Whole Plant Extracts for Neurocognitive Disorders: A Narrative Review of Neuropsychological and Preclinical Studies
Abstract
1. Introduction
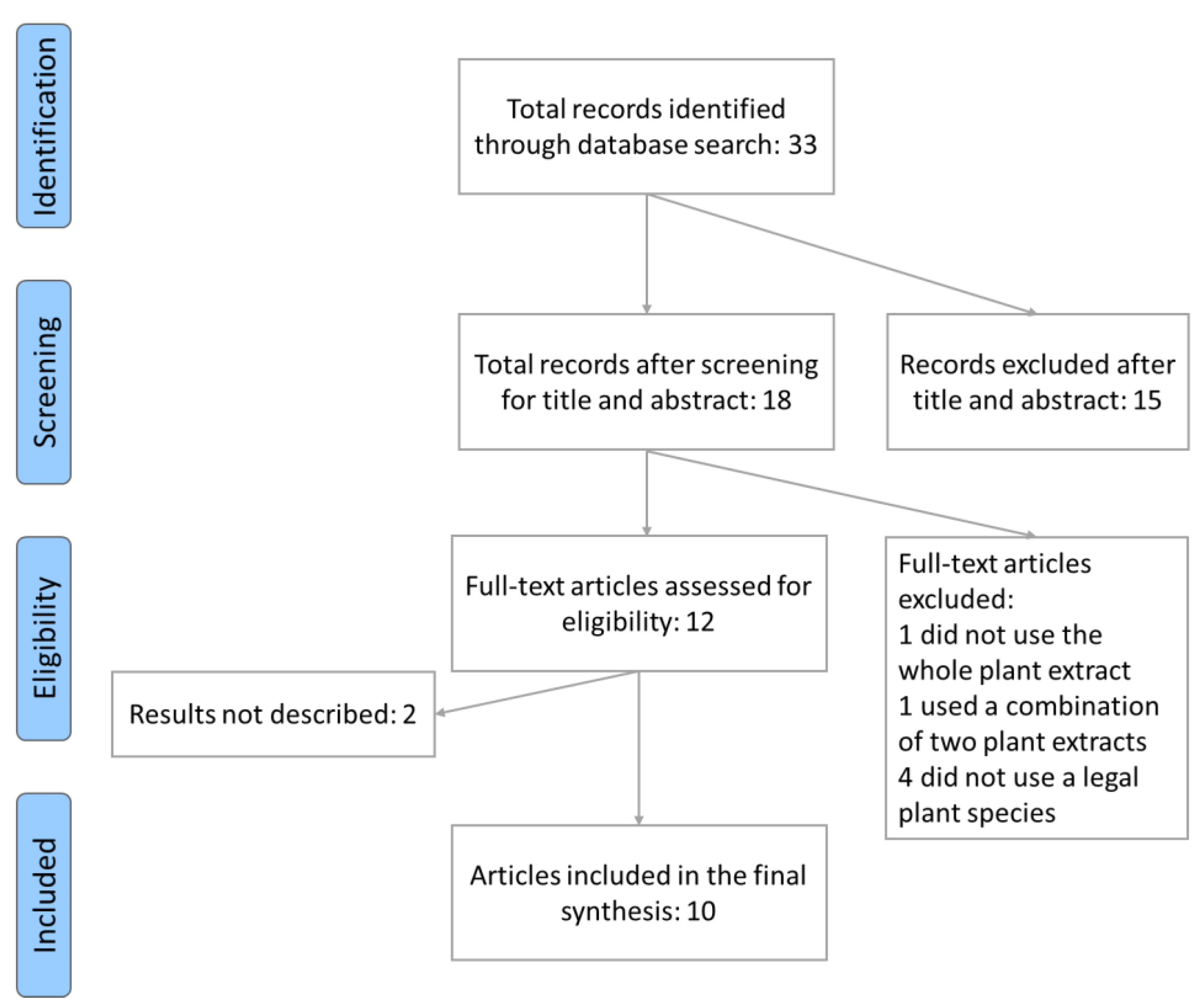
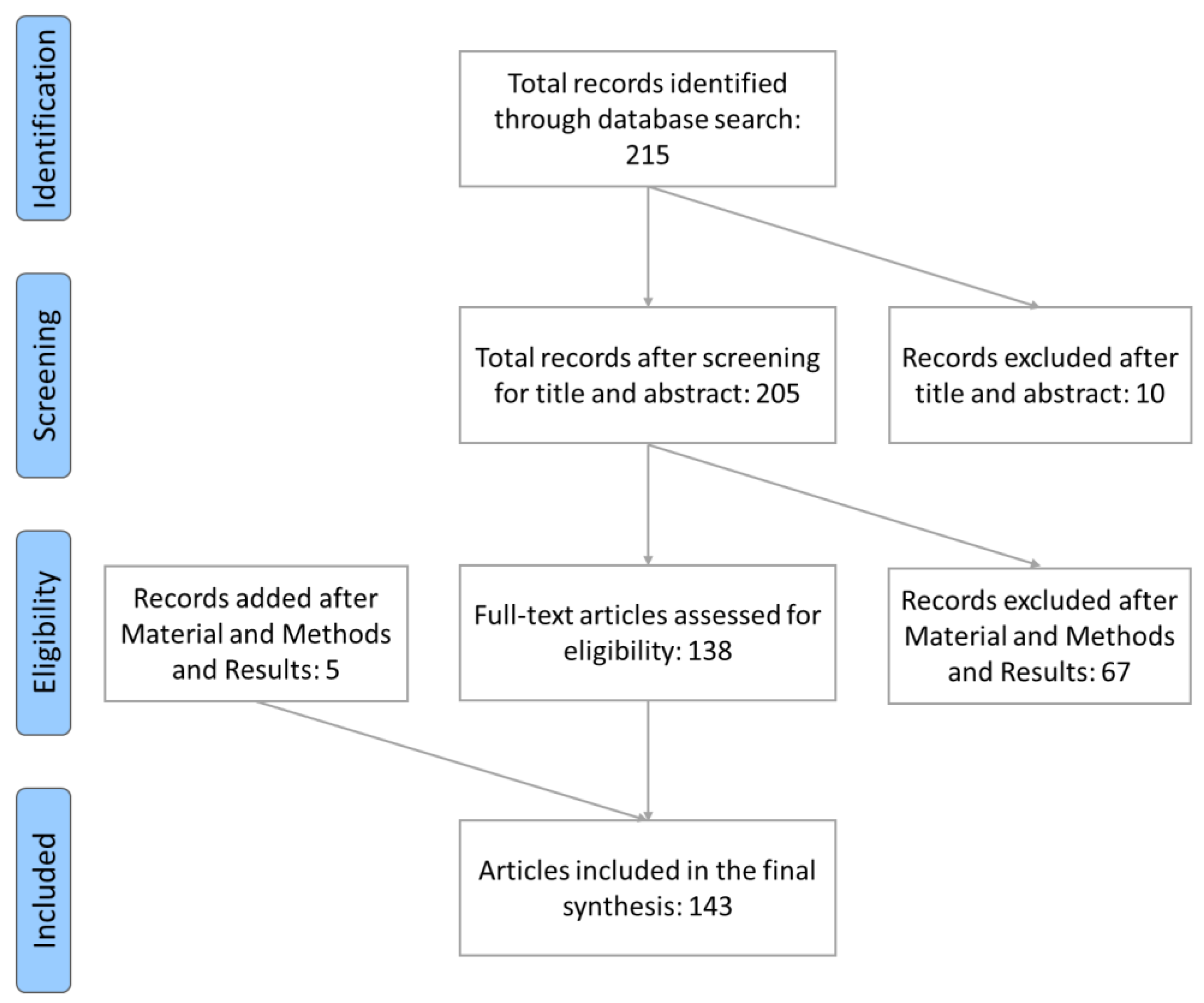
2. Materials and Methods
2.1. Search Criteria for Human Literature
2.2. Search Criteria for Preclinical Literature
3. Results
