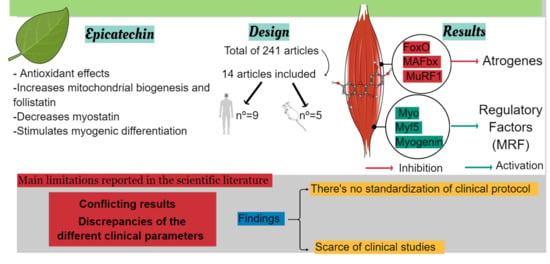
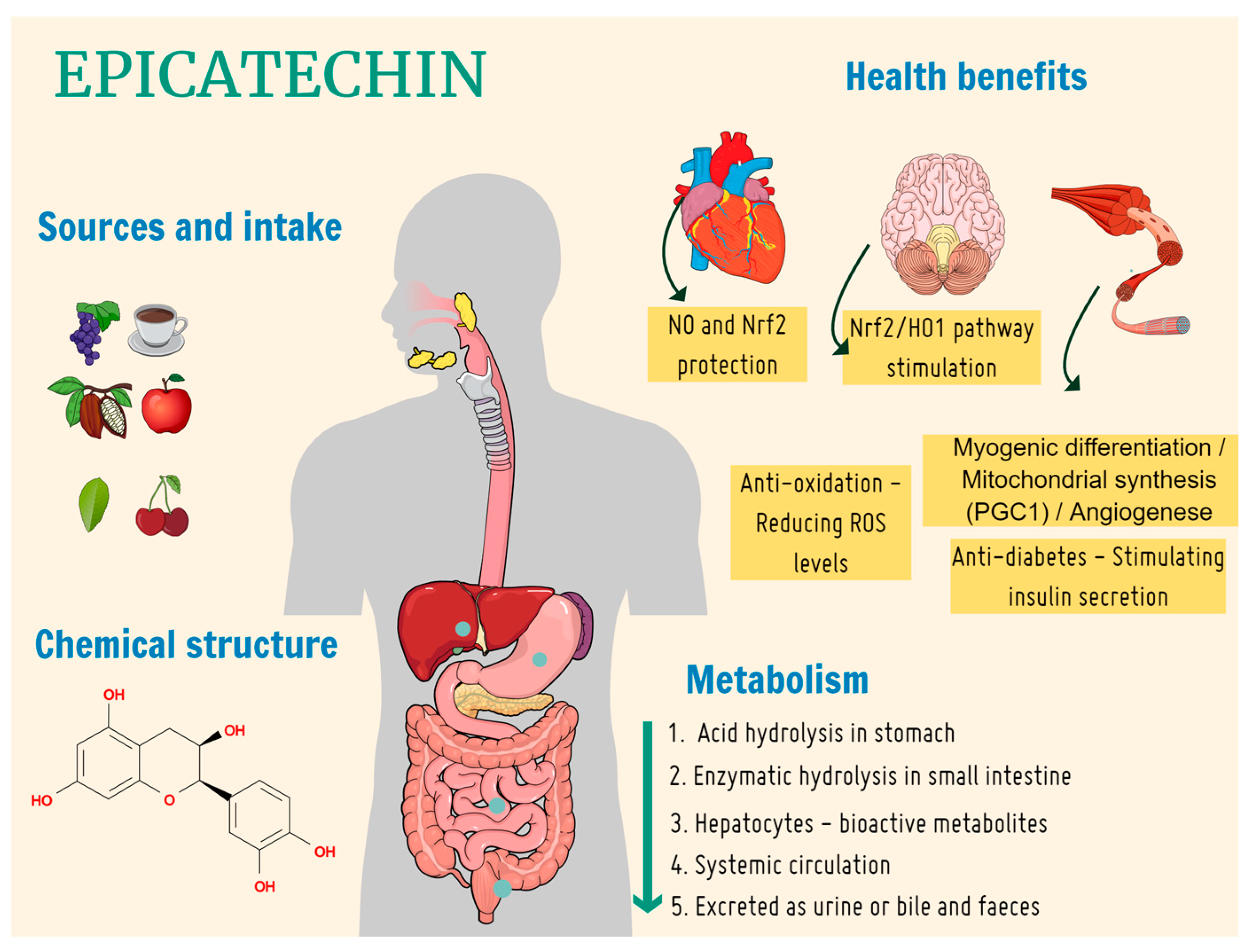
New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin
Abstract
1. Introduction
- mTOR signaling: many dietary supplements target the mammalian rapamycin (mTOR) signaling pathway, related to protein turnover and autophagy (a process of recycling resulting in degradation of the body’s own tissue) [24];
- AMP-activated protein kinase (AMPK) pathway: AMPK is a metabolic pathway that regulates energy metabolism and cellular energy homeostasis. Some supplements can activate the AMPK pathway to promote processes using fat as an energy source [25];
- nuclear transcription factor kappa B (NF-kB) pathway: NF-kB is a pathway in inflammatory and immune response. Supplements can modulate the NF-kB pathway to regulate inflammation and promote a healthy immune response [26];
- peroxisome proliferator-activated receptors (PPAR) pathway: PPARs are a family of receptors that regulate metabolism of lipids and energy homeostasis. Oral nutrition supplements can activate PPARs to modulate metabolism and the inflammatory response [27].
2. Materials and Methods
2.1. Data Extraction Methods
2.2. Inclusion Criteria
- In vivo and in vitro studies that evaluated EC in the treatment of muscular atrophy.
- Studies with specifications of the dosage of EC used, treatment time, and administration route.
- Systematic literature reviews.
2.3. Exclusion Criteria
- Articles that used another type of catechin or flavonoid.
- Duplicated articles.
- Studies that did not analyze EC effects on skeletal musculature.
3. Results
3.1. Data Synthesis Methods-Search Results
3.2. Risk of Bias in the Studies
Risk of Bias Assessment Methods
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, M.; Zhang, J.; Polster, B.M.; Elustondo, P.A.; Thirumaran, A.; Pavlov, E.V.; Robertson, G.S. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. J. Neurosci. 2015, 12, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [PubMed]
- Araújo, C.R.R.; de Melo Silva, T.; Dos Santos, M.G.; Ottoni, M.H.F.; de Souza Fagundes, E.M.; de Sousa Fontoura, H.; de Melo, G.E.B.A.; de Carvalho Alcântara, A.F. Anti-inflammatory and cytotoxic activities of the extracts, fractions, and chemical constituents isolated from luehea ochrophylla mart. BMC Complement. Altern. Med. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Han, X.; Li, Q.; Wang, J. Epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via nrf2 and ap-1 signaling pathways. Mol. Neurobiol. 2017, 54, 7898–7907. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Heyman, E.; Burelle, Y. Effects of epicatechin on mitochondria. Nutr. Rev. 2021, 79, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Yazdi, H.S.; Samarghandian, S. The protective effects of green tea catechins in the management of neurodegenerative diseases: A review. Curr. Drug Discov. Technol. 2019, 16, 57–65. [Google Scholar] [CrossRef]
- Lee, S.J.; Leem, Y.E.; Go, G.Y.; Choi, Y.; Song, Y.J.; Kim, I.; Kim, D.Y.; Kim, Y.K.; Seo, D.W.; Kang, J.S.; et al. Epicatechin elicits myod-dependent myoblast differentiation and myogenic conversion of fibroblasts. PLoS ONE 2017, 12, e0175271. [Google Scholar] [CrossRef]
- Zbinden-Foncea, H.; Castro-Sepulveda, M.; Fuentes, J.; Speisky, H. Effect of epicatechin on skeletal muscle. Curr. Med. Chem. 2022, 29, 1110–1123. [Google Scholar] [CrossRef]
- Munguia, L.; Ortiz, M.; González, C.; Portilla, A.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. Beneficial effects of flavonoids on skeletal muscle health: A systematic review and meta-analysis. J. Med. Food 2022, 25, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; León Idougourram, S.; Muñoz Jiménez, C.; Rodríguez-Alonso, R.; Alonso Echague, R.; Chica Palomino, S.; Sanz Sanz, A.; Manzano García, G.; Gálvez Moreno, M.Á.; Calañas Continente, A.; et al. Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients 2023, 15, 2726. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M. Autophagy and aging: Keeping that old broom working. Trends Genet. 2008, 24, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 2659–2674. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study. Nutrients 2023, 15, 2703. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Gordon, P.L.; Sakkas, G.K.; Doyle, J.W.; Shubert, T.; Johansen, K.L. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Ren. Nutr. 2007, 17, 397–407. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Saif, N.; Ariza, I.E.; Isaacson, R.S. Precision Nutrition for Alzheimer’s Prevention in ApoE4 Carriers. Nutrients 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Savary-Auzeloux, I.; Magne, H.; Migné, C.; Oberli, M.; Breuillé, D.; Faure, M.; Vidal, K.; Perrot, M.; Rémond, D.; Combaret, L.; et al. A dietary supplementation with leucine and antioxidants is capable to accelerate muscle mass recovery after immobilization in adult rats. PLoS ONE 2013, 8, e81495. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Barnett, C.F.; Moreno-Ulloa, A.; Shiva, S.; Ramirez-Sanchez, I.; Taub, P.R.; Su, Y.; Ceballos, G.; Dugar, S.; Schreiner, G.; Villarreal, F. Pharmacokinetic, partial pharmacodynamic and initial safety analysis of epicatechin in healthy volunteers. Food Funct. 2015, 6, 824–833. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.; Oskarsson, B. Epicatechin enhances mitochondrial biogenesis, increases dystrophin and utrophin, increases follistatin while decreasing myostatin, and improves skeletal muscle exercise response in adults with becker muscular dystrophy (BMD). Neuromuscul. Disord. 2015, 25, S314–S315. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The green tea polyphenol epigallocatechin-3-gallate (EGCG) attenuates skeletal muscle atrophy in a rat model of sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Catechins activate muscle stem cells by myf5 induction and stimulate muscle regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.B.; Lee, H.S.; Kim, D.H.; Moon, J.M.; Park, Y. Tannase-converted green tea extract with high epicatechin inhibits skeletal muscle mass in aged mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 4319398. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Gonzalez-Basurto, S.; Coral-Vazquez, R.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and type 2 diabetes: Restorative effects of epicatechin-rich cocoa. Clin. Sci. 2013, 125, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.A.; Blahnik, Z.J.; Prahadeeswaran, S.; McKinley-Barnard, S.K.; Holden, S.L.; Waldhelm, A. (-)-Epicatechin Supplementation Inhibits Aerobic Adaptations to Cycling Exercise in Humans. Front. Nutr. 2018, 5, 132. [Google Scholar] [CrossRef]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Corr, L.D.; Field, A.; Pufal, D.; Killey, J.; Clifford, T.; Harper, L.D.; Naughton, R.J. Acute consumption of varied doses of cocoa flavanols does not influence exercise-induced muscle damage. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 338–344. [Google Scholar] [CrossRef]
