Belief That Caffeine Ingestion Improves Performance in a 6-Minute Time Trial Test without Affecting Pacing Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Design
2.3. Procedure
2.4. Statistical Analysis
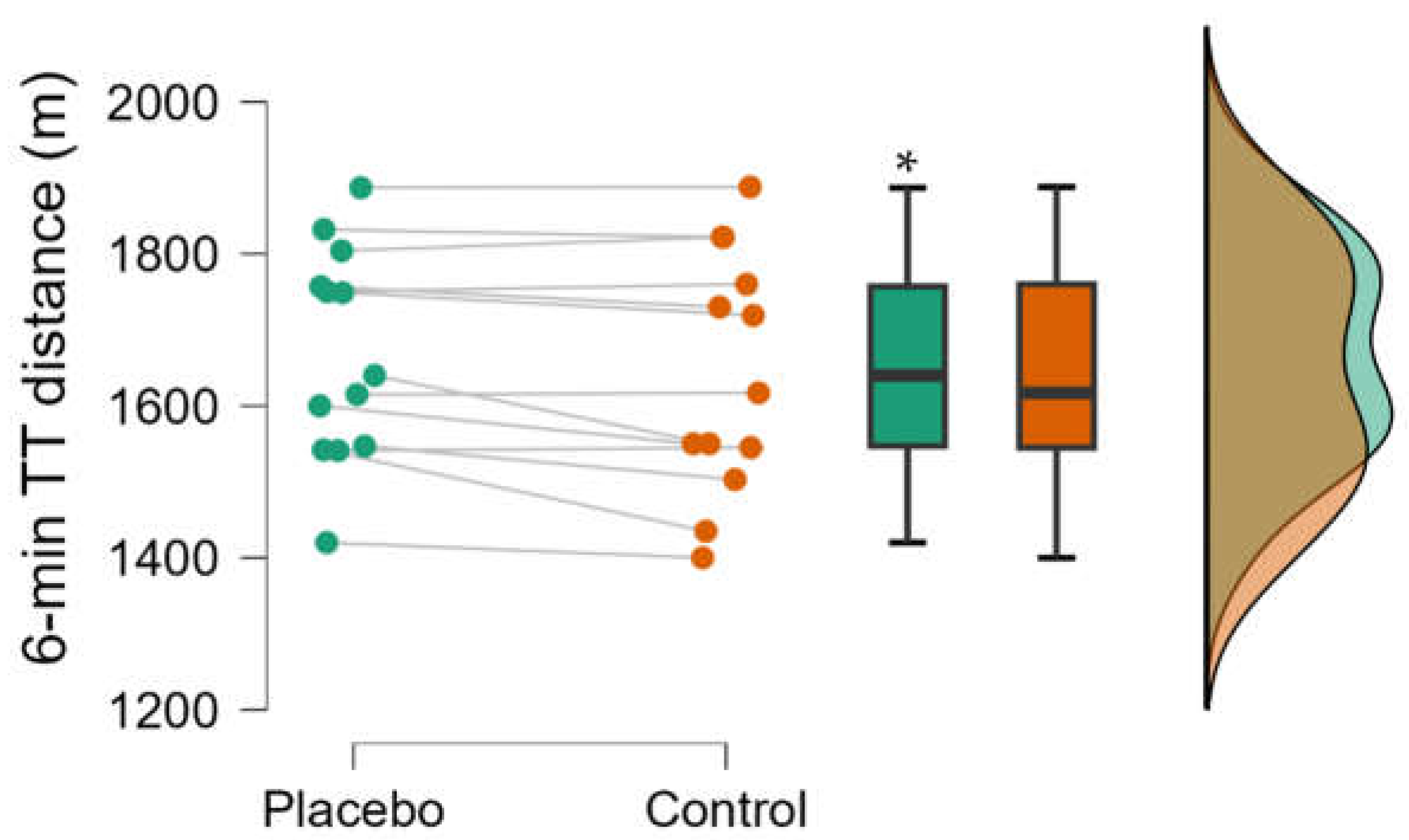
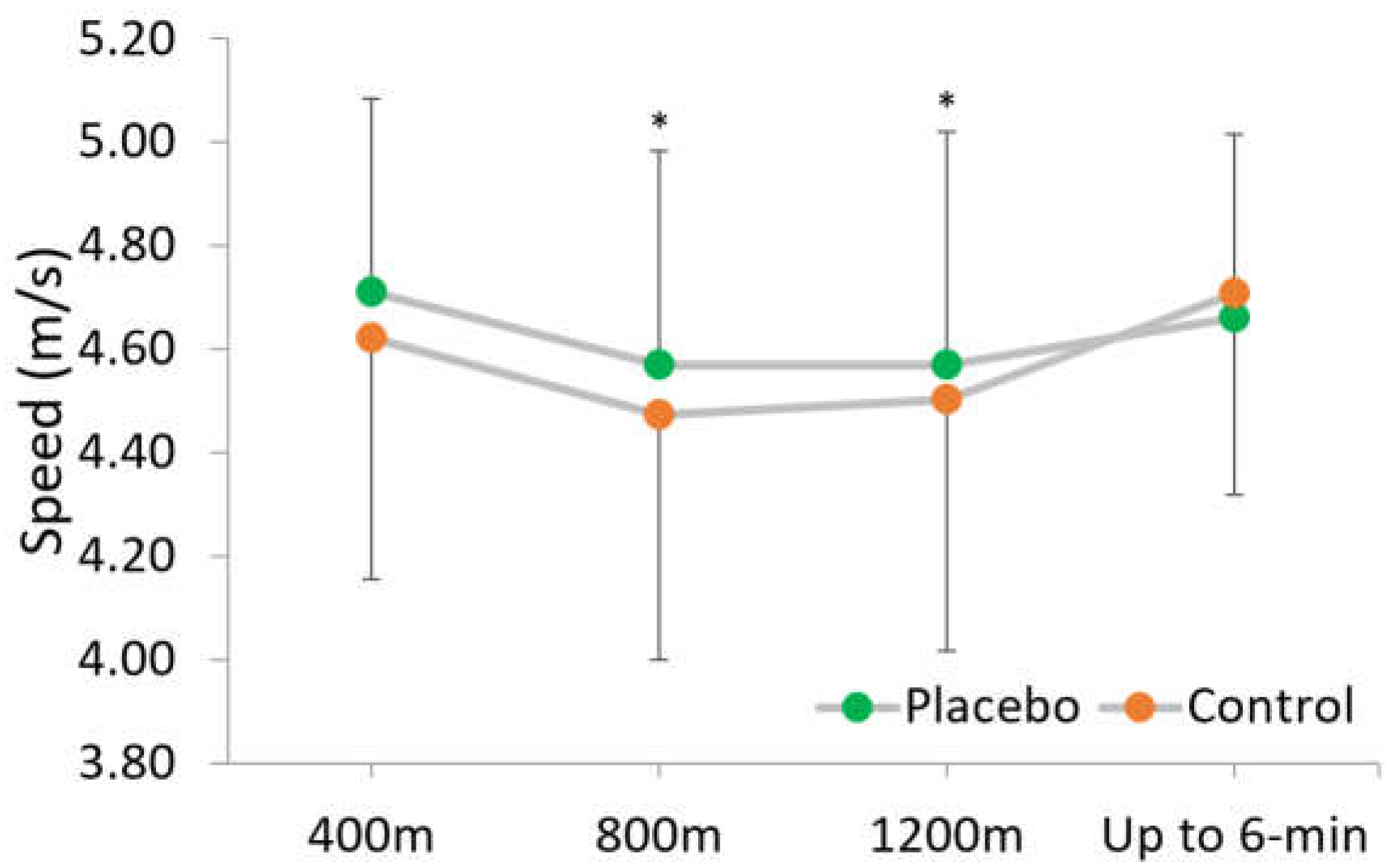
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frisaldi, E.; Shaibani, A.; Benedetti, F.; Pagnini, F. Placebo and nocebo effects and mechanisms associated with pharmacological interventions: An umbrella review. BMJ Open 2023, 13, e077243. [Google Scholar] [CrossRef] [PubMed]
- Beedie, C.J.; Foad, A.J. The Placebo Effect in Sports Performance. Sports Med. 2009, 39, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Hurst, P.; Schipof-Godart, L.; Szabo, A.; Raglin, J.; Hettinga, F.; Roelands, B.; Lane, A.; Foad, A.; Coleman, D.; Beedie, C.J. The Placebo and Nocebo effect on sports performance: A systematic review. Eur. J. Sport Sci. 2020, 20, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.R.; Hopkins, W.G.; Hawley, J.A.; Burke, L.M. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med. Sci. Sports Exerc. 2000, 32, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Kalasountas, V.; Reed, J.; Fitzpatrick, J. The Effect of Placebo-Induced Changes in Expectancies on Maximal Force Production in College Students. J. Appl. Sport Psychol. 2007, 19, 116–124. [Google Scholar] [CrossRef]
- McClung, M.; Collins, D. “Because I know it will!”: Placebo effects of an ergogenic aid on athletic performance. J. Sport Exerc. Psychol. 2007, 29, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Salinero, J.J.; Lara, B.; Del Coso, J. Effects of acute ingestion of caffeine on team sports performance: A systematic review and meta-analysis. Res. Sports Med. 2019, 27, 238–256. [Google Scholar] [CrossRef]
