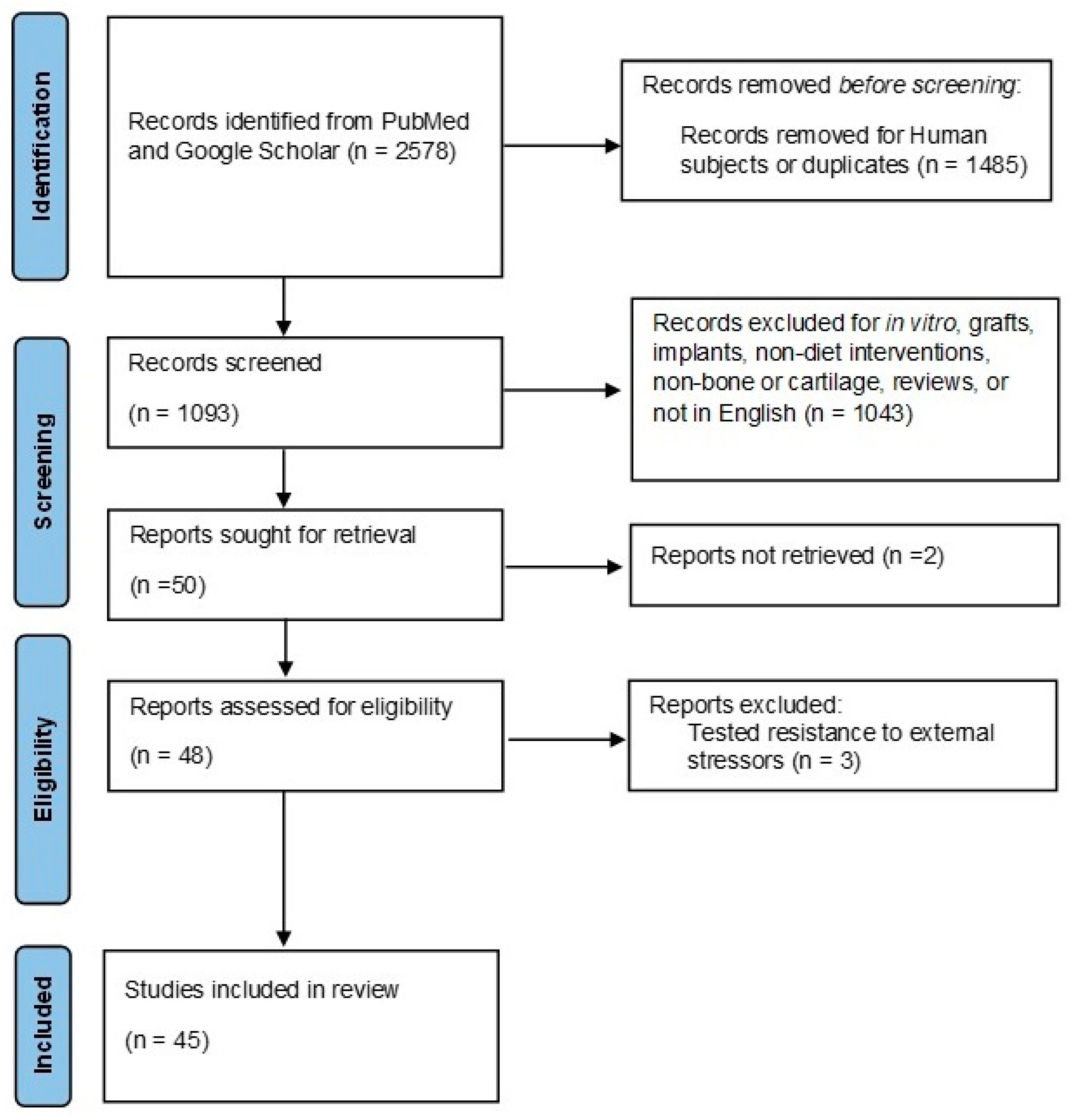
2.2. Search Strategy
2.3. Article Selection
2.4. Data Extraction
2.5. Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Carlisle, E.M. Silicon: An Essential Element for the Chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Milne, D.B. Growth-Promoting Effects of Silicon in Rats. Nature 1972, 239, 333–334. [Google Scholar] [CrossRef]
- Carlisle, E.M. A Silicon Requirement for Normal Skull Formation in Chicks. J. Nutr. 1980, 110, 352–359. [Google Scholar] [CrossRef]
- Carlisle, E.M. Biochemical and Morphological Changes Associated with Long Bone Abnormalities in Silicon Deficiency. J. Nutr. 1980, 110, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Watson, A.I.E.; Bhattacharya, P.; van Lenthe, G.H.; Powell, J.J. Positive Association between Serum Silicon Levels and Bone Mineral Density in Female Rats Following Oral Silicon Supplementation with Monomethylsilanetriol. Osteoporos. Int. 2015, 26, 1405–1415. [Google Scholar] [CrossRef]
- Carlisle, E.M. In Vivo Requirement for Silicon in Articular Cartilage and Connective Tissue Formation in the Chick. J. Nutr. 1976, 106, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Watson, A.I.E.; Pedro, L.D.; Powell, J.J. The Decrease in Silicon Concentration of the Connective Tissues with Age in Rats Is a Marker of Connective Tissue Turnover. Bone 2015, 75, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic Acid Stimulates Collagen Type 1 Synthesis and Osteoblastic Differentiation in Human Osteoblast-like Cells In Vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Bu, S.Y.; Sung, M.K.; Choi, M.K. Effects of Silicon on Osteoblast Activity and Bone Mineralization of MC3T3-E1 Cells. Biol. Trace Elem. Res. 2013, 152, 105–112. [Google Scholar] [CrossRef]
- Matsko, N.B.; Žnidaršič, N.; Letofsky-Papst, I.; Dittrich, M.; Grogger, W.; Štrus, J.; Hofer, F. Silicon: The Key Element in Early Stages of Biocalcification. J. Struct. Biol. 2011, 174, 180–186. [Google Scholar] [CrossRef]
- Nielsen, B.D.; Potter, G.D.; Morris, E.L.; Odom, T.W.; Senor, D.M.; Reynolds, J.A.; Smith, W.B.; Martin, M.T.; Bird, E.H. Training Distance to Failure in Young Racing Quarter Horses Fed Sodium Zeolite A. J. Equine Vet. Sci. 1993, 13, 562–567. [Google Scholar] [CrossRef]
- Lang, K.J.; Nielsen, B.D.; Waite, K.L.; Hill, G.A.; Orth, M.W. Increased Plasma Silicon Concentrations and Altered Bone Resorption in Response to Sodium Zeolite a Supplementation in Yearling Horses. J. Equine Vet. Sci. 2001, 21, 550–555. [Google Scholar] [CrossRef]
- Calomme, M.; Vanden Berghe, D. Supplementation of Calves with Stabilized Orthosilicic Acid: Effect on the Si, Ca, Mg, and P Concentrations in Serum and the Collagen Concentration in Skin and Cartilage. Biol. Trace Elem. Res. 1997, 56, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Jiao, G.; Liu, H.; Wu, W.; Li, S.; Wang, Q.; Xu, D.; Li, X.; Liu, H.; Chen, Y. Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-like Cells through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol. Trace Elem. Res. 2016, 173, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Bae, Y.J.; Choi, M.K.; Chung, Y.S. Silicon Supplementation Improves the Bone Mineral Density of Calcium-Deficient Ovariectomized Rats by Reducing Bone Resorption. Biol. Trace Elem. Res. 2009, 128, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, Ž.; Johansson, A.; Willman, B.; Shahabi, K.; Björn, E.; Ransjö, M. Soluble Silica Inhibits Osteoclast Formation and Bone Resorption in Vitro. Acta Biomater. 2014, 10, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Maehira, F.; Miyagi, I.; Eguchi, Y. Effects of Calcium Sources and Soluble Silicate on Bone Metabolism and the Related Gene Expression in Mice. Nutrition 2009, 25, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The Role of Silicon in Osteoblast-like Cell Proliferation and Apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent Advances in the Application of Mesoporous Silica-Based Nanomaterials for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 107, 11026. [Google Scholar] [CrossRef]
