Association of Serum Bile Acid Profile with Diet and Physical Activity Habits in Japanese Middle-Aged Men
Abstract
1. Introduction
2. Methods
2.1. Participants and Experimental Design
2.2. Serum BA Analysis
2.3. Data Collection and Measurements
2.4. Statistical Analysis
3. Results
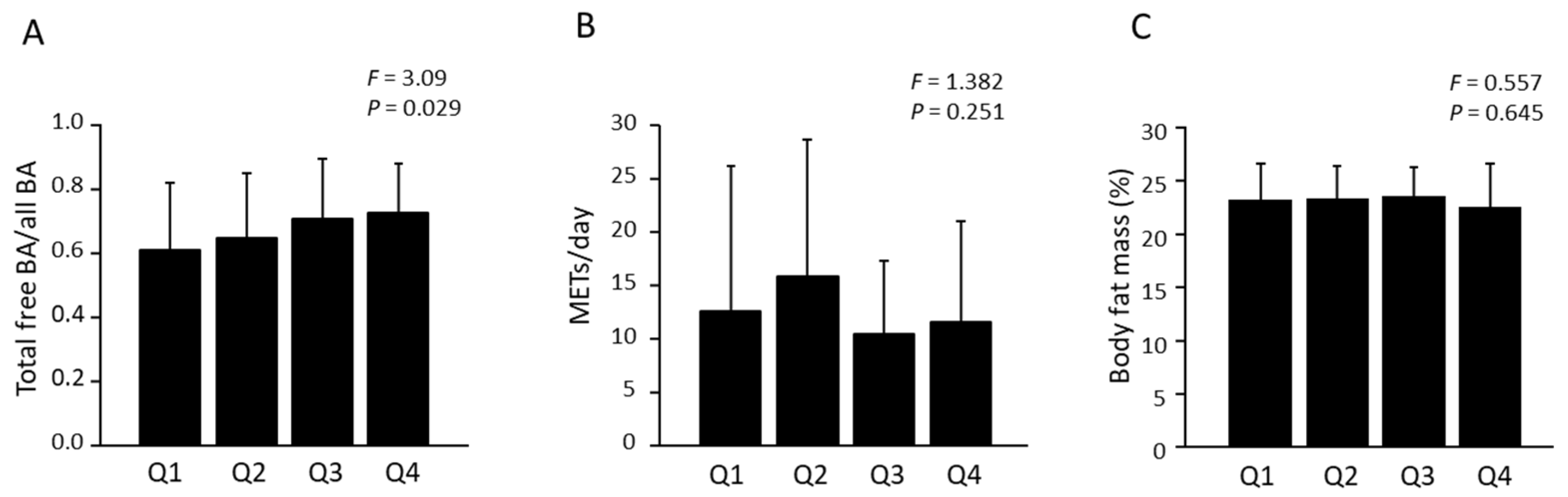
3.1. Association of BA Profile with Dietary Habits
3.2. Association of BA Profile with Fitness Parameters and Physical Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Yang, C.; Wai, S.T.C.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; San Cheang, W.; Liu, B.; Carpéné, C.; et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic functions of flavonoids: From human epidemiology to molecular mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Wood Dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, J.A.; Lima Ribeiro, M. Effects of polyphenols on thermogenesis and mitochondrial biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 2016, 34, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Priest, C.; Tontonoz, P. Inter-organ cross-talk in metabolic syndrome. Nat. Metab. 2019, 1, 1177–1188. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Jadhav, K.; Xu, Y.; Xu, Y.; Li, Y.; Xu, J.; Zhu, Y.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 131–140. [Google Scholar] [CrossRef]
- Aoi, W.; Inoue, R.; Mizushima, K.; Honda, A.; Björnholm, M.; Takagi, T.; Naito, Y. Exercise-acclimated microbiota improves skeletal muscle metabolism via circulating bile acid deconjugation. iScience 2023, 26, 106251. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Schlegel, J.; Thomalla, M.; Karrasch, T.; Schäffler, A. Evidence of functional bile acid signaling pathways in adipocytes. Mol. Cell. Endocrinol. 2019, 483, 1–10. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Gnewuch, C.; Liebisch, G.; Langmann, T.; Dieplinger, B.; Mueller, T.; Haltmayer, M.; Dieplinger, H.; Zahn, A.; Stremmel, W.; Rogler, G.; et al. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 3134–3141. [Google Scholar] [CrossRef]
- Naito, Y.; Ushiroda, C.; Mizushima, K.; Inoue, R.; Yasukawa, Z.; Abe, A.; Takagi, T. Epigallocatechin-3-gallate (EGCG) attenuates non-alcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J. Clin. Biochem. Nutr. 2020, 67, 2–9. [Google Scholar] [CrossRef]
- Ma, E.; Ohira, T.; Fukasawa, M.; Yasumura, S.; Miyazaki, M.; Suzuki, T.; Furuyama, A.; Kataoka, M.; Hosoya, M. Prevalence trends of metabolic syndrome in residents of postdisaster Fukushima: A longitudinal analysis of Fukushima Health Database 2012–2019. Public Health 2023, 217, 115–124. [Google Scholar] [CrossRef]