3.1. Evidence of Plant Extract Effects on Human Cognition
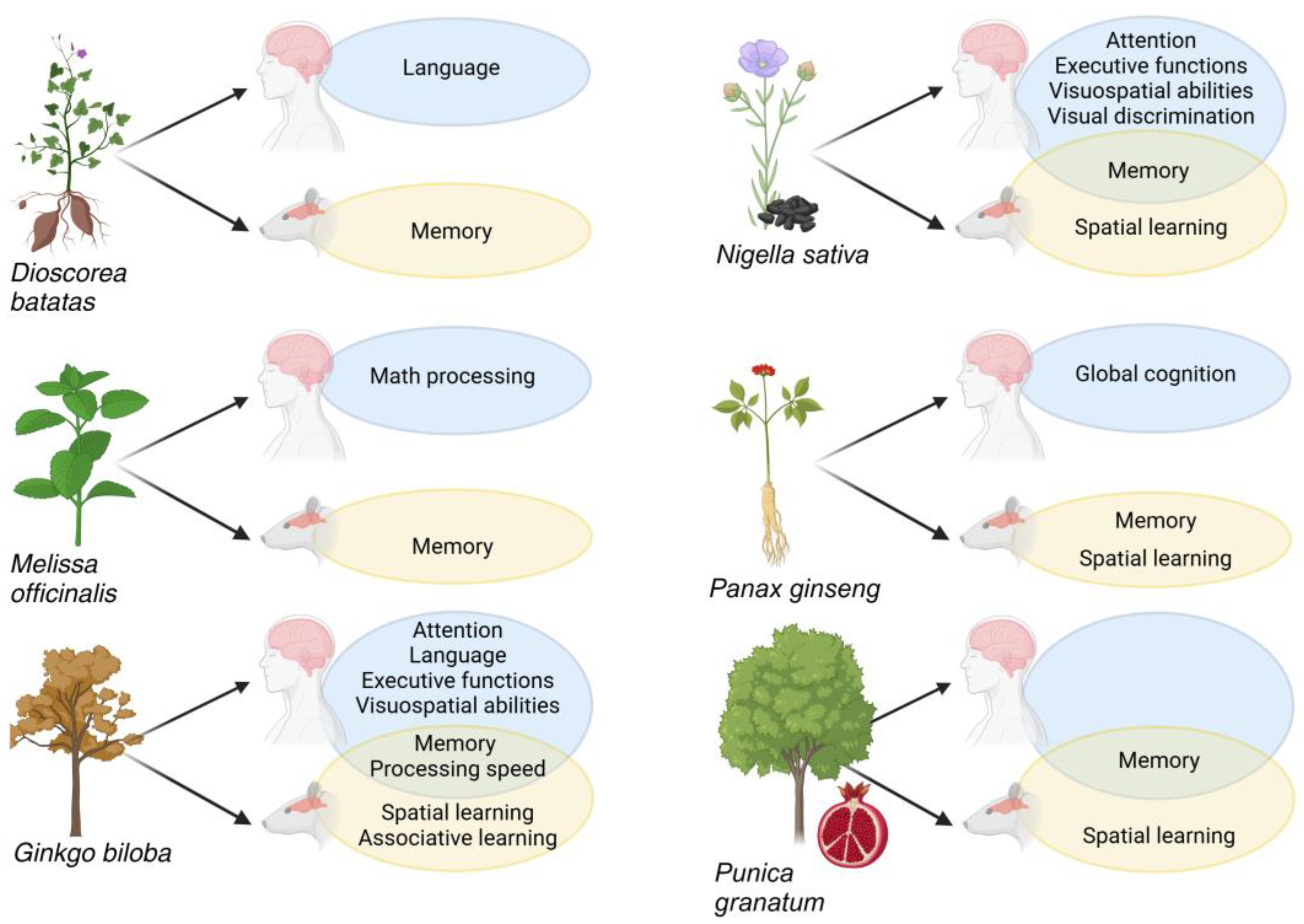
3.1.1. Dioscorea batatas
3.1.2. Ginkgo biloba
3.1.3. Melissa officinalis
3.1.4. Nigella sativa
3.1.5. Olea europaea
3.1.6. Panax ginseng
3.1.7. Punica granatum
3.1.8. Vitis vinifera
3.2. Evidence of Plant Extract Effects on Rodents Cognition
3.2.1. Dioscorea batatas
3.2.2. Ginkgo biloba
3.2.3. Melissa officinalis
3.2.4. Nigella sativa
3.2.5. Olea europaea
3.2.6. Panax ginseng
3.2.7. Punica granatum
3.2.8. Vitis vinifera
4. Discussion
Supplementary Materials
Author Contributions
Funding
Award Number
Data Availability Statement
Conflicts of Interest
References
- WHO. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 December 2023).