- Arora, M.; Arora, E. The Promise of Silicon: Bone Regeneration and Increased Bone Density. J. Arthrosc. Jt. Surg. 2017, 4, 103–105. [Google Scholar] [CrossRef]
- Sripanyakorn, S.; Jugdaohsingh, R.; Thompson, R.P.H.; Powell, J.J. Dietary Silicon and Bone Health. Nutr. Bull. 2005, 30, 222–230. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and Bone Health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar] [PubMed]
- Nielsen, F.H. Update on the Possible Nutritional Importance of Silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Najda, J.; Gmiński, J.; Drózdz, M.; Danch, A. The Action of Excessive, Inorganic Silicon (Si) on the Mineral Metabolism of Calcium (Ca) and Magnesium (Mg). Biol. Trace Elem. Res. 1993, 37, 107–114. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.I.; Nielsen, B.D.; Woodward, A.D.; Spooner, H.S.; Ventura, B.A.; Turner, K.K. Mineral Balance in Horses Fed Two Supplemental Silicon Sources. J. Anim. Physiol. Anim. Nutr. 2008, 92, 173–181. [Google Scholar] [CrossRef]
- Kayongo-Male, H.; Julson, J.L. Effects of High Levels of Dietary Silicon on Bone Development of Growing Rats and Turkeys Fed Semi-Purified Diets. Biol. Trace Elem. Res. 2008, 123, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, E.J.; Jung, J.Y.; Choi, M.K. Effect of Water-Soluble Silicon Supplementation on Bone Status and Balance of Calcium and Magnesium in Male Mice. Biol. Trace Elem. Res. 2014, 158, 238–242. [Google Scholar] [CrossRef]
- Seaborn, C.D.; Nielsen, F.H. Effects of Germanium and Silicon on Bone Mineralization. Biol. Trace Elem. Res. 1994, 42, 151–164. [Google Scholar] [CrossRef]
- Seaborn, C.D.; Nielsen, F.H. Dietary Silicon and Arginine Affect Mineral Element Composition of Rat Femur and Vertebra. Biol. Trace Elem. Res. 2002, 89, 239–250. [Google Scholar] [CrossRef]
- Sgavioli, S.; de Faria Domingues, C.H.; Castiblanco, D.M.C.; Praes, M.F.F.M.; Andrade-garcia, G.M.; Santos, E.T.; Baraldi-Artoni, S.M.; Garcia, R.G.; Junqueira, O.M. Silicon in Broiler Drinking Water Promotes Bone Development in Broiler Chickens. Br. Poult. Sci. 2016, 57, 693–698. [Google Scholar] [CrossRef][Green Version]
- Nakhon, S.; Numthuam, S.; Charoensook, R.; Tartrakoon, W.; Incharoen, P.; Incharoen, T. Growth Performance, Meat Quality, and Bone-Breaking Strength in Broilers Fed Dietary Rice Hull Silicon. Anim. Nutr. 2019, 5, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Scholey, D.V.; Belton, D.J.; Burton, E.J.; Perry, C.C. Bioavailability of a Novel Form of Silicon Supplement. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Gasparri, C.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Silicon: A Neglected Micronutrient Essential for Bone Health. Exp. Biol. Med. 2021, 246, 1500–1511. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake Is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Anderson, S.H.C.; Tucker, K.L.; Elliott, H.; Kiel, D.P.; Thompson, R.P.H.; Powell, J.J. Dietary Silicon Intake and Absorption. Am. J. Clin. Nutr. 2002, 75, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.; Koptev, V.; Zaharova, V.; Reshetnikova, P.; Trofimova, E.; Bychkova, E.; Podgorbunskikh, E.; Lomovsky, O. Experimental Testing of the Action of Vitamin D and Silicon Chelates in Bone Fracture Healing and Bone Turnover in Mice and Rats. Nutrients 2022, 14, 1992. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A Requirement in Bone Formation Independent of Vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Merkley, J.W.; Miller, E.R. The Effect of Sodium Fluoride and Sodium Silicate on Growth and Bone Strength of Broilers. Poult. Sci. 1983, 62, 798–804. [Google Scholar] [CrossRef]