- Haraguchi, N.; Koyama, T.; Kuriyama, N.; Ozaki, E.; Matsui, D.; Watanabe, I.; Uehara, R.; Watanabe, Y. Assessment of anthropometric indices other than BMI to evaluate arterial stiffness. Hypertens. Res. 2019, 42, 1599–1605. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Hara, M.; Higaki, Y.; Taguchi, N.; Shinchi, K.; Morita, E.; Naito, M.; Hamajima, N.; Takashima, N.; Suzuki, S.; Nakamura, A.; et al. Effect of the PPARG2 Pro12Ala polymorphism and clinical risk factors for diabetes mellitus on HbA1c in the Japanese general population. J. Epidemiol. 2012, 22, 523–531. [Google Scholar] [CrossRef]
- Oshima, Y.; Shiga, T.; Namba, H.; Kuno, S. Estimation of whole-body skeletal muscle mass by bioelectrical impedance analysis in the standing position. Obes. Res. Clin. Pract. 2010, 4, e1–e82. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.H.R.; Hursel, R.; Westerterp-Plantenga, M.S. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol. Behav. 2016, 162, 83–87. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha green tea drinks enhance fat oxidation during brisk walking in females. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 536–541. [Google Scholar] [CrossRef]
- Shigeta, M.; Aoi, W.; Morita, C.; Soga, K.; Inoue, R.; Fukushima, Y.; Kobayashi, Y.; Kuwahata, M. Matcha green tea beverage moderates fatigue and supports resistance training-induced adaptation. Nutr. J. 2023, 22, 32. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of Matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Bancirova, M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Jin, J.S.; Touyama, M.; Hisada, T.; Benno, Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012, 56, 729–739. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, M.; Zhang, X.; Cao, J.; Wu, Z.; Weng, P. Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int. J. Food Sci. Technol. 2017, 52, 1723–1730. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does exercise alter gut microbial composition? A systematic review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of bile acids in metabolic control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Qiao, D.; Chen, W.; Stratagoules, E.D.; Martinez, J.D. Bile acid-induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. J. Biol. Chem. 2000, 275, 15090–15098. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Antiinflamm. Antiallergy. Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Yuan, F.; Dong, H.; Fang, K.; Gong, J.; Lu, F. Effects of green tea on lipid metabolism in overweight or obese people: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Biophys. Res. Commun. 2015, 461, 1–7. [Google Scholar] [CrossRef]
- Bakhtiyari, S.; Zaherara, M.; Haghani, K.; Khatami, M.; Rashidinejad, A. The phosphorylation of IRS1S307 and AktS473 molecules in insulin-resistant C2C12 cells induced with palmitate is influenced by epigallocatechin gallate from green tea. Lipids 2019, 54, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. B.M.C. Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef]