- Baltazar-Martins, J.G.; Brito De Souza, D.; Aguilar, M.; Grgic, J.; Del Coso, J. Infographic. The road to the ergogenic effect of caffeine on exercise performance. Br. J. Sports Med. 2020, 54, 618–619. [Google Scholar] [CrossRef]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1–37. [Google Scholar] [CrossRef]
- Roelands, B.; Hurst, P. The Placebo Effect in Sport: How Practitioners Can Inject Words to Improve Performance. Int. J. Sports Physiol. Perform. 2020, 15, 765–766. [Google Scholar] [CrossRef]
- Aguilar-Navarro, M.; Muñoz, G.; Salinero, J.J.; Muñoz-Guerra, J.; Fernández-álvarez, M.; Plata, M.D.M.; Del Coso, J. Urine Caffeine Concentration in Doping Control Samples from 2004 to 2015. Nutrients 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Mikulic, P. Effects of caffeine on rate of force development: A meta-analysis. Scand. J. Med. Sci. Sports 2021, 32, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Raya-González, J.; Rendo-Urteaga, T.; Domínguez, R.; Castillo, D.; Rodríguez-Fernández, A.; Grgic, J. Acute Effects of Caffeine Supplementation on Movement Velocity in Resistance Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Giraldez-Costas, V.; Del Coso, J.; Manas, A.; Salinero, J.J. The Long Way to Establish the Ergogenic Effect of Caffeine on Strength Performance: An Overview Review. Nutrients 2023, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Caffeine and sports performance. Appl. Physiol. Nutr. Metab. 2008, 33, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine and ephedrine: Physiological, metabolic and performance-enhancing effects. Sports Med. 2004, 34, 871–889. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine and Exercise. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef]
- Tallis, J.; Muhammad, B.; Islam, M.; Duncan, M.J. Placebo effects of caffeine on maximal voluntary concentric force of the knee flexors and extensors. Muscle Nerve 2016, 54, 479–486. [Google Scholar] [CrossRef]
- Pollo, A.; Carlino, E.; Benedetti, F. The top-down influence of ergogenic placebos on muscle work and fatigue. Eur. J. Neurosci. 2008, 28, 379–388. [Google Scholar] [CrossRef]
- Duncan, M.J.; Lyons, M.; Hankey, J. Placebo effects of caffeine on short-term resistance exercise to failure. Int. J. Sports Physiol. Perform. 2009, 4, 244–253. [Google Scholar] [CrossRef]
- Pires, F.O.; Dos Anjos, C.A.S.; Covolan, R.J.M.; Fontes, E.B.; Noakes, T.D.; St Clair Gibson, A.; Magalhaes, F.H.; Ugrinowitsch, C. Caffeine and Placebo Improved Maximal Exercise Performance Despite Unchanged Motor Cortex Activation and Greater Prefrontal Cortex Deoxygenation. Front. Physiol. 2018, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Beedie, C.J.; Stuart, E.M.; Coleman, D.A.; Foad, A.J. Placebo effects of caffeine on cycling performance. Med. Sci. Sports Exerc. 2006, 38, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Foad, A.J.; Beedie, C.J.; Coleman, D.A. Pharmacological and psychological effects of caffeine ingestion in 40-km cycling performance. Med. Sci. Sports Exerc. 2008, 40, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; de Oliveira, L.F.; da Silva, R.P.; de Salles Painelli, V.; Gonçalves, L.S.; Yamaguchi, G.; Mutti, T.; Maciel, E.; Roschel, H.; Artioli, G.G.; et al. Placebo in sports nutrition: A proof-of-principle study involving caffeine supplementation. Scand. J. Med. Sci. Sports 2017, 27, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Rohloff, G.; Souza, D.B.; Ruiz-Moreno, C.; Del Coso, J.; Polito, M.D. Stimulus Expectancy and Stimulus Response of Caffeine on 4-km Running Performance: A Randomized, Double-blind, Placebo-controlled and Crossover Study. Int. J. Exerc. Sci. 2022, 15, 645–654. [Google Scholar] [PubMed]