- Watkins, K.L.; Vagnoni, D.B.; Southern, L.L. Effect of Dietary Sodium Zeolite A and Excess Calcium on Growth and Tibia Calcium and Phosphorus Concentration in Uninfected and Eimeria Acervulina-Infected Chicks. Poult. Sci. 1989, 68, 1236–1240. [Google Scholar] [CrossRef]
- Elliot, M.A.; Edwards, H.M. Effect of Dietary Silicon on Growth and Skeletal Development in Chickens. J. Nutr. 1991, 121, 201–207. [Google Scholar] [CrossRef]
- Watkins, K.L.; Southern, L.L. Effect of Dietary Sodium Zeolite A and Graded Levels of Calcium and Phosphorus on Growth, Plasma, and Tibia Characteristics of Chicks. Poult. Sci. 1992, 71, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, S.E. Effects of Various Types of Aluminosilicates and Aflatoxin B1 on Aflatoxin Toxicity, Chick Performance, and Mineral Status. Poult. Sci. 1993, 72, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.; Robison, C.; Nguyen, T.; Nielsen, B.D. Silicon Supplementation Affects Mineral Metabolism but Not Bone Density or Strength in Male Broilers. PLoS ONE 2020, 15, e0243007. [Google Scholar] [CrossRef] [PubMed]
- Hott, M.; de Pollak, C.; Modrowski, D.; Marie, P.J. Short-Term Effects of Organic Silicon on Trabecular Bone in Mature Ovariectomized Rats. Calcif. Tissue Int. 1993, 53, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Firling, C.E.; Evans, G.L.; Wakley, G.K.; Sibonga, J.; Turner, R.T. Lack of an Effect of Sodium Zeolite A on Rat Tibia Histomorphometry. J. Bone Miner. Res. 1996, 11, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Rico, H.; Gallego-Lago, J.L.; Hernandez, E.R.; Villa, L.F.; Sanchez-Atrio, A.; Seco, C.; Gervas, J.J. Effect of Silicon Supplementation on Osteopenia Induced by Ovariectomy in Rats. Calcif. Tissue Int. 2000, 66, 53–55. [Google Scholar] [CrossRef]
- Seaborn, C.D.; Nielsen, F.H. Silicon Deprivation Decreases Collagen Formation in Wounds and Bone, and Ornithine Transaminase Enzyme Activity in Liver. Biol. Trace Elem. Res. 2002, 89, 251–261. [Google Scholar] [CrossRef]
- Calomme, M.; Geusens, P.; Demeester, N.; Behets, G.J.; D’Haese, P.; Sindambiwe, J.B.; Van Hoof, V.; Vanden Berghe, D. Partial Prevention of Long-Term Femoral Bone Loss in Aged Ovariectomized Rats Supplemented with Choline-Stabilized Orthosilicic Acid. Calcif. Tissue Int. 2006, 78, 227–232. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, J.Y.; Choi, M.K.; Chung, Y.S.; Kim, M.H. Short-Term Administration of Water-Soluble Silicon Improves Mineral Density of the Femur and Tibia in Ovariectomized Rats. Biol. Trace Elem. Res. 2008, 124, 157–163. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Calomme, M.R.; Robinson, K.; Nielsen, F.; Anderson, S.H.C.; D’Haese, P.; Geusens, P.; Loveridge, N.; Thompson, R.P.H.; Powell, J.J. Increased Longitudinal Growth in Rats on a Silicon-Depleted Diet. Bone 2008, 43, 596–606. [Google Scholar] [CrossRef]
- Maehira, F.; Iinuma, Y.; Eguchi, Y.; Miyagi, I.; Teruya, S. Effects of Soluble Silicon Compound and Deep-Sea Water on Biochemical and Mechanical Properties of Bone and the Related Gene Expression in Mice. J. Bone Miner. Metab. 2008, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Kim, M.H.; Choi, M.K. Effect of Silicon Supplementation on Bone Status in Ovariectomized Rats Under Calcium-Replete Condition. Biol. Trace Elem. Res. 2016, 171, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zheng, H. Combined Effects of Phytoestrogen Genistein and Silicon on Ovariectomy-Induced Bone Loss in Rat. Biol. Trace Elem. Res. 2017, 177, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zheng, H.; Qi, S. Genistein and Silicon Synergistically Protects Against Ovariectomy-Induced Bone Loss Through Upregulating OPG/RANKL Ratio. Biol. Trace Elem. Res. 2019, 188, 441–450. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, M.K. Effect of Silicon Supplementation in Diets with Different Calcium Levels on Balance of Calcium, Silicon and Magnesium, and Bone Status in Growing Female Rats. Biol. Trace Elem. Res. 2021, 199, 258–266. [Google Scholar] [CrossRef]
- Ward, T.L.; Watkins, K.L.; Southern, L.L.; Hoyt, P.G.; French, D.D. Interactive Effects of Sodium Zeolite-A and Copper in Growing Swine: Growth, and Bone and Tissue Mineral Concentrations. J. Anim. Sci. 1991, 69, 726–733. [Google Scholar] [CrossRef]
- Frey, K.S.; Potter, G.D.; Odom, T.W.; Senor, D.M.; Reagan, V.D.; Weir, V.H.; Elslander, J.; Webb, S.P.; Morris, E.L.; Smith, W.B.; et al. Plasma Silicon and Radiographic Bone Density in Weanling Quarter Horses Fed Sodium Zeolite A1. J. Equine Vet. Sci. 1992, 12, 292–296. [Google Scholar] [CrossRef]
- Lang, K.J.; Nielsen, B.D.; Waite, K.L.; Hill, G.M.; Orth, M.W. Supplemental Silicon Increases Plasma and Milk Silicon Concentrations in Horses. J. Anim. Sci. 2001, 79, 2627–2633. [Google Scholar] [CrossRef][Green Version]
- Turner, K.K.; Nielsen, B.D.; O’Connor-Robison, C.I.; Rosenstein, D.S.; Marks, B.P.; Nielsen, F.H.; Orth, M.W. Sodium Zeolite A Supplementation and Its Impact on the Skeleton of Dairy Calves. Biol. Trace Elem. Res. 2008, 121, 149–159. [Google Scholar] [CrossRef]
- Pritchard, A.; Nielsen, B.D.; Robison, C.; Manfredi, J.M. Low Dietary Silicon Supplementation May Not Affect Bone and Cartilage in Mature, Sedentary Horses. J. Anim. Sci. 2020, 98, skaa377. [Google Scholar] [CrossRef]
- Pritchard, A.; Nielsen, B.; Robison, C.; Manfredi, J. Bioavailable Silicon Supplementation May Influence Biomarkers, but Not Lameness, in Mature Horses. J. Equine Vet. Sci. 2019, 76, 83–84. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Powell, J.J.; McNaughton, S.A.; Jugdaohsingh, R.; Anderson, S.H.C.; Dear, J.; Khot, F.; Mowatt, L.; Gleason, K.L.; Sykes, M.; Thompson, R.P.H.; et al. A Provisional Database for the Silicon Content of Foods in the United Kingdom. Br. J. Nutr. 2005, 94, 804–812. [Google Scholar] [CrossRef] [PubMed]
| Reference | Silicon Form | Group | Daily Intake from Diet | Silicon Dose per Day | Total Silicon mg/kg BW | Animal | Age at Start | Results |
|---|---|---|---|---|---|---|---|---|
| Carlisle, 1972 [1] | Sodium metasilicate | Control | 1 ppm | X | - | Chicks | 1 day | Reduced growth rate, shorter leg bones with smaller circumferences and thinner cortices in control, control tibias and femurs fracture more easily |
| Supplemented | 100 ppm | |||||||
| Carlisle 1976 [6] | Sodium metasilicate | Control | <3 ppm | 0 mg | - | Chicks | 1 day | Better growth, higher hexosamine content and percent in articular cartilage, greater silicon content in comb, less water content in tibia and femur with supplementation, no difference in percent ash |
| Supplemented | 100 ppm | |||||||
| Carlisle, 1980a [4] | Sodium metasilicate | Control | 1 ppm | X | - | Chicks | 1 day | Greater percentage and total amount of hexosamine and greater percentage of collagen in tibias from supplemented vs control, Si-deficient tibias had lesions and changes in epiphyseal cartilage especially in proliferative zone |
| Supplemented | 250 ppm | |||||||
| Carlisle, 1980b [3] | Sodium metasilicate | Control | 1 ppm | X | - | Chicks | 1 day | Si-deficient skulls had less trabeculae and calcification, reduced collagen content |
| Supplemented | 250 ppm | |||||||
| Carlisle, 1981 [37] | Sodium metasilicate | Control | 1 ppm | X | - | Chicks | 1 day | Skull abnormalities in Si-deficient chicks from less collagen concentration in bones |
| Supplemented | 250 ppm | |||||||
| Merkley and Miller, 1983 [38] | Sodium metasilicate | Control | - | X | - | Chicks | 1 day | Humeri strength decreased during immobilization in control but remained similar to unrestricted humeri strength with metasilicate |
| Sodium metasilicate | 74 ppm | |||||||
| Watkins, Vagnoni, and Southern, 1989 [39] | Sodium zeolite A | 0% | - | 0 mg | - | Chicks | 4 days | SZA with excess Ca decreased weight gain and tibia ash |
| 0.75% | 90.3 mg | |||||||
| Elliot and Edwards, 1991 [40] | Sodium metasilicate | Basal 1 | 0.01 mg | 0 mg | 0.02 | Chicks | 1 day | High silicon inclusion reduced feed efficiency, no difference in tibial ash |
| 25 1 | 0.20 mg | 0.66 | ||||||
| 50 1 | 0.46 mg | 1.53 | ||||||
| 150 1 | 1.44 mg | 4.78 | ||||||
| 250 1 | 2.72 mg | 7.62 | ||||||
| Watkins and Southern, 1992 [41] | Sodium zeolite A | 0% SZA | - | X | - | Chicks | 4 days | Plasma Ca or alkaline phosphatase unaffected by SZA, reduction in plasma P but increase in tibia Mn, Zn, Cu, and Al with SZA |
| 0.75% SZA | ||||||||
| Scheidler, 1993 [42] | Aluminosilicates | Control | - | X | - | Chicks | 1 day | Novasil increased bone ash, Ethacal decreased bone ash, supplementation decreased serum Cl |
| Ethacal | 163 mg | 279 | ||||||
| Novasil | 288 mg | 506 | ||||||
| Perlite | 357 mg | 543 | ||||||
| Zeobrite | 333 mg | 514 | ||||||
| Kayongo-Male and Julson, 2008 [26] | Tetraethyl-orthosilicate | Groups based on supplemented Si levels | 0 ppm | - | Turkeys | 1 day | Moment of inertia and plasma calcium lower with high supplementation, no differences in other physical or mechanical properties | |
| 135 ppm | ||||||||
| 270 ppm | ||||||||
| 540 ppm | ||||||||
| Sgavioli et al., 2016 [30] | Not given | 0 mg Supplement | - | X | - | Chicks | 1 day | Si supplementation had no effect on bone density or breaking strength, bone ash, phosphorus, zinc, and manganese increased without increasing bone calcium |
| 0.5 mg Supplement | 244 mg | 150 | ||||||
| 1.0 mg Supplement | 488 mg | 300 | ||||||
| 1.5 mg Supplement | 740 mg | 450 | ||||||
| Scholey et al., 2018 [32] | Monomeric silicic acid | Control 1 | 55.8 mg | X | 114 | Chicks | 1 day | Improved tibia breaking strength and tibial Si at 1000 mg/L supplementation, foot and tibia ash increased in the 500 mg/L, no other significant differences in bone measures |
| 200 mg/L | 16.2 mg | 138 | ||||||
| 500 mg/L | 39.5 mg | 166 | ||||||
| 1000 mg/L 1 | 79.5 mg | 280 | ||||||
| Pritchard et al., 2020 [43] | Orthosilicic acid | Control | 2.9 mg | X | 4.1 | Chicks | 1 day | Supplementation reduced serum boron and increased serum calcium; bone density, morphology, and strength measures were similar among groups |
| Normal | 133 mg | 147 | ||||||
| High | 804 mg | 863 |
| Reference | Silicon Form | Group | Daily Intake from Diet | Silicon Dose per Day | Total Silicon mg/kg BW | Animal | Age at Start | Results |
|---|---|---|---|---|---|---|---|---|
| Schwarz and Milne, 1972 [2] | Sodium metasilicate | Control | <5 ppm | X | - | Rats | 20 days | Improved growth rates across two different diet compositions, improved incisor pigmentation and skull bone structure |
| Supplemented | 500 ppm | |||||||
| Najda et al., 1993 [24] | Sodium metasilicate | Control | - | 0 mg | - | Rats | 2 months | Supplementation increased serum Ca and tissue Mg |
| Supplemented | 0.7 mg/g BW | |||||||
| Hott et al., 1993 [44] | Silanol | Sham operated | - | X | - | Rats | 3 months | Silanol decreased osteoclast surface and number of osteoclast, higher dose increased mineral apposition rate and bone formation rate, no effect on the periosteal apposition rate with silanol |
| Ovariectomized | X | - | ||||||
| Ovariectomized + low silanol | 0.1 mg/kg | - | ||||||
| Ovariectomized + high silanol | 1.0 mg/kg | - | ||||||
| Firling et al., 1996 [45] | Sodium zeolite A | Normal Ca, 30 mg SZA/kg BW | - | X | 9.9 | Rats | - | No effect of SZA on cortical or cancellous bone formation and mass |
| Normal Ca, 100 mg SZA/kg BW | - | 33 | ||||||
| Normal Ca, 500 mg SZA/kg BW | - | 165 | ||||||
| Low Ca, 0 mg SZA/kg BW | - | 0 | ||||||
| Low Ca, 125 mg SZA/kg BW | - | 41.3 | ||||||
| Low Ca, 617 mg SZA/kg BW | - | 204 | ||||||
| Rico et al., 2000 [46] | Sodium metasilicate | OVX | - | X | - | Rats | 100 days | Attenuated bone loss in vertebra and femur in OVX + Si |
| OVX-Sham | - | |||||||
| OVX + Si | 50 g/100 g diet | |||||||
| Seaborn and Nielsen, 2002 [47] | Sodium metasilicate | −Si | 2.3 μg/g diet | 0 μg/g | - | Rats | 21 days | Tibial hydroxyproline lower and decreased liver ornithine aminotransferase in deficient rats |
| +Si | 10 μg/g | |||||||
| Seaborn and Nielsen, 2002 [29] | Sodium metasilicate | −Si | 2.3 μg/g | 0 μg/g | - | Rats | 21 days | Depressed growth, lower plasma Si, and lower femoral Ca concentrations in −Si, Lower alkaline phosphatase in +Si |
| +Si | 25 μg/g | |||||||
| Calomme et al., 2006 [48] | Orthosilicic acid | Sham | - | X | - | Rats | 9 months | OSA supplementation partially reversed the decrease in Ca excretion seen in OVX, tended to reduce bone turnover, increased total femoral BMC and BMD, marginally increased total lumbar BMD |
| OVX | X | - | ||||||
| OVX-Si | 1 mg/kg BW | - | ||||||
| Bae et al., 2008 [49] | Sodium metasilicate | Sham | 0.09 mg | X | 0.3 | Rats | 17 weeks | Supplementation increased femur and tibia BMD and serum CTx and decreased urinary Ca and P excretion compared to OVX |
| OVX | 0.11 mg | X | 0.4 | |||||
| OVX-Si | 0.10 mg | 6.21 mg | 65.4 | |||||
| Jugdaohsingh et al., 2008 [50] | Sodium silicate | Si-Deprived | 0.05 mg | X | 0.2 | Rats | 3 weeks | Serum Si concentrations and urinary excretion lower in Si-deprived vs Si-supplemented, tibia Si lower in Si-deprived and Si-supplemented than Normal, Si-deprived showed reduced bone growth plate thickness, increased in chondrocyte density and lower tibia phosphorus concentrations |
| Si-Supplemented | 0.05 mg | 53.2 μg/g water | 4.1 | |||||
| Normal | 5.46 mg | X | 18.5 | |||||
| Maehira et al., 2008 [51] | Sodium metasilicate/ Monosilicic acid | Tap Water (Control) | 9.4 μg | X | - | Mice | - | DW and Si improved bone bio- chemical indices such as femoral weight, mineral and collagen content, and marker enzymes of bone formation and resorption as well as mechanical properties as compared to TW |
| Deep Sea Water | 15.7 μg | |||||||
| Surface Sea Water | 9.9 μg | |||||||
| Tap + 200 ppm Si | 20.0 μg | |||||||
| Kayongo-Male and Julson, 2008 [26] | Tetraethyl-orthosilicate | Groups based on supplemented Si levels | 5 ppm | 0 ppm | - | Rats | - | Moment of inertia lower and trend for reduced plasma Mg with supplementation, no other physical or mechanical differences |
| 500 ppm | ||||||||
| Kim et al., 2009 [15] | Sodium metasilicate | Low Ca | 0.08 mg | X | 0.39 | Rats | 6 weeks | Supplementation increased BMD in femur and tibia of Ca-deficient ovariectomized rats, lower serum CTX in Si low calcium group but higher CTX in adequate calcium group |
| Low Ca + Si Supplement | 80.1 mg | 398 | ||||||
| Adequate Ca | 0.09 mg | 0.42 | ||||||
| Adequate Ca + Si Supplement | 81.9 mg | 408 | ||||||
| High Ca | 0.08 mg | 0.41 | ||||||
| High Ca + Si Supplement | 90.9 mg | 443 | ||||||
| Maehira et al., 2009 [17] | Sodium metasilicate/ Monosilicic acid | Control (CT) | 0.84 μg | X | - | Mice | 1 month | Femoral collagen content increased while OHProline urinary excretion decreased in Si, increased strength and structural stiffness in Si |
| CT + Si | 213.1 μg | |||||||
| Coral Sand (CS) | 2.12 μg | |||||||