- Gao, R.; Tao, Y.; Zhou, C.; Li, J.; Wang, X.; Chen, L.; Li, F.; Guo, L. Exercise therapy in patients with constipation: A systematic review and meta-analysis of randomized controlled trials. Scand. J. Gastroenterol. 2019, 54, 169–177. [Google Scholar] [CrossRef]
- Farahnak, Z.; Magri Tomaz, L.; Bergeron, R.; Chapados, N.; Lavoie, J.M. The effect of exercise training on upregulation of molecular markers of bile acid metabolism in the liver of ovariectomized rats fed a cholesterol-rich diet. ARYA Atheroscler. 2017, 13, 184–192. [Google Scholar] [PubMed]
- Meissner, M.; Lombardo, E.; Havinga, R.; Tietge, U.J.F.; Kuipers, F.; Groen, A.K. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011, 218, 323–329. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary bile acids and tumorigenesis in colorectal cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Lizarbe, M.A.; Turnay, J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol. In Vitro 2013, 27, 964–977. [Google Scholar] [CrossRef]
- Dermadi, D.; Valo, S.; Ollila, S.; Soliymani, R.; Sipari, N.; Pussila, M.; Sarantaus, L.; Linden, J.; Baumann, M.; Nyström, M. Western diet deregulates bile acid homeostasis, cell proliferation, and tumorigenesis in colon. Cancer Res. 2017, 77, 3352–3363. [Google Scholar] [CrossRef]
- The World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Colorectal Cancer; The World Cancer Research Fund: London, UK; American Institute for Cancer Research: Arlington, VA, USA, 2017. [Google Scholar]
- Kim, H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Protective effect of green tea consumption on colorectal cancer varies by lifestyle factors. Nutrients 2019, 11, 2612. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Yagi, N.; Mizushima, K.; Higashimura, Y.; Hirai, Y.; Dohi, O.; Okayama, T.; Yoshida, N.; Katada, K.; et al. Identification of colorectal neoplasia by using serum bile acid profile. Biomarkers 2021, 26, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
| Energy Intake | Fat Intake | Cholesterol Intake | Dietary Fiber Intake | Green Tea Intake | Coffee Intake | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variables | β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Free form | |||||||||||||
| Free CA | 0.045 | 0.651 | 0.005 | 0.970 | −0.065 | 0.534 | −0.019 | 0.862 | 0.067 | 0.450 | −0.212 | 0.015 | |
| Free CDCA | 0.180 | 0.071 | −0.003 | 0.980 | −0.087 | 0.407 | −0.125 | 0.264 | 0.025 | 0.778 | −0.191 | 0.028 | |
| Free DCA | −0.060 | 0.531 | −0.007 | 0.959 | −0.068 | 0.502 | 0.240 | 0.029 | −0.152 | 0.078 | 0.134 | 0.112 | |
| Free LCA | −0.096 | 0.326 | −0.045 | 0.728 | −0.033 | 0.751 | 0.241 | 0.030 | −0.091 | 0.296 | 0.104 | 0.221 | |
| Free UDCA | 0.143 | 0.143 | −0.165 | 0.200 | 0.149 | 0.149 | −0.083 | 0.449 | −0.074 | 0.391 | −0.100 | 0.240 | |
| Total primary free BAs | 0.132 | 0.185 | 0 | 0.999 | −0.082 | 0.437 | −0.087 | 0.437 | 0.043 | 0.626 | −0.207 | 0.018 | |
| Total free BAs | 0.172 | 0.083 | −0.061 | 0.638 | 0.083 | 0.429 | −0.066 | 0.555 | −0.064 | 0.466 | −0.146 | 0.093 | |
| Conjugated form | |||||||||||||
| Glyco-CA | −0.073 | 0.447 | −0.225 | 0.075 | 0.202 | 0.047 | 0.019 | 0.863 | −0.025 | 0.769 | −0.041 | 0.624 | |
| Glyco-CDCA | −0.132 | 0.174 | −0.101 | 0.428 | 0.089 | 0.386 | −0.058 | 0.593 | −0.062 | 0.469 | −0.081 | 0.338 | |
| Glyco-DCA | −0.114 | 0.237 | −0.06 | 0.633 | −0.129 | 0.206 | 0.285 | 0.010 | −0.170 | 0.048 | 0.129 | 0.125 | |
| Glyco-LCA | −0.137 | 0.164 | 0.145 | 0.260 | −0.060 | 0.564 | 0.154 | 0.165 | −0.135 | 0.124 | 0.075 | 0.379 | |
| Glyco-UDCA | 0.067 | 0.486 | −0.168 | 0.188 | 0.160 | 0.120 | −0.069 | 0.530 | −0.089 | 0.301 | −0.092 | 0.278 | |
| Total primary glyco-BA | −0.118 | 0.219 | −0.147 | 0.245 | 0.131 | 0.198 | −0.035 | 0.744 | −0.053 | 0.537 | −0.071 | 0.395 | |
| Total glyco-BA | −0.173 | 0.074 | −0.055 | 0.665 | −0.073 | 0.472 | 0.045 | 0.676 | −0.187 | 0.030 | 0.101 | 0.229 | |
| Tauro-CA | −0.005 | 0.954 | −0.203 | 0.103 | 0.263 | 0.009 | −0.025 | 0.816 | −0.062 | 0.463 | −0.066 | 0.426 | |
| Tauro-CDCA | −0.11 | 0.233 | −0.212 | 0.081 | 0.276 | 0.005 | −0.087 | 0.404 | −0.067 | 0.415 | −0.15 | 0.063 | |
| Tauro-DCA | −0.185 | 0.051 | −0.053 | 0.666 | −0.036 | 0.720 | 0.252 | 0.019 | −0.175 | 0.037 | 0.179 | 0.031 | |
| Tauro-LCA | −0.039 | 0.694 | 0.085 | 0.521 | 0.153 | 0.150 | −0.114 | 0.317 | −0.006 | 0.943 | 0.096 | 0.274 | |
| Tauro-UDCA | 0.082 | 0.398 | −0.18 | 0.157 | 0.212 | 0.039 | −0.088 | 0.421 | −0.063 | 0.461 | −0.064 | 0.447 | |
| Total primary tauro-BA | −0.094 | 0.306 | −0.217 | 0.074 | 0.282 | 0.004 | −0.078 | 0.452 | −0.068 | 0.407 | −0.140 | 0.084 | |
| Total tauro-BA | −0.150 | 0.117 | −0.265 | 0.037 | 0.144 | 0.156 | 0.121 | 0.263 | −0.138 | 0.107 | 0.059 | 0.482 | |
| All BA | 0.062 | 0.523 | −0.158 | 0.217 | 0.150 | 0.146 | −0.054 | 0.620 | −0.100 | 0.248 | −0.129 | 0.129 | |
| Total free BA/All BA | 0.177 | 0.067 | 0.075 | 0.551 | 0.057 | 0.577 | −0.054 | 0.621 | 0.189 | 0.028 | −0.101 | 0.229 | |
| Total glyco-BA/Total tauro-BA | 0.052 | 0.606 | 0.073 | 0.583 | −0.071 | 0.506 | 0.026 | 0.818 | −0.040 | 0.661 | −0.123 | 0.164 | |
| Total primary BA/All primary BA | 0.168 | 0.089 | −0.028 | 0.829 | 0.066 | 0.522 | −0.017 | 0.877 | 0.141 | 0.107 | −0.148 | 0.084 | |
| BFM | SMM | METs | Grip Strength | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent Variables | β | p-Value | Β | p-Value | β | p-Value | β | p-Value | |
| Free form | |||||||||
| Free CA | −0.048 | 0.754 | 0.079 | 0.516 | −0.043 | 0.634 | −0.076 | 0.394 | |
| Free CDCA | 0.021 | 0.750 | 0.030 | 0.334 | 0.004 | 0.723 | 0.005 | 0.694 | |
| Free DCA | 0.014 | 0.270 | 0.020 | 0.284 | −0.145 | 0.098 | 0.004 | 0.880 | |
| Free LCA | 0.085 | 0.570 | 0.088 | 0.464 | −0.234 | 0.009 | −0.103 | 0.245 | |