- McDermott, M.M.; Criqui, M.H.; Domanchuk, K.; Ferrucci, L.; Guralnik, J.M.; Kibbe, M.R.; Kosmac, K.; Kramer, C.M.; Leeuwenburgh, C.; Li, L.; et al. Cocoa to improve walking performance in older people with peripheral artery disease: The cocoa-pad pilot randomized clinical trial. Circ. Res. 2020, 126, 589–599. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.; et al. Epicatechin induces mitochondrial biogenesis and markers of muscle regeneration in adults with Becker muscular dystrophy. Muscle Nerve 2021, 63, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.Y.; Patterson, M.C.; Clark, V.; Johnson, J.N.; Moutvic, M.A.; Driscoll, S.W.; Kemppainen, J.L.; Huston, J., 3rd; Anderson, J.R.; Badley, A.D.; et al. Safety and efficacy of (+)-epicatechin in subjects with Friedreich’s ataxia: A phase II, open-label, prospective study. J. Inherit. Metab. Dis. 2021, 44, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Fu, Z.; Babu, P.V.; Zhen, W.; Leroith, T.; Meaney, M.P.; Voelker, K.A.; Jia, Z.; Grange, R.W.; Liu, D. Dietary epicatechin promotes survival of obese diabetic mice and drosophila melanogaster. J. Nutr. 2011, 141, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Lee, I.; Malek, M.H. (-)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J. 2012, 26, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Nogueira, L.; Moreno, A.; Murphy, A.; Taub, P.; Perkins, G.; Ceballos, G.M.; Hogan, M.; Malek, M.; Villarreal, F. Stimulatory effects of the flavanol (-)-epicatechin on cardiac angiogenesis: Additive effects with exercise. J. Cardiovasc. Pharmacol. 2012, 60, 429–438. [Google Scholar] [CrossRef]
- Hüttemann, M.; Lee, I.; Perkins, G.A.; Britton, S.L.; Koch, L.G.; Malek, M.H. Epicatechin is associated with increased angiogenic and mitochondrial signalling in the hindlimb of rats selectively bred for innate low running capacity. Clin. Sci. 2013, 124, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Salmean, G.; Ciaraldi, T.P.; Nogueira, L.; Barboza, J.; Taub, P.R.; Hogan, M.C.; Henry, R.R.; Meaney, E.; Villarreal, F.; Ceballos, G.; et al. Effects of epicatechin on molecular modulators of skeletal muscle growth and differentiation. J. Nutr. Biochem. 2014, 25, 91–94. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M.; Kruger, A.; Bollig-Fischer, A.; Malek, M.H. Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Front. Pharmacol. 2015, 13, 43. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Nogueira, L.; Rodriguez, A.; Barboza, J.; Hogan, M.C.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging with (-)-Epicatechin in Senile Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1370–1378. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M.; Malek, M.H. Epicatechin attenuates degradation of mouse oxidative muscle following hindlimb suspension. J. Strength Cond. Res. 2016, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.D.; Admed, B.; LeRoith, T.; Ansah, T.A.; Zhang, L.; Li, J.; Ordovás, J.M.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019, 33, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ruiz, C.; Cordero-Anguiano, P.; Morales-Guadarrama, A.; Mondragón-Lozano, R.; Sánchez-Torres, S.; Salgado-Ceballos, H.; Villarreal, F.; Meaney, E.; Ceballos, G.; Najera, N. Epicatechin reduces muscle waste after complete spinal cord transection in a murine model: Role of ubiquitin-proteasome system. Mol. Biol. Rep. 2020, 47, 8975–8985. [Google Scholar] [CrossRef] [PubMed]
- Munguia, L.; Ramirez-Sanchez, I.; Meaney, E.; Villarreal, F.; Ceballos, G.; Najera, N. Flavonoids from dark chocolate and epicatechin ameliorate high-fat diet-induced decreases in mobility and muscle damage in aging mice. Food Biosci. 2020, 37, 100710. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Navarrete-Yañez, V.; Garate-Carrillo, A.; Lara-Hernandez, M.; Espinosa-Raya, J.; Moreno-Ulloa, A.; Gomez-Diaz, B.; Cedeño-Garcidueñas, A.L.; Ceballos, G.; Villarreal, F. Restorative potential of epicatechin in a rat model of gulf war illness muscle atrophy and fatigue. Sci. Rep. 2021, 11, 21861. [Google Scholar] [CrossRef]
- Ramírez-Ramírez, M.; Fernández-Valverde, F.; Reséndiz-García, A.; Martínez-Damas, M.G.; Cano-Martínez, L.J.; Zentella-Dehesa, A.; Coral-Vázquez, R.M. (-)-Epicatechin improves Tibialis anterior muscle repair in CD1 mice with BaCl2-induced damage. J. Nutr. Biochem. 2022, 107, 109069. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Liu, D.; Qin, C.; Yan, X.; Pang, P.; Yun, Y.; Wang, L.; Nie, G. Dietary Epicatechin supplementation regulates myofiber development, fillet quality, and antioxidant status of Yellow River carp (Cyprinus carpio). Aquaculture 2023, 572, 739542. [Google Scholar] [CrossRef]
- Palma-Flores, C.; Zárate-Segura, P.B.; Hernández-Hernández, J.M.; de Los Santos, S.; Tejeda-Gómez, A.S.; Cano-Martínez, L.J.; Canto, P.; Garcia-Rebollar, J.O.; Coral-Vázquez, R.M. (-)-Epicatechin modulates the expression of myomiRs implicated in exercise response in mouse skeletal muscle. Gene 2023, 849, 146907. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Miranda-Cervantes, A.; Licea-Navarro, A.; Mansour, C.; Beltrán-Partida, E.; Donis-Maturano, L.; Delgado De la Herrán, H.C.; Villarreal, F.; Álvarez-Delgado, C. (-)-Epicatechin stimulates mitochondrial biogenesis and cell growth in C2C12 myotubes via the G-protein coupled estrogen receptor. Eur. J. Pharmacol. 2018, 822, 95–107. [Google Scholar] [CrossRef]
- Ortiz-Flores, M.; Portilla-Martínez, A.; Cabrera-Pérez, F.; Nájera, N.; Meaney, E.; Villarreal, F.; Pérez-Durán, J.; Ceballos, G. PXR is a target of (-)-epicatechin in skeletal muscle. Heliyon 2020, 6, e05357. [Google Scholar] [CrossRef]
- Edwards, S.J.; Carter, S.; Nicholson, T.; Allen, S.L.; Morgan, P.T.; Jones, S.W.; Rendeiro, C.; Breen, L. (-)-Epicatechin and its colonic metabolite hippuric acid protect against dexamethasone-induced atrophy in skeletal muscle cells. J. Nutr. Biochem. 2022, 110, 109150. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and its impact on quality of life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [PubMed]
- Beaudart, C.; Biver, E.; Bruyère, O.; Cooper, C.; Al-Daghri, N.; Reginster, J.Y.; Rizzoli, R. Quality of life assessment in musculo-skeletal health. Aging Clin. Exp. Res. 2018, 30, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Fraga, C.G.; Oteiza, P.I. Epicatechin in the control of glucose homeostasis: Involvement of redox-regulated mechanisms. Free Radic. Biol. Med. 2019, 130, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Kumar, A.; Prajapati, P.; Raj, V.; Kim, S.C.; Mishra, V.; Raorane, C.J.; Raj, R.; Kumar, D.; Kushwaha, S. Salbutamol ameliorates skeletal muscle wasting and inflammatory markers in streptozotocin (STZ)-induced diabetic rats. Int. Immunopharmacol. 2023, 124, 110883. [Google Scholar] [CrossRef]