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Gonzalez Kelso, I.; Tadi, P. Cognitive Assessment. 2022 Nov 7. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Hanell, A.; Marklund, N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef]
- Young, J.W.; Jentsch, J.D.; Bussey, T.J.; Wallace, T.L.; Hutcheson, D.M. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci. Biobehav. Rev. 2013, 37, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef]
- Lorca, C.; Mulet, M.; Arevalo-Caro, C.; Sanchez, M.A.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martinez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-derived nootropics and human cognition: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5521–5545. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014, 72 Pt A, 22–36. [Google Scholar] [CrossRef]
- Howes, M.R.; Perry, N.S.L.; Vasquez-Londono, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Baroni, L.; Sarni, A.R.; Zuliani, C. Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Yang, X.; Matsui, M.; Inada, Y.; Kadomoto, E.; Nakada, S.; Watari, H.; Shibahara, N. Diosgenin-Rich Yam Extract Enhances Cognitive Function: A Placebo-Controlled, Randomized, Double-Blind, Crossover Study of Healthy Adults. Nutrients 2017, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Mix, J.A.; Crews, W.D., Jr. An examination of the efficacy of Ginkgo biloba extract EGb761 on the neuropsychologic functioning of cognitively intact older adults. J. Altern. Complement. Med. 2000, 6, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, S.; Hartley, D.E.; Ali, O.; Williamson, E.M.; File, S.E. Differential cognitive effects of Ginkgo biloba after acute and chronic treatment in healthy young volunteers. Psychopharmacology 2005, 179, 437–446. [Google Scholar] [CrossRef]
- Santos, R.F.; Galduroz, J.C.; Barbieri, A.; Castiglioni, M.L.; Ytaya, L.Y.; Bueno, O.F. Cognitive performance, SPECT, and blood viscosity in elderly non-demented people using Ginkgo biloba. Pharmacopsychiatry 2003, 36, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Asaduzzaman, M.; Morshed, H.; Hossain, M.M.; Kadir, M.F.; Rahman, M.R. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J. Ethnopharmacol. 2013, 148, 780–786. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the replacement of dietary vegetable oils with a low dose of extravirgin olive oil in the Mediterranean Diet on cognitive functions in the elderly. J. Transl. Med. 2018, 16, 10. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Petrou, P.; Ginzberg, A.; Binyamin, O.; Karussis, D. Beneficial effects of a nano formulation of pomegranate seed oil, GranaGard, on the cognitive function of multiple sclerosis patients. Mult. Scler. Relat. Disord. 2021, 54, 103103. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; pp. 154–167. [Google Scholar]
- Liu, J.; Ye, T.; Zhang, Y.; Zhang, R.; Kong, Y.; Zhang, Y.; Sun, J. Protective Effect of Ginkgolide B against Cognitive Impairment in Mice via Regulation of Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12230–12240. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y.; Zhao, L. Ginkgolide B Alleviates Learning and Memory Impairment in Rats With Vascular Dementia by Reducing Neuroinflammation via Regulating NF-kappaB Pathway. Front. Pharmacol. 2021, 12, 676392. [Google Scholar] [CrossRef]