| Free UDCA | 0.180 | 0.231 | 0.162 | 0.179 | −0.026 | 0.772 | −0.245 | 0.006 | |
| Total primary free BA | −0.050 | 0.742 | 0.107 | 0.382 | −0.038 | 0.676 | −0.054 | 0.551 | |
| Total free BA | 0.157 | 0.304 | 0.208 | 0.089 | −0.083 | 0.358 | −0.193 | 0.033 | |
| Conjugated form | |||||||||
| Glyco-CA | 0.009 | 0.941 | 0.191 | 0.195 | 0.043 | 0.718 | 0.233 | 0.008 | |
| Glyco-CDCA | 0.051 | 0.664 | 0.098 | 0.509 | 0.051 | 0.667 | 0.201 | 0.023 | |
| Glyco-DCA | 0.245 | 0.099 | 0.147 | 0.217 | −0.012 | 0.892 | −0.036 | 0.681 | |
| Glyco-LCA | −0.085 | 0.575 | −0.100 | 0.406 | −0.078 | 0.380 | −0.080 | 0.368 | |
| Glyco-UDCA | 0.174 | 0.245 | 0.142 | 0.234 | 0.003 | 0.975 | −0.253 | 0.004 | |
| Total primary glyco-BA | 0.134 | 0.365 | 0.051 | 0.667 | 0.221 | 0.012 | −0.102 | 0.241 | |
| Total glyco-BA | 0.104 | 0.483 | 0.031 | 0.793 | 0.193 | 0.028 | −0.035 | 0.687 | |
| Tauro-CA | 0.066 | 0.647 | 0.013 | 0.912 | 0.290 | 0.001 | −0.091 | 0.287 | |
| Tauro-CDCA | 0.035 | 0.807 | 0.016 | 0.888 | 0.215 | 0.011 | −0.174 | 0.038 | |
| Tauro-DCA | −0.014 | 0.925 | −0.102 | 0.378 | −0.123 | 0.150 | −0.175 | 0.041 | |
| Tauro-LCA | −0.076 | 0.625 | 0.024 | 0.848 | −0.143 | 0.118 | 0.048 | 0.595 | |
| Tauro-UDCA | 0.143 | 0.337 | 0.138 | 0.246 | 0.020 | 0.819 | −0.248 | 0.005 | |
| Total primary tauro-BA | 0.041 | 0.770 | 0.016 | 0.888 | 0.235 | 0.005 | −0.165 | 0.050 | |
| Total tauro-BA | 0.108 | 0.462 | −0.031 | 0.790 | 0.105 | 0.227 | −0.092 | 0.291 | |
| All BA | 0.211 | 0.159 | 0.189 | 0.115 | 0.041 | 0.641 | −0.240 | 0.007 | |
| Total free BA/All BA | −0.108 | 0.466 | −0.027 | 0.822 | −0.192 | 0.029 | 0.041 | 0.635 | |
| Total glyco-BA/Total tauro-BA | 0.132 | 0.396 | 0.149 | 0.234 | 0.113 | 0.220 | −0.022 | 0.806 | |
| Total primary BA/All primary BA | −0.070 | 0.642 | 0.062 | 0.606 | −0.121 | 0.174 | 0.072 | 0.420 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoi, W.; Koyama, T.; Honda, A.; Takagi, T.; Naito, Y. Association of Serum Bile Acid Profile with Diet and Physical Activity Habits in Japanese Middle-Aged Men. Nutrients 2024, 16, 3381. https://doi.org/10.3390/nu16193381
Aoi W, Koyama T, Honda A, Takagi T, Naito Y. Association of Serum Bile Acid Profile with Diet and Physical Activity Habits in Japanese Middle-Aged Men. Nutrients. 2024; 16(19):3381. https://doi.org/10.3390/nu16193381
Chicago/Turabian StyleAoi, Wataru, Teruhide Koyama, Akira Honda, Tomohisa Takagi, and Yuji Naito. 2024. "Association of Serum Bile Acid Profile with Diet and Physical Activity Habits in Japanese Middle-Aged Men" Nutrients 16, no. 19: 3381. https://doi.org/10.3390/nu16193381
APA StyleAoi, W., Koyama, T., Honda, A., Takagi, T., & Naito, Y. (2024). Association of Serum Bile Acid Profile with Diet and Physical Activity Habits in Japanese Middle-Aged Men. Nutrients, 16(19), 3381. https://doi.org/10.3390/nu16193381