| Fossil Stony Coral (FCS) | 1.26 μg | |||||||
| Fish Bone (FC) | 2.17 μg | |||||||
| Eggshell (EC) | 0.94 μg | |||||||
| Kim et al., 2014 [27] | Sodium metasilicate | Control | 22.97 μg | X | 0.55 | Mice | 9 weeks | No difference in BMD in femur and tibia, adjusted BMD for final BW higher in Si50, femur area was higher in Si50 and Si150 than in control, supplementation decreased Mg retention without changing Ca retention, and decreased ALP |
| Si50 | 1958 μg | 48.5 | ||||||
| Si100 | 2877 μg | 74.6 | ||||||
| Si150 | 3636 μg | 89.4 | ||||||
| Jugdaohsingh et al., 2015a [5] | Monomethyl-silanetriol | Group 1 | 16.5 mg | X | 44.6 | Rats | 2 months | Si supplementation increased fasting serum and tissue Si concentrations, trend for serum OC concentration in female rats to show a dose-response increase, strong significant associations between serum Si concentrations and bone quality in female rats |
| Group 2 | 2.98 mg | 53.4 | ||||||
| Group 3 | 16.1 mg | 90.7 | ||||||
| Jugdaohsingh et al., 2015b [7] | - | Groups divided by age | 628 μg/g diet + 3.9 μg/mL water | X | - | Rats | 23 days | Higher Si concentrations (depending on age) found in connective tissues with highest amount found in the 3 or 5 wk old rats, Si decreased with age except in skin, decreases occurred pre-puberty and stabilize in adulthood, higher serum Si in younger animals, Total Si increases with growth of organ, linear association with bone, difference in total body Si between weanling and adult is less than 100 μg |
| Bu, Kim, and Choi, 2016 [52] | Metasilicate | Control | 0.09 mg | X | 0.36 | Rats | 7 weeks | Si supplementation unable to restore ovariectomy induced BMD decreases with Ca-replete diet, OVXVHSi increased OPG expression and decreased RANKL/OPG ratio in mRNA expression comparable to levels of sham-controls |
| OVXNSi (OVX control) | 0.09 mg | 0.36 | ||||||
| OVXHSi | 4.29 mg | 17.8 | ||||||
| OVXVHSi | 12.8 mg | 53.1 | ||||||
| Qi and Zheng, 2017 [53] | Sodium metasilicate | OVX | - | X | - | Rats | 3 months | Si improved BMD, bone histological and serum biochemical parameters in ovariectomized rats |
| OVX-Si | 5.44 mg | 20 | ||||||
| OVX-GEN-Si | 5.15 mg | 20 | ||||||
| Chen, Zheng, and Qi, 2019 [54] | Sodium metasilicate | Control | 0.06 mg | X | - | Rats | 3 months | Si improved BMD, bone histological and serum biochemical parameters in ovariectomized rats |
| Supplemented | 4.65 mg | 20 | ||||||
| Kim and Choi, 2021 [55] | Sodium metasilicate | Low Ca + Adequate Si | 0.07 mg | X | 0.4 | Rats | 6 wks | Si supplementation decreased serum CTx and increased serum Mg in low Ca, reduced BMD at femur and tibia in high Ca, and increased tibia strength in adequate Ca |
| Low Ca + High Si | 7.28 mg | 38.4 | ||||||
| Adequate Ca + Adequate Si | 0.08 mg | 0.4 | ||||||
| Adequate Ca + High Si | 7.44 mg | 38.7 | ||||||
| High Ca + Adequate Si | 0.07 mg | 0.4 | ||||||
| High Ca + High Si | 7.62 mg | 40.2 | ||||||
| Bychkov et al., 2022 [36] | Chelated silica | Control | - | X | - | Mice and Rats | 12 wks and 4 wks | Increase in Alkaline phosphatase in chelated silica supplemented mice; otherwise, no differences between silicon-chelated supplemented and control animals |
| Chelated Silica | 6 mg (Mice) | |||||||
| 24 mg (Rats) |
| Reference | Silicon Form | Group | Daily Intake from Diet | Silicon Dose per Day | Total Silicon mg/kg BW | Animal | Age at Start | Results |
|---|---|---|---|---|---|---|---|---|
| Ward et al., 1991 [56] | Sodium zeolite A | 0% SZA | - | X | - | Pigs | 31 days | SZA increased serum alkaline phosphatase and liver and bone Zn content, decreased serum Ca and inorganic P concentrations |
| 0.5% SZA | 3080 mg | 122 | ||||||