- Zheng, L.F.; Chen, P.J.; Xiao, W.H. Signaling pathways controlling skeletal muscle mass. Sheng Li Xue Bao 2019, 71, 671–679. [Google Scholar]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Sharma, M.; McFarlane, C.; Kambadur, R.; Kukreti, H.; Bonala, S.; Srinivasan, S. Myostatin: Expanding horizons. IUBMB Life 2015, 67, 589–600. [Google Scholar] [CrossRef]
- Gazdanova, A.A.; Kukes, V.G.; Parfenova, O.K.; Sidorov, N.G.; Perkov, A.V.; Solovieva, S.A.; Ryazantceva, O.V.; Lenkova, N.I. Myostatin—A modern understanding of the physiological role and significance in the development of age-associated diseases. Adv. Gerontol. 2021, 34, 701–706. [Google Scholar] [PubMed]
- Chen, M.M.; Zhao, Y.P.; Zhao, Y.; Deng, S.L.; Yu, K. Regulation of myostatin on the growth and development of skeletal muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.L.; Pu, H.F.; Chen, S.Y.; Wang, S.W.; Wang, P.S. Effects of catechin, epicatechin and epigallocatechin gallate on testosterone production in rat leydig cells. J. Cell. Biochem. 2010, 110, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Collier, L.; Pan, J.; Qin, W.; Bauman, W.A.; Cardozo, C.P. Testosterone reduced methylprednisolone-induced muscle atrophy in spinal cord-injured rats. Spinal Cord 2012, 50, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Chen, C.S.; Cheng, M.C.; Wu, M.F.; Cheng, F.T.; Hsu, C.L. Effects of resveratrol, epigallocatechin gallate, and epicatechin on mitochondrial functions in c2c12 myotubes. J. Funct. Foods 2017, 35, 507–512. [Google Scholar] [CrossRef]
- Sharma, G.; Prossnitz, E.R. G-protein-coupled estrogen receptor (GPER) and sex-specific metabolic homeostasis. Adv. Exp. Med. Biol. 2017, 1043, 427–453. [Google Scholar] [PubMed]
- Davison, G.; Callister, R.; Williamson, G.; Cooper, K.A.; Gleeson, M. The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur. J. Nutr. 2012, 51, 69–79. [Google Scholar] [CrossRef]
- Peschek, K.; Pritchett, R.; Bergman, E.; Pritchett, K. The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients 2013, 6, 50–62. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Godin, J.P.; Chou, C.J.; Grathwohl, D.; Ross, A.B.; Cooper, K.A.; Williamson, G.; Actis-Goretta, L. The effect of acute dark chocolate consumption on carbohydrate metabolism and performance during rest and exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 173–182. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.W.; Inagaki, T.; White, M.J.; Hirai, D.M.; Ferguson, S.K.; Holdsworth, C.T.; Sims, G.E.; Poole, D.C.; Musch, T.I. Epicatechin administration and exercising skeletal muscle vascular control and microvascular oxygenation in healthy rats. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H206–H214. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-A epicatequina medeia os efeitos benéficos do cacau rico em flavanol na função vascular em humanos. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Mikuriya, H.; Tayama, K.; Takahashi, H.; Nagasawa, A.; Yano, N.; Yuzawa, K.; Ogata, A.; Aoki, N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch. Toxicol. 2001, 75, 591–596. [Google Scholar] [PubMed]
- Yun, S.Y.; Kim, S.P.; Song, D.K. Effects of (-)-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006, 541, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Qi, C.; Liu, G.; Ping, Y.; Yang, K.; Tan, Q.; Zhang, Y.; Chen, G.; Huang, X.; Xu, D.A. Comprehensive review of nano-delivery system for tea polyphenols: Construction, applications, and challenges. Food Chem. X 2023, 17, 100571. [Google Scholar] [CrossRef]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Effect of liposomal encapsulation on the recovery and antioxidant properties of green tea catechins incorporated into a hard low-fat cheese following in vitro simulated gastrointestinal digestion. Food Bioprod. Process. 2016, 100, 238–245. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic potential of epigallocatechin gallate nanodelivery systems. Biomed. Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Chandran, S.P.; Vinukonda, A.; Dharmalingam, S.R. Green tea catechin loaded nanodelivery systems for the treatment of pandemic diseases. Asian J. Pharm. Clin. Res. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Lushchak, O.; Strilbytska, O.; Koliada, A.; Zayachkivska, A.; Burdyliuk, N.; Yurkevych, I.; Storey, K.B.; Vaiserman, A. Nanodelivery of phytobioactive compounds for treating aging associated disorders. Geroscience 2020, 42, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nano-technological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Messina, S.; Bitto, A.; Aguennouz, M.; Mazzeo, A.; Migliorato, A.; Polito, F.; Irrera, N.; Altavilla, D.; Vita, G.L.; Russo, M.; et al. Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: A comparison study with methylprednisolone. Exp Neurol. 2009, 220, 349–358. [Google Scholar] [CrossRef]
- Vita, G.L.; Sframeli, M.; Licata, N.; Bitto, A.; Romeo, S.; Frisone, F.; Ciranni, A.; Pallio, G.; Mannino, F.; Aguennouz, M.; et al. A Phase 1/2 Study of Flavocoxid, an Oral NF-κB Inhibitor, in Duchenne Muscular Dystrophy. Brain Sci. 2021, 16, 115. [Google Scholar] [CrossRef]


| First Author and Year | Manufacturer | Participants | Gender/ Age | Objective | Groups | Dosage | Experimental Time | Route of Administration | Procedure | Effects of EC (Main Results) |
|---|---|---|---|---|---|---|---|---|---|---|
| Taub et al., 2013 [37] | Hershey’s® 60% Dark chocolate | 5 participants | Male/ 47–71 years old | To evaluate the skeletal muscle growth with cocoa enriched with EC in patients with heart failure and type 2 diabetes. | -Control group: Patients aged 50–53 years with no disease. -Experimental group: Patients aged 47–71 years. | 100 mg a day | 3 months | Oral route | The patients underwent femoral quadriceps muscle biopsies before and after consuming cocoa enriched with EC. | There was a decrease in myostatin; however, it remained elevated compared to the control group. Follistatin increased above the controls with the treatment. The myogenin, MyoD, MEF2, and Myf5 levels were significantly stimulated with the EC treatment. p < 0.05 |