- Hurst, P.; Schipof-Godart, L.; Hettinga, F.; Roelands, B.; Beedie, C.J. Improved 1000-m Running Performance and Pacing Strategy With Caffeine and Placebo: A Balanced Placebo Design Study. Int. J. Sports Physiol. Perform. 2019, 15, 483–488. [Google Scholar] [CrossRef]
- Salinero, J.J.; Lara, B.; Abian-Vicen, J.; Gonzalez-Millán, C.; Areces, F.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Del Coso, J. The use of energy drinks in sport: Perceived ergogenicity and side effects in male and female athletes. Br. J. Nutr. 2014, 112, 1494–1502. [Google Scholar] [CrossRef]
- Pallarés, J.G.; Fernández-Elías, V.E.; Ortega, J.F.; Muñoz, G.; Muñoz-Guerra, J.; Mora-Rodríguez, R. Neuromuscular responses to incremental caffeine doses: Performance and side effects. Med. Sci. Sports Exerc. 2013, 45, 2184–2192. [Google Scholar] [CrossRef]
- Blondel, N.; Berthoin, S.; Billat, V.; Lensel, G. Relationship between run times to exhaustion at 90, 100, 120, and 140% of vVO2max and velocity expressed relatively to critical velocity and maximal velocity. Int. J. Sports Med. 2001, 22, 27–33. [Google Scholar] [CrossRef]
- Huerta Ojeda, Á.; Galdames-Maliqueo, S.; Peña Pizarro, J.; Fuentes Kloss, R. Validation of the 6-min race test as a predictor of maximal aerobic speed in university endurance athletes. Isokinet. Exerc. Sci. 2020, 28, 383–390. [Google Scholar] [CrossRef]
- Garcia-Pinillos, F.; Roche-Seruendo, L.E.; Marcen-Cinca, N.; Marco-Contreras, L.A.; Latorre-Roman, P.A. Absolute Reliability and Concurrent Validity of the Stryd System for the Assessment of Running Stride Kinematics at Different Velocities. J. Strength Cond. Res. 2021, 35, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. A Scale of Magnitudes for Effect Statistics. Available online: http://www.sportsci.org/resource/stats/ (accessed on 12 December 2023).
- Billat, V.; Renoux, J.C.; Pinoteau, J.; Petit, B.; Koralsztein, J.P. Times to exhaustion at 90, 100 and 105% of velocity at VO2max (maximal aerobic speed) and critical speed in elite long-distance runners. Arch. Physiol. Biochem. 1995, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.; Koralsztein, J.P. Significance of the velocity at VO2max and time to exhaustion at this velocity. Sports Med. 1996, 22, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Hurst, P.; Foad, A.; Coleman, D.; Beedie, C.J. Athletes Intending to Use Sports Supplements Are More Likely to Respond to a Placebo. Med. Sci. Sports Exerc. 2017, 49, 1877–1883. [Google Scholar] [CrossRef]
- Casado, A.; Hanley, B.; Jimenez-Reyes, P.; Renfree, A. Pacing profiles and tactical behaviors of elite runners. J. Sport Health Sci. 2021, 10, 537–549. [Google Scholar] [CrossRef]
- Enck, P.; Klosterhalfen, S. Does Sex/Gender Play a Role in Placebo and Nocebo Effects? Conflicting Evidence From Clinical Trials and Experimental Studies. Front. Neurosci. 2019, 13, 160. [Google Scholar] [CrossRef]
| Control | Placebo | Statistic | p | Cohen’s d | ||
|---|---|---|---|---|---|---|
| Heart rate (bpm) | 400 m | 147.6 ± 12.3 | 148.8 ± 14.0 | 0.54 | 0.48 | 0.22 |
| 800 m | 165.2 ± 12.1 † | 167.1 ± 12.9 † | 1.78 | 0.21 | 0.40 | |
| 1200 m | 168.6 ± 12.0 † | 170.7 ± 13.1 † | 2.32 | 0.27 | 0.34 | |
| Rest | 171.8 ± 11.8 † | 173.2 ± 12.5 † | 0.98 | 0.35 | 0.30 | |
| Overall | 162.9 ± 12.1 | 165.0 ± 13.6 | 1.49 | 0.12 | 0.45 | |
| Rate of perceived exertion (a.u.) | Overall | 9.2 ± 0.6 | 9.3 ± 0.5 | 0.90 | 0.39 | 0.25 |
| Step frequency (spm) | 400 m | 181.8 ± 10.2 | 183.6 ± 8.5 | 1.49 | 0.25 | 0.37 |
| 800 m | 180.6 ± 9.8 | 182.4 ± 8.0 | 1.61 | 0.23 | 0.38 | |
| 1200 m | 181.4 ± 9.9 | 182.6 ± 8.5 | 1.82 | 0.21 | 0.41 | |
| Rest | 183.6 ± 9.3 | 184.2 ± 8.7 | 0.58 | 0.47 | 0.23 | |
| Overall | 181.9 ± 9.8 | 183.1 ± 8.1 | 1.08 | 0.31 | 0.33 | |
| Vertical oscillation (cm) | 400 m | 8.30 ± 0.95 | 8.26 ± 0.95 | 0.10 | 0.76 | 0.10 |
| 800 m | 8.35 ± 1.04 | 8.26 ± 0.95 | 0.48 | 0.50 | 0.21 | |
| 1200 m | 8.27 ± 1.07 | 8.21 ± 1.02 | 0.32 | 0.58 | 0.17 | |
| Rest | 8.11 ± 1.07 | 8.12 ± 1.05 | 0.03 | 0.88 | 0.05 | |
| Overall | 8.27 ± 1.02 | 8.21 ± 0.96 | 0.66 | 0.53 | 0.20 | |
| Contact time (ms) | 400 m | 170.6 ± 20.6 | 164.3 ± 14.4 | 4.27 | 0.07 | 0.62 |
| 800 m | 173.6 ± 18.9 | 169.3 ± 14.6 | 3.80 | 0.08 | 0.59 | |
| 1200 m | 173.7 ± 17.2 | 170.7 ± 14.5 | 1.80 | 0.21 | 0.41 | |
| Rest | 170.1 ± 15.0 | 168.0 ± 13.2 | 1.24 | 0.29 | 0.34 | |
| Overall | 171.9 ± 18.4 | 168.7 ± 13.3 | 1.37 | 0.33 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, F.; González-Mohíno, F.; Salinero, J.J. Belief That Caffeine Ingestion Improves Performance in a 6-Minute Time Trial Test without Affecting Pacing Strategy. Nutrients 2024, 16, 327. https://doi.org/10.3390/nu16020327
Valero F, González-Mohíno F, Salinero JJ. Belief That Caffeine Ingestion Improves Performance in a 6-Minute Time Trial Test without Affecting Pacing Strategy. Nutrients. 2024; 16(2):327. https://doi.org/10.3390/nu16020327
Chicago/Turabian StyleValero, Fernando, Fernando González-Mohíno, and Juan José Salinero. 2024. "Belief That Caffeine Ingestion Improves Performance in a 6-Minute Time Trial Test without Affecting Pacing Strategy" Nutrients 16, no. 2: 327. https://doi.org/10.3390/nu16020327
APA StyleValero, F., González-Mohíno, F., & Salinero, J. J. (2024). Belief That Caffeine Ingestion Improves Performance in a 6-Minute Time Trial Test without Affecting Pacing Strategy. Nutrients, 16(2), 327. https://doi.org/10.3390/nu16020327