| Frey et al., 1992 [57] | Sodium zeolite A | 0% SZA | - | 0 mg | - | Horses | 6 months | Increased plasma silicon concentrations with supplementation, gain in BMC for first 56 days greatest in 2.0% SZA but no differences among treatments in BMC over the course of the study |
| 0.66% SZA | 4.3 mg | |||||||
| 1.32% SZA | 8.7 mg | |||||||
| 2.0% SZA | 12.5 mg | |||||||
| Nielsen et al., 1993 [11] | Sodium zeolite A | 0% SZA | - | 0 mg | - | Horses | 18 months | Increased plasma silicon concentrations and faster average race times, 1.86% and 2.8% increased distance and training/racing cycles prior to injury |
| 0.92% SZA | 10.3 mg | |||||||
| 1.86% SZA | 20.8 mg | |||||||
| 2.8% SZA | 31.4 mg | |||||||
| Calome and Vanden Berghe, 1997 [13] | Orthosilicic acid | Control | 360 mg | 0 mg | 4.3 | Calves | 1 week | Increased Si serum and collagen dermis concentration |
| Supplemented | 378 mg | 17.5 to 70 mg | 4.9 | |||||
| Lang et al., 2001 [58] | Sodium zeolite A | Control | 10.8 g | X | - | Horses | Supplemented mares had higher plasma and milk Si concentrations, foals of Supplemented mares had higher plasma Si concentrations but did not influence bone metabolism in foals | |
| Supplemented | 44.3 g | |||||||
| Lang et al., 2001 [12] | Sodium zeolite A | Control | 9.25 g | X | 27.0 | Horses | 1 year | Higher plasma Si concentrations and lower ICTP in Si treated group, no differences for OC or PYD |
| Si Treated | 30.57 g | 87.2 | ||||||
| O’Connor et al., 2007 [25] | Sodium aluminum silicate/orthosilicic acid | Control | 874 mg | X | 1.7 | Horses | 10 years | SA increased Si excretion and calcium retention and apparent digestion, OSA increased Ca and B retention, apparent B and Si digestion, plasma Si, and tended to increase Si retention |
| SA | 124 mg | 1.9 | ||||||
| OSA | 137 mg | 2.2 | ||||||
| Frantz et al., 2008 | - | Control | 0 mg | X | 0 | Pigs | - | Si Diet had lower overall osteochondrosis incidence scores than Control |
| Si Diet | 2790 mg | 46.1 | ||||||
| Turner et al., 2008 [59] | Sodium zeolite A | Control (CO) | 2.7 g | X | 41.2 | Calves | 3 days | No differences in OC concentrations, OC:DPD ratio, bone architecture, mechanical properties, or glycosaminoglycan concentration in cartilage or synovial fluid CO had lower DPD concentrations, SS had greater cortical bone and articular cartilage aluminum content |
| Supplemented (SS) | 6.5 g | 138 | ||||||
| Pritchard et al., 2020 [60] | Silicon-collagen | Control | 1.8 mg | X | 0.003 | Horses | 13 years | No differences |
| Supplemented | 52.7 mg | 0.1 |
| Min | Max | Median | Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Con | Si | Con | Si | Con | Si | Con | Si | |
| Daily Dietary Si Intake (mg) | 0.0008 | 0.01 | 17 | 2790 | 0.08 | 7.4 | 1 ± 4 | 175 ± 534 |
| Daily Si Supplementation Dose (mg) | - | 0.2 | - | 804 | - | 16.1 | - | 81 ± 187 |
| Standardized Total Si Intake (mg/kg BW) | 0.003 | 0.1 | 114 | 863 | 0.41 | 47.3 | 12 ± 27 | 120 ± 189 |
| No Effect | Positive Effect | |
|---|---|---|
| Daily Si Supplementation Dose (mg) | 12 ± 21 | 116 ± 223 |
| Standardized Total Si Intake (mg/kg BW) | 73 ± 140 | 139 ± 214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritchard, A.; Nielsen, B.D. Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans. Nutrients 2024, 16, 339. https://doi.org/10.3390/nu16030339
Pritchard A, Nielsen BD. Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans. Nutrients. 2024; 16(3):339. https://doi.org/10.3390/nu16030339
Chicago/Turabian StylePritchard, Abby, and Brian D. Nielsen. 2024. "Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans" Nutrients 16, no. 3: 339. https://doi.org/10.3390/nu16030339
APA StylePritchard, A., & Nielsen, B. D. (2024). Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans. Nutrients, 16(3), 339. https://doi.org/10.3390/nu16030339