| Schwarz et al., 2018 [38] | 20 participants | Active men and women/Between 18 and 30 years old | To determine if EC supplementation increases the performance of cycling exercise. | EPI group or PLA (Placebo) group. | 200 mg twice daily | 4 weeks anaerobic and aerobic cycle training protocol. | Oral capsules | The cycling exercise sessions were conducted per week for 4 weeks (total of 16 sessions) with EC supplementation and a placebo. | EC supplementation did not affect myostatin expression but suppressed mitochondrial adaptations to exercise training. p ≤ 0.05 | |
| Mafi et al., 2019 [39] | Sigma-Aldrich, St. Louis, MO, USA | 62 participants | Male /68 ± 2.86 years old | To evaluate the plasma levels of follistatin and myostatin in men with sarcopenia under training and EC supplementation. | RT: Resistance training, EP: EC, RT + EP: Resistance training + EC, PL: Double-blind placebo. | 1 mg·kg−1·a day | 8 weeks | Oral route(Daily capsules with 200 mL of water). | The training groups’ subjects conducted the protocol at 05:00 p.m. (45 min, 3 sets, 8–12 repetitions). The placebo group received starch capsules. | Follistatin significantly increased in the RT + EP groups compared to PL group. In comparison, myostatin reduced in the RT + EP and in RT groups. The maximum supine strength significantly improved in RT + EP and RT participants. p ≤ 0.05 |
| Corr et al., 2020 [40] | Chococru®/EC | 23 participants | 13 women and 10 men/24 years. | To investigate if an acute dose of flavonoid cocoa (FC) may help in muscle recovery following EIMD. | CON: Control group: Did not receive FC, n = 8; CF830: High FC dose 830 mg group, n = 8; CF1245: FC overdose group 1245 mg, n = 7. | 830 mg and 1245 mg | 5 days (2 adaptation days and 3 days of EC) | Oral route | The EIMD protocol consisted in the hip fastening to the dynamometer at 85° of bending using straps to isolate the knee (5 series of 10 maximum concentric and eccentric contractions of the knee. | No significant modifications were observed between the groups for all the measures in the bending exercises. The FC did not show benefits in muscle recovery after 24 h, 48 h, and 72 h after EIMD protocol. p ≤ 0.05 |
| McDermott et al., 2020 [41] | Hershey’s Co®. | 44 participants | Male and female/≥60 years old. | To evaluate if cocoa with EC improves walking performance in aged people with peripheral artery disease. | Cocoa drink/Epi (n = 23) versus placebo drink (n = 21) (did not contain cocoa or EC). | 75 mg | 6 months | Oral route | The physical activity was conducted over 7 days with Accelerometer ActiGraph placed on the right hip. | Statistical differences were observed in the Cocoa/Epi group versus the placebo group in the 6-min walk test 2.5 h after consuming the drink. These results suggest a therapeutic effect of cocoa/Epi in the walk performance. However, cocoa/Epi did not significantly affect myostatin, follistatin, and Pax7. p < 0.10 |
| McDonald et al., 2021 [42] | cGMP facility (Syngene, Karnatak, India) | 7 participants | Male/18–60 years old | To evaluate EC capacity in mitochondrial biogenesis and in the muscle markers. | Nonrandomized clinical trial (before and after). | 50 mg twice a day | 8 weeks | Oral route (gelatin capsules). | The participants received two capsules in the morning and two in the evening. The brachial biceps muscle biopsies were collected pre- and post-treatment. | Follistatin significantly increased, while myostatin decreased. There was a significant increment of tissue markers Myf5, MyoD, myogenin, and MEF2a. EC stimulated PGC1α (a coactivator of mitochondrial biogenesis). p < 0.05 |
| Qureshi et al., 2021 [43] | Epirium Bio, Inc. | 10 participants | Both/10 to 22 years old | To analyze the efficacy of EC in patients with Friedreich’s ataxia. | Prospective, nonrandomized, open-label study | 75 mg/daily | 12 and 24 weeks | Oral route | Subjects received 25-mg capsule, 3/daily (75 mg daily) to assess clinical and biochemical parameters. Mitochondrial function pre- and post- EPI treatment and oxidative damage were measured. | Follistatin was higher at 12 and 24 weeks after consumption (12 weeks, p = 0.020; 24 weeks, p = 0.016). However, myostatin levels demonstrated no significant differences at 12 or 24 weeks. p < 0.05 |
| First Author and Year | Manufacturer | Population | Gender/Age | Objective | Groups | Dosage | Experimental Time | Route of Administration | Procedure | Effects of EC (Main Results) |
|---|---|---|---|---|---|---|---|---|---|---|
| Si et al., 2011 [44] | Sigma-Aldrich | 29 C57BLKS/J and KS.Cg-m +/+Lepr db/J, db/db Mice | Male/ 5 weeks of age | To investigate the effects of EC in obese diabetic mice. | Con: n = 12 Control group: C57BLKS/J Mice; db: n = 6: Diabetic rats without EC. db + EC: n = 11: 0.25%: Diabetic rats + EC. | 0.25% every other day | 15 weeks | Oral route | To determine the contractile function, the EDL muscles were excised and attached by means of a suture to a servomotor (Aurora Scientific). | EC significantly decreased the inflammatory markers (C-reactive protein) in diabetic rats. The GSK antioxidant concentration and AMPKa phosphorylation were considerably higher than db group. p < 0.05 |
| Hüttemann et al., 2012 [45] | Sigma Aldrich, USA | C57BL/6, n = 32 | Male mice/5-month-old | To determine whether EC could enhance endurance capacity on detraining hindlimb muscles of mice. | Four groups:
| 1 mg/kg twice daily | 14 days | Oral gavage | Groups 2, 3, and 4 performed a training (treadmill) 5 times a week for 5 weeks with a pre- and post-training analysis 48 h after the exercise test. Animals underwent a third incremental treadmill test. The plantaris and quadriceps femoris muscles were collected for analysis. | In the DT-14-W and DT-14-Epi (groups 2 and 3), the VEGF-A protein was higher compared to groups 1 and 2. Complex I expression was increased in the DT-14-Epi group compared to the group 1. However, the expression of complex III protein was significantly greater in the group 4. The fiber area was greater in the trained and group 4. p ≤ 0.05 |
| Ramirez-Sanchez et al., 2012 [46] | Sigma-Aldrich | 25 C57BL/6N | Male mice/One-year-old | To examine the Epi effect on cardiac angiogenesis and plantaris muscle when Epi and exercise are combined. | Four groups: (1) Water; (2) Water exercise (W-Ex); (3) EC (Epicatechin) and (4) EC exercise (Epi Ex). | 1 mg/kg twice a day | 15 consecutive days | Oral gavage | All animals exercised on a treadmill at a slow speed and at 10° inclination angle for 5–10 min until exhaustion. Plantaris muscle was collected for further analysis. | Plantaris muscle capillary was increased by EC. VEGF protein was significantly enhanced by Epi and Exercise alone, but when combined, VEGF was enhanced (10%). p-PI3K was increased further on the Epi-Ex group (~80%). p ≤ 0.05 |
| Hüttemann et al., 2013 [47] | Sigma- Aldrich | 21 LCR rats (rats grown for low capacity to run) with congenital muscle dysfunction. | Males/5 months of age | To determine the action of EC on angiogenesis and mitochondrial proliferation. | Control: Water group for 30 days; Epi 30d: EC for 30 days; post-Epi 15d: EC for 30 days and 15 days without EC. | 1 mg/kg twice a day | EC for 30 days, followed by 15 days without EC. | Gavage | The plantar muscle was analyzed in order to determine the effects of EC on a glycolytic muscle fiber. | EC increased in capillarity and mitochondrial biogenesis in the 15-day treatment period, including in the 15-day period of treatment interruption. EC increased VEGF and reduced CD47 and the receptor TSP1, and it also activated the P38 MAPK pathways. p ≤ 0.05 |
| Gutierrez-Salmean et al., 2014 [48] | Sigma-Aldrich | 20 C57BL/6 Mice n = 20 5/group | Young males/6 months and senile males/26 months | To examine the changes to the protein levels in the skeletal muscle of young vs senile humans and mice. | Ctrl (Young), Epi (Senile), Ctrl (Senile), Epi (Young) | 1 mg/kg | 2 weeks | Gavage | The control groups received water through gavage. Quadriceps muscle samples were obtained from the mice. | Epicatechin significantly decreased the myostatin levels 15% (young) and 21% (aged). Follistatin increased 56% in the senile group. Myogenin significantly increased in young and senile animals (16%, 21%, respectively), while MyoD increased 19% in senile rats. Myf5 incremented 12% (young) and 15% (senile), and MEF2 10%, 19%, respectively. p < 0.05 |
| 12 participants | Gender not reported/Young adults: 28 years old, n = 6 Aged: 62 years old, n = 6 | To evaluate the effects of the treatment with epicatechin on muscle strength and on the plasma levels of myostatin and follistatin. | Young adults’ group (n = 6) Senile group (n = 6) | 25 mg/day | 1 week | Oral route (capsule) | The muscle strength was evaluated by hand grip dynamometry (three times with each hand, alternating the hand and resting for 10 s to prevent fatigue). | The treatment with epicatechin increases the hand’s muscle strength by 7%. With age, there was a significant increase in myostatin (28%, 48%). The treatment with EC significantly increased the plasma levels of follistatin (49%). p < 0.05 | ||
| Lee et al., 2015 [49] | Sigma-Aldrich, St. Louis, MO, USA | 34 C57BL/6N Mice | Males/14 months of age | To determine the effect of epicatechin on angiogenesis and mitochondrial biogenesis protein markers. | C: control group; CE: control with resistance training; Epi: epicatechin; Epi-Ex: epicatechin + training. | 1 mg/kg twice a day | 8 weeks | Gavage | The training groups’ mice were submitted to training on a treadmill for 8 weeks (5 times/week for 60 min/session). | The Epi-Ex showed better resistance performance, and a significantly higher VEGF-R2 expression, and increased PGC-1b and TFAM. FoxO1 expression was reduced in the experimental groups. p ≤ 0.05 |
| Moreno-Ulloa et al., 2015 [50] | Not reported | 15 C57BL/6 | Male mice/26-month-old | To compare the protein levels in senile mice versus young mice on skeletal muscle, heart, kidney, and brain. | (1) Y mice: (6-month-old), n = 5; (2) S mice: (26-month-old), n = 5; (3) S mice: treated with EC, n = 5. | 1 mg/kg twice daily | 2 weeks | Gavage | Muscle biopsy tissue was processed for analysis. | EC re-establish GSH in skeletal muscle (SkM). Aging biomarkers were reduced in old mice. In SkM, Epi administration increased complex I protein levels (C-I) and significantly decreased SA-β-gal protein. p < 0.05 |
| Lee et al., 2016 [51] | Sigma-Aldrich, St. Louis, MO, USA | 25 C57BL/6N Mice | Males/6 months of age | To determine if the treatment with EC may mitigate the muscle mass loss in skeletal muscle. | C: Control (water); HS-V: Suspension of the hind limbs + water; HS-EC: Suspension of the hind limbs + EC. | 1.0 mg/kg twice a day (Morning and evening). | 14 consecutive days | Gavage | For the hind limb suspension protocol, the animals were placed in a cage with a steel bar. The soleus, medial, and gastrocnemius muscles were removed from both hind limbs. | HS-EC showed significantly higher FCSA. In HS-Epi there was a slight decrease in FP compared to the control group. VEGF-A was lower in the vehicle or epicatechin groups. HS-Epi showed a significant increase in mTOR, Akt, and TFAM. PGC-1β was only induced in HS-Epi, and CcO was similar to the control. FoxO and GSK-3β were induced in HS-V. p ≤ 0.05 |
| Si et al., 2019 [52] | Millipore Sigma, Burlington, MA, USA | 33 C57BL/6 Mice | Males/ 9 months and 20 months of age | To investigate the effects of EC on the survival rate and on the physical performance in aged mice. | OC: Control (aged mice); YC: Young control: 9-month-old mice; EC: 0.25% epicatechin. | 0.25% | 37 weeks and 44 weeks | Oral route | The samples were collected following 37 weeks, and the rest was treated for one additional week (on week 44). | EC attenuated the deterioration of the muscle; in addition, it improved physical activity, and delayed the degeneration of the quadriceps. E in senile mice presented a survival rate (69%) compared to the control group (39%). p < 0.05 |
| Gonzalez-Ruiz et al., 2020 [53] | Sigma-Aldrich | 36 Long-Evans Rats | Females/11 weeks | To analyze the effects of epicatechin on the regulation of UPS proteins in the hind limbs. | SCI + water 7 days: n = 6; SCI + Epi 7 days: n = 6; SCI + water 30 days: n = 9; SCI + Epi 30 days: n = 9; Sham: Only laminectomy n = 6. | 1 mg/kg/day | 1 week and at 30 days | Gavage | The spinal cord was sectioned (region of the T8 to T10 vertebrae). The left side gastrocnemius and soleus muscles were dissected. | At 30 days, the injury group lost 49.52% of the cross-sectional area of the muscles, and the epicatechin groups lost 24.28 ± 15.45%. After 7 days, the SCI + EC had only one significant difference in MuRF. The treatment with EC induced a significant decrease in atrophy markers FOXO, MAFbx, and MuRF1 compared to the control group (VEH) after 7 and 30 days from the lesion. p < 0.05 |
| Munguia et al., 2020 [54] | Sigma-Aldrich Co. (St. Louis, MO, USA) | 15 C57BL/6 Mice induced to a high-fat diet | Males/10 weeks | To evaluate the benefits of the flavonoids in the improvement of the physical activity decreased by age/high-fat diet. | Three interventions: Control: Water; High-flavonoid dark chocolate; (DC) drink: 2 mg EC + 12.8 mg procyanidins/kg); EC: Epicatechin (2 mg EC/kg). | 2 mg EC/kg | 5 weeks of treatment with EC. Week 64–Change from normal diet + 5 weeks of treatment. Total: 69 weeks. | Gavage | Gastrocnemius were collected. The inverted screen and front limbs functional test consisted in the longest time hanging, establishing a fixed time of 120 s and 130 s, respectively. | EC increased follistatin and myocyte enhancer factor 2A (MEF2A) expression. DC and EC decreased FoxO and MURF; however, MAFbx was not significant. DC and EC reduced the fat content and increased physical performance compared to the control. p < 0.05 |
| Ramirez-Sanchez et al., 2021 [55] | Sigma-Aldrich, Inc./Hershey, PA, USA | 30 Wistar Rats | Male/3 months of age | To examine the potential restorative effects of epicatechin in muscular atrophy-induced rats. | Control group (n = 15): Without physical restriction (water): The experimental group (n = 15): Physical restriction (2 weeks). Rats were divided into two groups: Epi GWI-Epi group (n = 8) and Water GWI group (n = 7). | 1 mg/kg/day | 2 weeks of EC. Atrophy induction (3 weeks) + 1 maintenance week + 2 weeks of EC. On week 6–Functional test and euthanasia. | Gavage | Atrophy induction protocol: pyridostigmine bromide (PB) 1.3 mg/kg/day through the oral route, permethrin 0.13 mg/kg/day, and DEET 40 mg/kg/day. The animals were physically contained for 5 min/day for 3 weeks. | The treatment with epicatechin induced a partial recovery of muscle strength and run distance on treadmill. MURF, Fbox40, and atrogin-1 were partially recovered by EC. Epicatechin significantly increased AKT and mTORC1 activation. p < 0.05 |
| Ramírez-Ramírez et al., 2022 [56] | Sigma-Aldrich, St. Louis, MO, USA | One hundred twenty-six 132 CD-1 mice | Male /10-weeks-old | To examine the effects of EC treatment in the Tibialis anterior muscle repair process. | Two treatments: Vehicle treatment: right leg injured with BaCl2 (WI-E) and left leg without damage (WOI-E). EC treatment: right leg injured with BaCl2 (WI + E) and left leg without injury (WOI + E). | 1 mg/kg EC/kg | C was administrated every 12 h and animals were sacrificed at 12 and 24 h, 2 days, 4 days and 15 days. | Oral gavage twice daily | Hind legs tibialis anterior muscles were collected for histological analyses. | EC significant increased MyoD and Myogenin at 24 h (h) after injury compared to the other groups. Histological lesion in WI + E presented a smaller lesion area after 24 h (p= < 0.05), and also more significant reduction after two days (p = 0.0149). The number of central nuclei were increased only at 12 h post-injury in WI + E. p < 0.05 |
| Mi et al., 2023 [57] | Nanjing Daosifu Biotech Co., Ltd., Nanjing, China. | 300 fish (16.27 ± 0.24 g). | Not reported/Juvenile yellow river carp. | To investigate the antioxidant and muscle fiber growth effects of EC. | The groups were divided according to the amount of epicatechin present in the diet, as follows: EC (0, 100, 500, and 1000 mg/kg). | 0, 100, 500, and 1000 mg/kg. | The juvenile carp were fed three times a day for 60 days | Hand-fed | Juvenile carp were randomly allocated in 3 tanks per group. Four blocks of muscle were collected from the bilateral dorsal fin. | EC activated AMPKα2 and PGC-1α. EC 500 and EC 1000 groups increased muscle hardness and SOD activity. EC 1000 group upregulated MyoD, and myogenin and downregulated Myostatin b (mstnb). p < 0.05 |
| Palma-Flores et al., 2023 [58] | Sigma-Aldrich, St. Louis, MO, USA | Twelve CD-1 mice | Not reported/2.5 months old | To determine the potential activity of epicatechin on the expression of miRNAs in skeletal muscle growth and regeneration. | Two groups: Control, Ctrl: Water-treated and Epi-treated (Epi). | 1 mg/kg EC/kg | Two weeks | Oral gavage twice daily | After treatment, the quadriceps muscles samples were excised and stored for further analysis. | MyoD and myogenin were increased by EC. p < 0.05 |
| First Author and Year | Manufacturer | Type of Muscle Cells | Objective | Groups | Dosage | Experimental Time | Procedure | Effects of EC (Main Results) |
|---|---|---|---|---|---|---|---|---|
| Moreno-Ulloa et al., 2018 [59] | EPI, MISSION® siRNA Universal Negative Control #1 | C2C12 myoblasts | To analyze if EC stimulates mitochondrial biogenesis (MiB). | Control group: Dimethyl sulfoxide DMSO used as vehicle; Epi 3 µm: Treatment with 3 µm of EC; Epi 10 µm: Treatment with 10 µm of epicatechin. | EPI (3 µM and 10 µM | 48 h | Myotubes in DMEM were treated with an incubation time of 48 h. | COX-I/SDH-A was increased by epicatechin, indicating the effect of Epi on mitochondrial biogenesis. Epi increased the width and length of C2C12 myotubes. p ≤ 0.05 |
| Ortiz-Flores et al., 2020 [60] | Merck KGaA, Darmstadt, Germany | Mouse myoblast (C2C12 cells) | To demonstrate that EC probably activates PXR as a target in C2C12 myoblasts. | Control: FBS 10%; Positive control: 2% horse serum EC: 1 μM; EC + Keto: 1 μM + 10 μM ketoconazole (PXR’s antagonist); PCN (PXR activator): 1Μm of Pregnenolone-16a-carbonitrile (PCN); PCN + Keto: 1 μM of PCN + 10 μM ketoconazole. | EC 1 μM | 30 min | C2C12 cells were cultured in DMEM-F12. After C2C12 differentiation assay, myogenin was quantified. | EC activated PXR, promoting muscle cell differentiation and increasing myogenin and Cyp3a11 expression in C2C12 cultured cells. p < 0.05 |
| Edwards et al., 2022 [61] | Epi: #E1753 Sigma | Mouse skeletal muscle C2C12 myoblasts | To investigate the effects of EC and HA (hippuric acid) on skeletal muscle morphology and metabolism investigating an in vitro model of muscle atrophy with dexamethasone. | Divided into 6 groups: VC-CTRL: vehicle control. VC-DEX: cells incubated in dexamethasone; EPI-CTL: cells incubated with 25 μM EC; EPI + DEX: cells were incubated in 25 μM EC and 100 μM DEX; HA-CTL: cells incubated in 25 μM HA.; HA + DEX: cells incubated with 25 μM HA and 100 μM DEX | 25 μM EPI and 100 μM DEX. | 24h of treatment protocol | Cells were incubated in DMEM (5mM glucose), followed by 6 days of differentiation, and received 24 h of treatment. | PGC1 α, ACC, and TFAM (regulators of mitochondrial function) were significantly lower in DEX-treated versus CTL cells (Control). However, EPI or HA partially attenuated the proteolysis in DEX-treated groups by preserving the expression of LC3 and caspase-3 protein. Myotube diameter was significantly greater in EPI-DEX and HA- DEX. p ≤ 0.05 |
| Risk of Bias | Taub et al., 2013 [37] | Schwarz et al., 2018 [38] | Mafi et al., 2019 [39] | Corr et al., 2020 [40] | McDermott et al., 2020 [41] | McDonald et al., 2021 [42] | Qureshi et al., 2021 [43] | Si et al., 2011 [44] | Hüttemann et al., 2012 [45] | Ramirez-Sanchez et al., 2012 [46] | Hüttemann et al., 2013 [47] | Gutierrez-Salmean et al., 2014 [48] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced sample size |  |  |  | ||||||||||
| Reduced evaluation time |  | ||||||||||||
| Failure to collect the participants’ diet |  |  | |||||||||||
| Only one period was evaluated |  |  |  |  |  |  |  |  |  |  | |||
| Only one dose was studied |  |  |  |  |  |  |  |  |  |  |  | ||
| Absence of plasma (epicatechin) measured. |  |  |  |  |  |  |  |  |  | ||||
| Difference of the euthanasia periods | |||||||||||||
| Choice of the animal model |  | ||||||||||||
| Epicatechin interruption was not evaluated |  |  |  |  |  |  |  |  |  |  |  | ||
| Participants’ gender not reported |  | ||||||||||||
| No control group |  | ||||||||||||
| Reduced sample size | |||||||||||||
| Reduced evaluation time | |||||||||||||
| Failure to collect the participants’ diet | N/A | N/A | N/A | ||||||||||
| Only one period was evaluated |  |  |  |  |  |  |  |  |  |  | |||
| Only one dose was studied |  |  |  |  |  |  |  |  |  |  |  | ||
| Absence of plasma (epicatechin) measured. |  |  |  |  |  |  |  |  |  |  | N/A | N/A | N/A |
| Difference of the euthanasia periods |  | N/A | N/A | N/A | |||||||||
| Choice of the animal model | N/A | N/A | N/A | ||||||||||
| Epicatechin interruption was not evaluated |  |  |  |  |  |  |  |  |  |  | |||
| Participants’ gender not reported | N/A | N/A | N/A | ||||||||||
| No control group | |||||||||||||
| Risk of Bias | Lee et al., 2015 [49] | Moreno-Ulloa et al., 2015 [50] | Lee et al., 2016 [51] | Si et al., 2019 [52] | Gonzalez-Ruiz et al., 2020 [53] | Munguia et al., 2020 [54] | Ramirez-Sanchez et al., 2021 [55] | Ramirez- Ramírez et al., 2022 [56] | Mi et al., 2023 [57] | Palma-Flores et al., 2023 [58] | Moreno-Ulloa et al., 2018 [59] | Ortiz-Flores et al., 2020 [60] | Edwards et al., 2022 [61] |
| Reduced sample size | |||||||||||||
| Reduced evaluation time | |||||||||||||
| Failure to collect the participants’ diet | N/A | N/A | N/A | ||||||||||
| Only one period was evaluated |  |  |  |  |  |  |  |  |  |  | |||
| Only one dose was studied |  |  |  |  |  |  |  |  |  |  |  | ||
| Absence of plasma (epicatechin) measured. |  |  |  |  |  |  |  |  |  |  | N/A | N/A | N/A |
| Difference of the euthanasia periods |  | N/A | N/A | N/A | |||||||||
| Choice of the animal model | N/A | N/A | N/A | ||||||||||
| Epicatechin interruption was not evaluated |  |  |  |  |  |  |  |  |  |  | |||
| Participants’ gender not reported | N/A | N/A | N/A | ||||||||||
| No control group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
German, I.J.S.; Pomini, K.T.; Andreo, J.C.; Shindo, J.V.T.C.; Castro, M.V.M.d.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Fornari Laurindo, L.; Bueno, P.C.d.S.; et al. New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients 2024, 16, 326. https://doi.org/10.3390/nu16020326
German IJS, Pomini KT, Andreo JC, Shindo JVTC, Castro MVMd, Detregiachi CRP, Araújo AC, Guiguer EL, Fornari Laurindo L, Bueno PCdS, et al. New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients. 2024; 16(2):326. https://doi.org/10.3390/nu16020326
Chicago/Turabian StyleGerman, Iris Jasmin Santos, Karina Torres Pomini, Jesus Carlos Andreo, João Vitor Tadashi Cosin Shindo, Marcela Vialogo Marques de Castro, Claudia Rucco P. Detregiachi, Adriano Cressoni Araújo, Elen Landgraf Guiguer, Lucas Fornari Laurindo, Patrícia Cincotto dos Santos Bueno, and et al. 2024. "New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin" Nutrients 16, no. 2: 326. https://doi.org/10.3390/nu16020326
APA StyleGerman, I. J. S., Pomini, K. T., Andreo, J. C., Shindo, J. V. T. C., Castro, M. V. M. d., Detregiachi, C. R. P., Araújo, A. C., Guiguer, E. L., Fornari Laurindo, L., Bueno, P. C. d. S., Souza, M. d. S. S. d., Gabaldi, M., Barbalho, S. M., & Shinohara, A. L. (2024). New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients, 16(2), 326. https://doi.org/10.3390/nu16020326