- Shao, L.; Dong, C.; Geng, D.; He, Q.; Shi, Y. Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem. Biophys. Res. Commun. 2021, 572, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Wu, W.Y.; Huang, H.; Wu, Y.Y.; Yin, Y.Y. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol. Med. Rep. 2013, 8, 935–941. [Google Scholar] [CrossRef]
- Wu, R.; Shui, L.; Wang, S.; Song, Z.; Tai, F. Bilobalide alleviates depression-like behavior and cognitive deficit induced by chronic unpredictable mild stress in mice. Behav. Pharmacol. 2016, 27, 596–605. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Dai, J.; Yang, L.; Zhang, L.; Shen, S.; Huang, P. Hyperbaric oxygen and Ginkgo biloba extract inhibit Abeta25-35-induced toxicity and oxidative stress in vivo: A potential role in Alzheimer’s disease. Int. J. Neurosci. 2012, 122, 563–569. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, L.; Wang, J.; Dai, J.; Shen, S.; Yang, L.; Huang, P. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Abeta25-35-induced oxidative stress and neuronal apoptosis in rats. Behav. Brain Res. 2013, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.; Ma, L.; Zhang, L.; Dai, J.G.; Chang, L.G.; Huang, P.L.; Tian, X.Q. Hyperbaric Oxygen and Ginkgo biloba Extract Ameliorate Cognitive and Memory Impairment via Nuclear Factor Kappa-B Pathway in Rat Model of Alzheimer’s Disease. Chin. Med. J. 2015, 128, 3088–3093. [Google Scholar] [CrossRef]
- Tu, J.L.; Chen, W.P.; Cheng, Z.J.; Zhang, G.; Luo, Q.H.; Li, M.; Liu, X. EGb761 ameliorates cell necroptosis by attenuating RIP1-mediated mitochondrial dysfunction and ROS production in both in vivo and in vitro models of Alzheimer’s disease. Brain Res. 2020, 1736, 146730. [Google Scholar] [CrossRef]
- Hoyer, S.; Lannert, H.; Noldner, M.; Chatterjee, S.S. Damaged neuronal energy metabolism and behavior are improved by Ginkgo biloba extract (EGb 761). J. Neural. Transm. 1999, 106, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schotz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Tomic, I.; Hao, W.; Menger, M.D.; Liu, C.; Fassbender, K.; Liu, Y. Ginkgo biloba Extract EGb 761 and Its Specific Components Elicit Protective Protein Clearance Through the Autophagy-Lysosomal Pathway in Tau-Transgenic Mice and Cultured Neurons. J. Alzheimers Dis. 2018, 65, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.; Yue, H.Y.; Zou, J.; Tang, M.; Zou, F.M.; Li, Z.L.; Jia, Q.Q.; Li, Y.B.; Kang, J.; Zuo, L.H. Comprehensive metabolomics and lipidomics profiling uncovering neuroprotective effects of Ginkgo biloba L. leaf extract on Alzheimer’s disease. Front. Pharmacol. 2022, 13, 1076960. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhang, C.; Danielsen, M.; Li, Q.; Chen, W.; Chan, Y.; Li, Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016, 81, 92–100. [Google Scholar] [CrossRef]
- Stackman, R.W.; Eckenstein, F.; Frei, B.; Kulhanek, D.; Nowlin, J.; Quinn, J.F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp. Neurol. 2003, 184, 510–520. [Google Scholar] [CrossRef]
- Ge, W.; Ren, C.; Xing, L.; Guan, L.; Zhang, C.; Sun, X.; Wang, G.; Niu, H.; Qun, S. Ginkgo biloba extract improves cognitive function and increases neurogenesis by reducing Abeta pathology in 5xFAD mice. Am. J. Transl. Res. 2021, 13, 1471–1482. [Google Scholar]
- Zeng, K.; Li, M.; Hu, J.; Mahaman, Y.A.R.; Bao, J.; Huang, F.; Xia, Y.; Liu, X.; Wang, Q.; Wang, J.Z.; et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr. