High-Protein Diets during either Resistance or Concurrent Training Have No Detrimental Effect on Bone Parameters in Resistance-Trained Males
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Anthropometry and Bone Parameters
2.4. Resistance Training
2.5. Concurrent Training
2.6. Training Volume
- RT volume = [repetitions (n) × sets (n) × load or selected weight (kg)].
- The volume of ET was determined using the following formula: Total ET volume: [work + rest].
- Work: [Intensity × maximum aerobic power (MAP) × (set × duration [as noted in the training protocol] × 0.06)].
- Rest: [Intensity × MAP × (set × duration [as noted in the training protocol] × 0.06)].
- Intensity: percent of MAP; Set: number of repetitions of each session; Duration: spent time (minutes); 0.06: Convert watts to kilojoules
2.7. Diet
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Bone Parameters
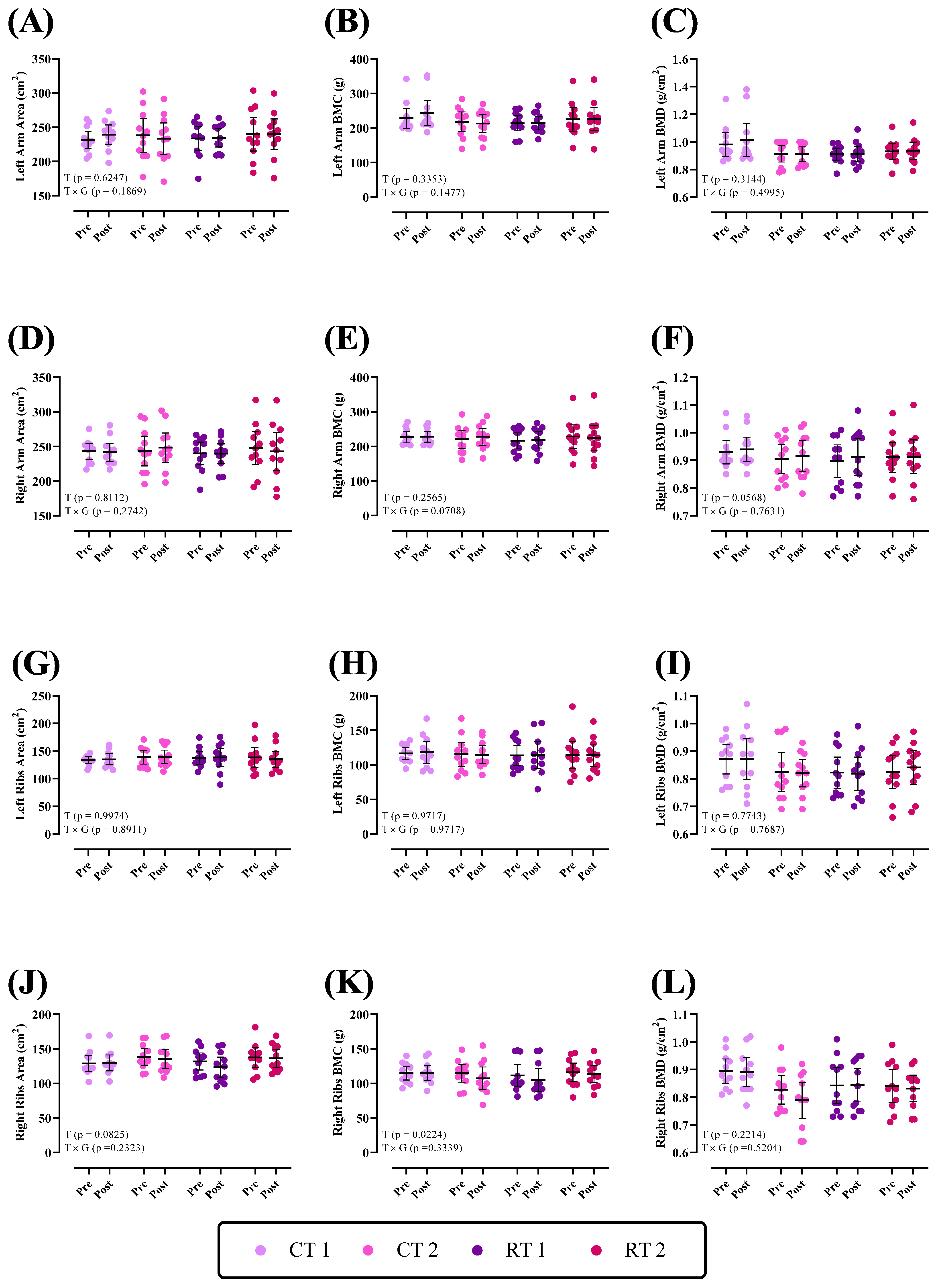
3.2.1. Upper Body
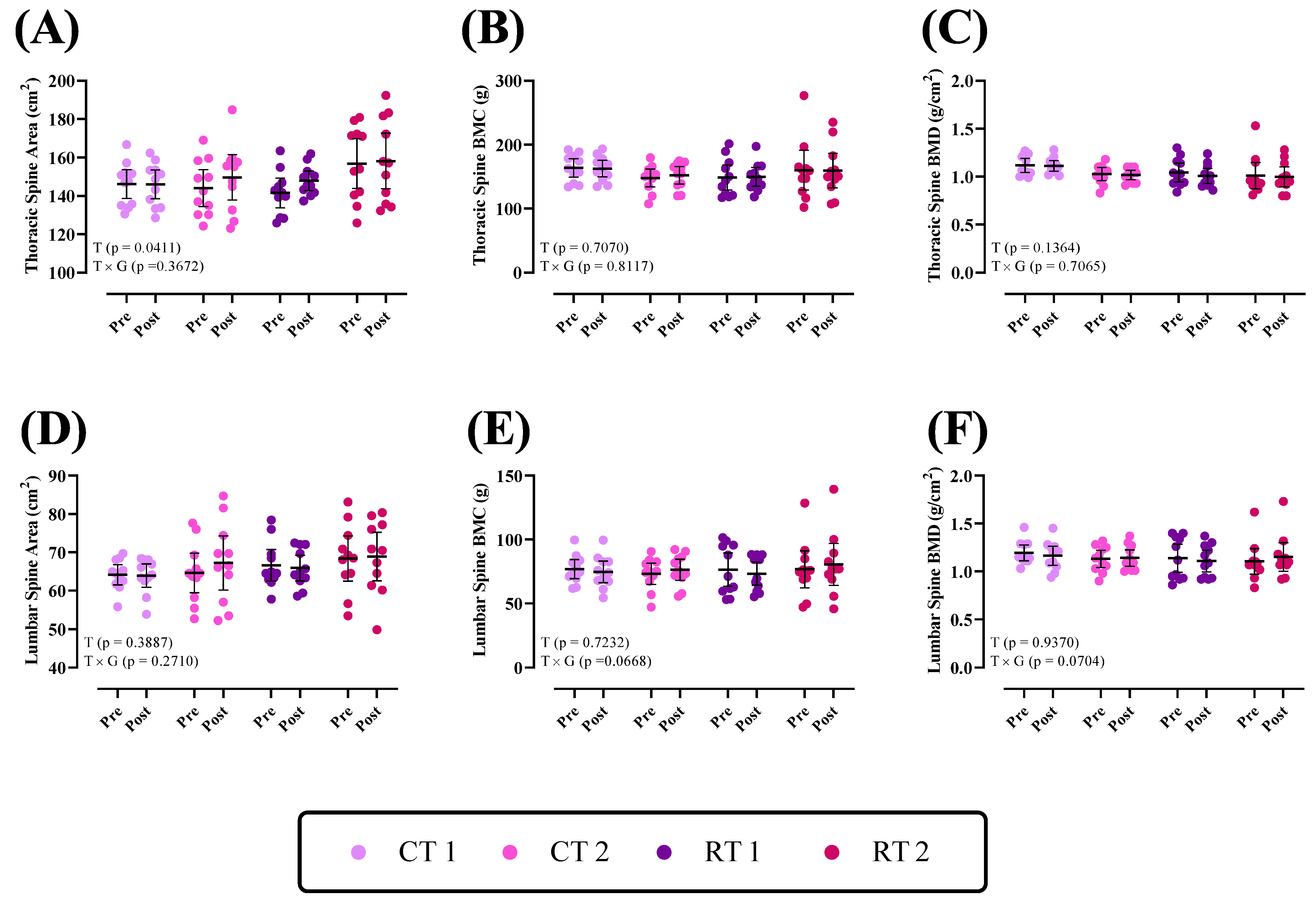
3.2.2. Thoracic Spine and Lumbar
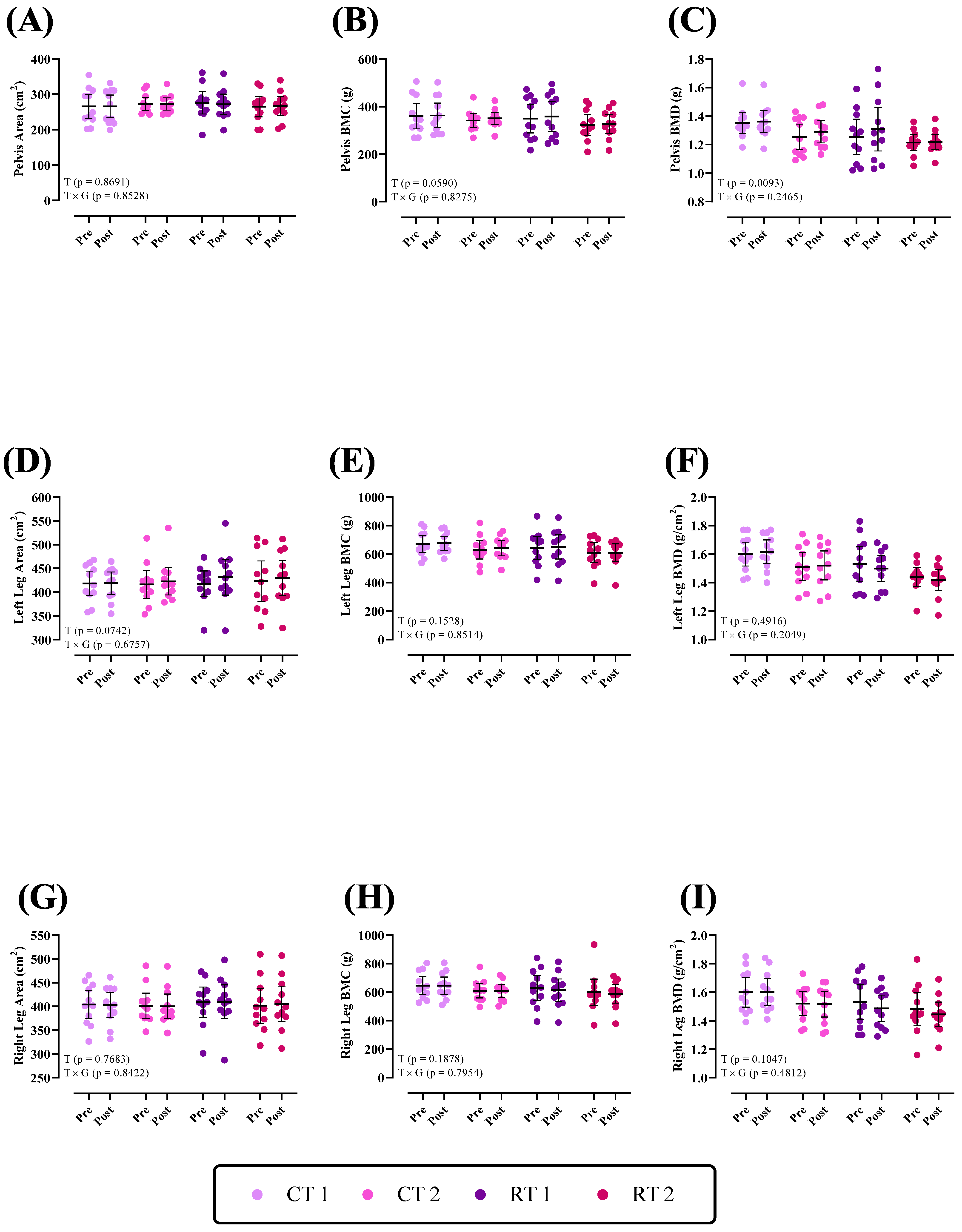
3.2.3. Lower Body
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A.; et al. Strategic ingestion of high-protein dairy milk during a resistance training program increases lean mass, strength, and power in trained young males. Nutrients 2021, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.J.; Wood, D.T.; Andrews, R.G.; Elkind, L.M.; Davis, W.B. Concurrent training enhances athletes’ strength, muscle endurance, and other measures. J. Strength Cond. Res. 2008, 22, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Sabag, A.; Najafi, A.; Michael, S.; Esgin, T.; Halaki, M.; Hackett, D. The compatibility of concurrent high intensity interval training and resistance training for muscular strength and hypertrophy: A systematic review and meta-analysis. J. Sports Sci. 2018, 36, 2472–2483. [Google Scholar] [CrossRef] [PubMed]
- Shamim, B.; Devlin, B.L.; Timmins, R.G.; Tofari, P.; Dow, C.L.; Coffey, V.G.; Hawley, J.A.; Camera, D.M. Adaptations to concurrent training in combination with high protein availability: A comparative trial in healthy, recreationally active men. Sports Med. 2018, 48, 2869–2883. [Google Scholar] [CrossRef]
- Cao, J.J. High dietary protein intake and protein-related acid load on bone health. Curr. Osteoporos. Rep. 2017, 15, 571–576. [Google Scholar] [CrossRef]
- Massini, D.A.; Nedog, F.H.; de Oliveira, T.P.; Almeida, T.A.F.; Santana, C.A.A.; Neiva, C.M.; Macedo, A.G.; Castro, E.A.; Espada, M.C.; Santos, F.J.; et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1129. [Google Scholar] [CrossRef]
- Larsen, M.N.; Nielsen, C.M.; Helge, E.W.; Madsen, M.; Manniche, V.; Hansen, L.; Hansen, R.; Bangsbo, J.; Krustrup, P. Positive effects on bone mineralisation and muscular fitness after 10 months of intense school-based physical training for children aged 8–10 years: The FIT FIRST randomised controlled trial. Br. J. Sports Med. 2018, 52, 254–260. [Google Scholar] [CrossRef]
- Skerry, T.M. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008, 473, 117–123. [Google Scholar] [CrossRef]
- Skerry, T.M.; Suva, L.J. Investigation of the regulation of bone mass by mechanical loading: From quantitative cytochemistry to gene array. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2003, 21, 223–229. [Google Scholar] [CrossRef]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Brandi, M.L. Muscle–bone interactions: Basic and clinical aspects. Endocrine 2014, 45, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Bettis, T.; Kim, B.-J.; Hamrick, M.W. Impact of muscle atrophy on bone metabolism and bone strength: Implications for muscle-bone crosstalk with aging and disuse. Osteoporos. Int. 2018, 29, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Beck, B.R.; Harding, A.T.; Watson, S.L.; Weeks, B.K. Regional changes in indices of bone strength of upper and lower limbs in response to high-intensity impact loading or high-intensity resistance training. Bone 2020, 132, 115192. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef]
- Beck, B.R. Muscle forces or gravity-what predominates mechanical loading on bone?: Introduction. Med. Sci. Sports Exerc. 2009, 41, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Wanderley, F.; Machado, L.; Sousa, F.; Viana, J.L.; Moreira-Goncalves, D.; Moreira, P.; Mota, J.; Carvalho, J. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 2011, 46, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Tran, Z.V. Resistance training and bone mineral density in women: A meta-analysis of controlled trials. Am. J. Phys. Med. Rehabil. 2001, 80, 65–77. [Google Scholar] [CrossRef]
- Layne, J.E.; Nelson, M.E. The effects of progressive resistance training on bone density: A review. Med. Sci. Sports Exerc. 1999, 31, 25–30. [Google Scholar] [CrossRef]
- Martyn-St James, M.; Carroll, S. High-intensity resistance training and postmenopausal bone loss: A meta-analysis. Osteoporos. Int. 2006, 17, 1225–1240. [Google Scholar] [CrossRef]
- Nickols-Richardson, S.M.; Miller, L.E.; Wootten, D.F.; Ramp, W.K.; Herbert, W.G. Concentric and eccentric isokinetic resistance training similarly increases muscular strength, fat-free soft tissue mass, and specific bone mineral measurements in young women. Osteoporos. Int. 2007, 18, 789–796. [Google Scholar] [CrossRef]
- Swift, J.M.; Nilsson, M.I.; Hogan, H.A.; Sumner, L.R.; Bloomfield, S.A. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J. Bone Miner. Res. 2010, 25, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Camargo Filho, J.C.S.; Ozaki, G.A.T.; Koike, T.E.; Castoldi, R.C.; Garçon, A.A.B.; Kodama, F.Y.; Watanabe, A.W.; Job, A.E.; Louzada, M.J.Q.; Camargo, E.C.T.; et al. Efeitos da remobilização por meio de exercício físico sobre a densidade óssea de ratos adultos e idosos. Motricidade 2014, 10, 71–78. [Google Scholar]
- Hong, A.R.; Kim, S.W. Effects of resistance exercise on bone health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Blimkie, C.; Rice, S.; Webber, C.; Martin, J.; Levy, D.; Gordon, C. Effects of resistance training on bone mineral content and density in adolescent females. Can. J. Physiol. Pharmacol. 1996, 74, 1025–1033. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Bonjour, J.P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C.; et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 2018, 29, 1933–1948. [Google Scholar] [PubMed]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Sun, X.-L.; Lv, Q.-B.; Zhou, Y.; Xia, D.-D.; Xu, H.-Z.; Huang, Q.-S.; Chi, Y.-L. The relationship between dietary protein consumption and risk of fracture: A subgroup and dose-response meta-analysis of prospective cohort studies. Sci. Rep. 2015, 5, 9151. [Google Scholar] [CrossRef]
- Bagheri, R.; Kargarfard, M.; Sadeghi, R.; Scott, D.; Camera, D.M. Effects of 16 weeks of two different high-protein diets with either resistance or concurrent training on body composition, muscular strength and performance, and markers of liver and kidney function in resistance-trained males. J. Int. Soc. Sports Nutr. 2023, 20, 2236053. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Taylor, L.W.; Outlaw, J.; Williams, L.; Campbell, B.; Foster, C.A.; Smith-Ryan, A.; Urbina, S.; Hayward, S. The effects of pre-and post-exercise whey vs. casein protein consumption on body composition and performance measures in collegiate female athletes. J. Sports Sci. Med. 2013, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Spillane, M.; Schwarz, N.; Willoughby, D.S. Heavy resistance training and peri-exercise ingestion of a multi-ingredient ergogenic nutritional supplement in males: Effects on body composition, muscle performance and markers of muscle protein synthesis. J. Sports Sci. Med. 2014, 13, 894. [Google Scholar] [PubMed]
- Haff, G.G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Vechin, F.C.; Conceição, M.S.; Telles, G.D.; Libardi, C.A.; Ugrinowitsch, C. Interference phenomenon with concurrent strength and high-intensity interval training-based aerobic training: An updated model. Sports Med. 2021, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.M.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Schoenfeld, B.J.; Antunes, M.; Gobbo, L.A.; Teixeira, D.; Cyrino, E.S. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: A randomized controlled trial. J. Strength Cond. Res. 2020, 34, 1008–1016. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Deveries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A high protein diet has no harmful effects: A one-year crossover study in resistance-trained males. J. Nutr. Metab. 2016, 2016, 9104792. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Snijders, T.; Res, P.T.; Smeets, J.S.; van Vliet, S.; Van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.; Van Loon, L.J.C. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef]
- Perez-Schindler, J.; Hamilton, D.L.; Moore, D.R.; Baar, K.; Philp, A. Nutritional strategies to support concurrent training. Eur. J. Sport Sci. 2015, 15, 41–52. [Google Scholar] [CrossRef]
- Hamilton, D.L.; Philp, A. Can AMPK mediated suppression of mTORC1 explain the concurrent training effect? Cell. Mol. Exerc. Physiol. 2013, 2, e4. [Google Scholar] [CrossRef]
- Colletti, L.A.; Edwards, J.; Gordon, L.; Shary, J.; Bell, N.H. The effects of muscle-building exercise on bone mineral density of the radius, spine, and hip in young men. Calcif. Tissue Int. 1989, 45, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Woitge, H.W.; Friedmann, B.; Suttner, S.; Farahmand, I.; Müller, M.; Schmidt-Gayk, H.; Baertsch, P.; Ziegler, R.; Seibel, M.J. Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J. Bone Miner. Res. 1998, 13, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Association NNSC. NSCA’s Essentials of Personal Training: Human Kinetics; Association NNSC: New York, NY, USA, 2011. [Google Scholar]
- Maïmoun, L.; Coste, O.; Philibert, P.; Briot, K.; Mura, T.; Galtier, F.; Mariano-Goulart, D.; Paris, F.; Sultan, C. Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism 2013, 62, 1088–1098. [Google Scholar] [CrossRef]
- Tournis, S.; Michopoulou, E.; Fatouros, I.G.; Paspati, I.; Michalopoulou, M.; Raptou, P.; Leontsini, D.; Avloniti, A.; Krekoukia, M.; Zouvelou, V.; et al. Effect of rhythmic gymnastics on volumetric bone mineral density and bone geometry in premenarcheal female athletes and controls. J. Clin. Endocrinol. Metab. 2010, 95, 2755–2762. [Google Scholar] [CrossRef][Green Version]
- Nowak, A.; Straburzyńska-Lupa, A.; Kusy, K.; Zieliński, J.; Felsenberg, D.; Rittweger, J.; Karolkiewicz, J.; Straburzyńska-Migaj, E.; Pilaczyńska-Szcześniak, Ł. Bone mineral density and bone turnover in male masters athletes aged 40–64. Aging Male 2010, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Varley, I.; Ackerman, K.E.; Pereira, R.M.R.; Elliott-Sale, K.J.; Sale, C. The Bone Metabolic Response to Exercise and Nutrition. Exerc. Sport Sci. Rev. 2020, 48, 49–58. [Google Scholar] [CrossRef]
- Gómez-Bruton, A.; Gónzalez-Agüero, A.; Gómez-Cabello, A.; Casajús, J.A.; Vicente-Rodríguez, G. Is Bone Tissue Really Affected by Swimming? A Systematic Review. PLoS ONE 2013, 8, e70119. [Google Scholar] [CrossRef]
- Paliologo, T.; Shimano, R.C.; Shimano, A.C.; Macedo, A.P.; Falcai, M.J.; Issa, J.P.M. Effects of swimming associated with risedronate in osteopenic bones: An experimental study with ovariectomized rats. Micron 2015, 78, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-I.; Sone, T.; Ohnaru, K.; Tanaka, K.; Fukunaga, M. Effect of swimming exercise on three-dimensional trabecular bone microarchitecture in ovariectomized rats. J. Appl. Physiol. 2015, 119, 990–997. [Google Scholar] [CrossRef]
- Mullins, N.M.; Sinning, W.E. Effects of resistance training and protein supplementation on bone turnover in young adult women. Nutr. Metab. 2005, 2, 19. [Google Scholar] [CrossRef]
- Ponzano, M.; Rodrigues, I.B.; Hosseini, Z.; Ashe, M.C.; A Butt, D.; Chilibeck, P.D.; Stapleton, J.; Thabane, L.; Wark, J.D.; Giangregorio, L.M. Progressive resistance training for improving health-related outcomes in people at risk of fracture: A systematic review and meta-analysis of randomized controlled trials. Phys. Ther. 2021, 101, pzaa221. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise Training and Nutritional Supplementation for Physical Frailty in Very Elderly People. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Seynnes, O.; Fiatarone Singh, M.A.; Hue, O.; Pras, P.; Legros, P.; Bernard, P.L. Physiological and Functional Responses to Low-Moderate Versus High-Intensity Progressive Resistance Training in Frail Elders. J. Gerontol. Ser. A 2004, 59, M503–M509. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.H.; Jones, G.R.; Rice, C.L. Ageing and physical activity: Evidence to develop exercise recommendations for older adults. Appl. Physiol. Nutr. Metab. 2007, 32, S69–S108. [Google Scholar] [CrossRef]
- Sinaki, M.; Itoi, E.; Wahner, H.; Wollan, P.; Gelzcer, R.; Mullan, B.; Collins, D.; Hodgson, S. Stronger back muscles reduce the incidence of vertebral fractures: A prospective 10 year follow-up of postmenopausal women. Bone 2002, 30, 836–841. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar]
- Iolascon, G.; Resmini, G.; Tarantino, U. Mechanobiology of bone. Aging Clin. Exp. Res. 2013, 25, 3–7. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.-M.; Kim, H.-H.; Lee, Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of Serum Ionized Calcium During Exercise Attenuates Parathyroid Hormone and Bone Resorption Responses. J. Bone Miner. Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Gentil, P.; Lima, R.M.; Jacó de Oliveira, R.; Pereira, R.W.; Reis, V.M. Association Between Femoral Neck Bone Mineral Density and Lower Limb Fat-Free Mass in Postmenopausal Women. J. Clin. Densitom. 2007, 10, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.; Ackland, T.R.; Dhaliwal, S.S.; James, A.P.; Woodhouse, J.J.; Price, R.; Prince, R.L.; Kerr, D.A. Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos. Int. 2010, 21, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Hong, A.; Lau, E.; Lynn, H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing 2007, 36, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Mera, P. Molecular bases of the crosstalk between bone and muscle. Bone 2018, 115, 43–49. [Google Scholar] [CrossRef]
- Kaji, H. Effects of myokines on bone. Bonekey Rep. 2016, 5, 826. [Google Scholar] [CrossRef]
- Lombardi, G.; Sanchis-Gomar, F.; Perego, S.; Sansoni, V.; Banfi, G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine 2016, 54, 284–305. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef]
- Bielemann, R.M.; Martinez-Mesa, J.; Gigante, D.P. Physical activity during life course and bone mass: A systematic review of methods and findings from cohort studies with young adults. BMC Musculoskelet. Disord. 2013, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Sanborn, C.; Love, A. Resistance training and bone mineral density in adolescent females. J. Pediatr. 2001, 139, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Carson, C. The Effects of a High-Protein Diet on Bone Mineral Density in Exercise-Trained Women: A 1-Year Investigation. J. Funct. Morphol. Kinesiol. 2018, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Sale, C. Protein and bone health across the lifespan. Proc. Nutr. Soc. 2019, 78, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Shikany, J.M.; Burghardt, A.J.; Cawthon, P.M.; Orwoll, E.S.; Cauley, J.A.; Taylor, B.C.; Schousboe, J.T.; Bauer, D.C.; Vo, T.N.; et al. High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: A cross-sectional study. Osteoporos. Int. 2018, 29, 69–77. [Google Scholar] [CrossRef]
- Darling, A.L.; Manders, R.J.F.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.L.; Clapper, J.A.; Specker, B.L.; Binkley, T.L.; Vukovich, M.D. Effect of protein supplementation during a 6-mo strength and conditioning program on insulin-like growth factor I and markers of bone turnover in young adults. Am. J. Clin. Nutr. 2005, 81, 1442–1448. [Google Scholar] [CrossRef]
- Cao, J.J.; Pasiakos, S.M.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Young, A.J.; Combs, G.F., Jr. Calcium homeostasis and bone metabolic responses to high-protein diets during energy deficit in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Josse, A.R.; Atkinson, S.A.; Tarnopolsky, M.A.; Phillips, S.M. Diets Higher in Dairy Foods and Dietary Protein Support Bone Health during Diet- and Exercise-Induced Weight Loss in Overweight and Obese Premenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 251–260. [Google Scholar] [CrossRef]
- Holm, L.; Olesen, J.L.; Matsumoto, K.; Doi, T.; Mizuno, M.; Alsted, T.J.; Mackey, A.L.; Schwarz, P.; Kjaer, M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J. Appl. Physiol. 2008, 105, 274–281. [Google Scholar] [CrossRef]
- Ginty, F. Dietary protein and bone health. Proc. Nutr. Soc. 2003, 62, 867–876. [Google Scholar] [CrossRef]
- Darling, A.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health: Towards a synthesised view. Proc. Nutr. Soc. 2021, 80, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Frankenfeld, C.L. Dietary Protein Intake above the Current RDA and Bone Health: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99 (Suppl. S5), 1256s–1262s. [Google Scholar] [CrossRef]
- Cao, J.J.; Johnson, L.K.; Hunt, J.R. A Diet High in Meat Protein and Potential Renal Acid Load Increases Fractional Calcium Absorption and Urinary Calcium Excretion without Affecting Markers of Bone Resorption or Formation in Postmenopausal Women. J. Nutr. 2011, 141, 391–397. [Google Scholar] [CrossRef]
- Sukumar, D.; Ambia-Sobhan, H.; Zurfluh, R.; Schlussel, Y.; Stahl, T.J.; Gordon, C.L.; Shapses, S.A. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: A randomized, controlled trial. J. Bone Miner. Res. 2011, 26, 1339–1348. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; O’Brien, K.O.; Insogna, K.L. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am. J. Clin. Nutr. 2003, 78, 584S–592S. [Google Scholar] [CrossRef]
- Promislow, J.H.E.; Goodman-Gruen, D.; Slymen, D.J.; Barrett-Connor, E. Protein Consumption and Bone Mineral Density in the Elderly: The Rancho Bernardo Study. Am. J. Epidemiol. 2002, 155, 636–644. [Google Scholar] [CrossRef]
- Alexy, U.; Remer, T.; Manz, F.; Neu, C.M.; Schoenau, E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am. J. Clin. Nutr. 2005, 82, 1107–1114. [Google Scholar] [CrossRef]
- Hannan, M.T.; Tucker, K.L.; Dawson-Hughes, B.; Cupples, L.A.; Felson, D.T.; Kiel, D.P. Effect of Dietary Protein on Bone Loss in Elderly Men and Women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2000, 15, 2504–2512. [Google Scholar] [CrossRef]
- Sellmeyer, D.E.; Stone, K.L.; Sebastian, A.; Cummings, S.R. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Krupp, D.; Shi, L. Dietary protein’s and dietary acid load’s influence on bone health. Crit. Rev. Food Sci. Nutr. 2014, 54, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
| CT1 | CT2 | RT1 | RT2 | |
|---|---|---|---|---|
| Measure | ||||
| Anthropometry and training experience | ||||
| Age (year) | 27 ± 6 | 25 ± 7 | 26 ± 6 | 28 ± 5 |
| Height (cm) | 178 ± 5 | 179 ± 8 | 180 ± 7 | 182 ± 6 |
| Body mass (kg) | 83.8 ± 10.6 | 81.6 ± 10.7 | 82.1 ± 9.1 | 85.2 ± 10.9 |
| BMI (kg.m−2) | 26.3 ± 3.4 | 25.2 ± 3.1 | 25.1 ± 2.3 | 25.7 ± 2.9 |
| Training experience (year) | 3.7 ± 2.2 | 4.6 ± 2.6 | 3.5 ± 1.7 | 4.8 ± 2.4 |
| Bone parameters | ||||
| Left Arm Area (cm2) | 231.5 ± 18.7 | 238.2 ± 36.6 | 233.6 ± 25.8 | 239.9 ± 36.5 |
| Left Arm BMC (g) | 228.1 ± 43.6 | 218.3 ± 42.3 | 213.9 ± 32.7 | 225.2 ± 50.7 |
| Left Arm BMD (g/cm2) | 0.98 ± 0.12 | 0.91 ± 0.08 | 0.91 ± 0.06 | 0.93 ± 0.08 |
| Right Arm Area (cm2) | 243.2 ± 17.03 | 243.4 ± 31.8 | 240.1 ± 24.1 | 247.7 ± 36 |
| Right Arm BMC (g) | 226.4 ± 23.4 | 220.5 ± 37.7 | 216.18 ± 34 | 228.3 ± 50.2 |
| Right Arm BMD (g/cm2) | 0.93 ± 0.06 | 0.90 ± 0.07 | 0.89 ± 0.08 | 0.91 ± 0.08 |
| Left Ribs Area (cm2) | 133.9 ± 8.7 | 138.7 ± 18.2 | 137.5 ± 17.4 | 138.5 ± 27.1 |
| Left Ribs BMC (g) | 116.6 ± 13.2 | 115.2 ± 25.6 | 113.6 ± 21.7 | 114.7 ± 29.2 |
| Left Ribs BMD (g/cm2) | 0.87 ± 0.07 | 0.82 ± 0.10 | 0.82 ± 0.08 | 0.82 ± 0.09 |
| Right Ribs Area (cm2) | 128.8 ± 17.8 | 138.3 ± 18.4 | 131.8 ± 18.4 | 137.5 ± 20.7 |
| Right Ribs BMC (g) | 114.8 ± 14.5 | 114.6 ± 18.9 | 111.6 ± 24 | 116 ± 20 |
| Right Ribs BMD (g/cm2) | 0.89 ± 0.06 | 0.82 ± 0.07 | 0.84 ± 0.09 | 0.84 ± 0.08 |
| Thoracic Spine Area (cm2) | 146.1 ± 11.2 | 144.1 ± 14.2 | 141.5 ± 11.3 | 156.8 ± 19.2 |
| Thoracic Spine BMC (g) | 163.6 ± 21.1 | 147.9 ± 20.4 | 148.7 ± 29.5 | 160.2 ± 46.5 |
| Thoracic Spine BMD (g/cm2) | 1.11 ± 0.10 | 1.02 ± 0.10 | 1.04 ± 0.14 | 1.01 ± 0.20 |
| Lumbar Spine Area (cm2) | 64.1 ± 3.9 | 64.6 ± 7.6 | 66.6 ± 6 | 68.3 ± 8.7 |
| Lumbar Spine BMC (g) | 76.9 ± 11.1 | 73.2 ± 12.4 | 76.4 ± 19.4 | 76.6 ± 21.6 |
| Lumbar Spine BMD (g/cm2) | 1.19 ± 0.12 | 1.13 ± 0.13 | 1.13 ± 0.21 | 1.10 ± 0.20 |
| Pelvis Area (cm2) | 265.9 ± 51.1 | 272.4 ± 27.7 | 275.8 ± 46.9 | 264.9 ± 42.7 |
| Pelvis BMC (g) | 360.1 ± 79.6 | 341.2 ± 44.2 | 349.1 ± 89.5 | 322.7 ± 65.1 |
| Pelvis BMD (g/cm2) | 1.35 ± 0.11 | 1.25 ± 0.13 | 1.25 ± 0.18 | 1.21 ± 0.08 |
| Left Leg Area (cm2) | 418.3 ± 38.6 | 416.3 ± 43.7 | 417.7 ± 39.7 | 423.2 ± 63.1 |
| Left Leg BMC (g) | 669.9 ± 89.2 | 629.7 ± 96.3 | 642.5 ± 122.3 | 610 ± 102.5 |
| Left Leg BMD (g/cm2) | 1.59 ± 0.12 | 1.51 ± 0.14 | 1.52 ± 0.18 | 1.43 ± 10 |
| Right Leg Area (cm2) | 404.2 ± 43.9 | 401.3 ± 39.6 | 408.6 ± 47.7 | 401.4 ± 54.9 |
| Right Leg BMC (g) | 646.1 ± 93.8 | 610.1 ± 75.8 | 630.1 ± 132.1 | 599.1 ± 137 |
| Right Leg BMD (g/cm2) | 1.59 ± 0.15 | 1.52 ± 0.12 | 1.52 ± 0.17 | 1.48 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, R.; Karimi, Z.; Mousavi, Z.; Ziaee Bashirzad, M.; Camera, D.M.; Sadeghi, R.; Dabbagh, V.R.; Kargarfard, M.; Dutheil, F. High-Protein Diets during either Resistance or Concurrent Training Have No Detrimental Effect on Bone Parameters in Resistance-Trained Males. Nutrients 2024, 16, 325. https://doi.org/10.3390/nu16020325
Bagheri R, Karimi Z, Mousavi Z, Ziaee Bashirzad M, Camera DM, Sadeghi R, Dabbagh VR, Kargarfard M, Dutheil F. High-Protein Diets during either Resistance or Concurrent Training Have No Detrimental Effect on Bone Parameters in Resistance-Trained Males. Nutrients. 2024; 16(2):325. https://doi.org/10.3390/nu16020325
Chicago/Turabian StyleBagheri, Reza, Zohreh Karimi, Zeynabalsadat Mousavi, Mahdi Ziaee Bashirzad, Donny M. Camera, Ramin Sadeghi, Vahid Reza Dabbagh, Mehdi Kargarfard, and Frederic Dutheil. 2024. "High-Protein Diets during either Resistance or Concurrent Training Have No Detrimental Effect on Bone Parameters in Resistance-Trained Males" Nutrients 16, no. 2: 325. https://doi.org/10.3390/nu16020325
APA StyleBagheri, R., Karimi, Z., Mousavi, Z., Ziaee Bashirzad, M., Camera, D. M., Sadeghi, R., Dabbagh, V. R., Kargarfard, M., & Dutheil, F. (2024). High-Protein Diets during either Resistance or Concurrent Training Have No Detrimental Effect on Bone Parameters in Resistance-Trained Males. Nutrients, 16(2), 325. https://doi.org/10.3390/nu16020325








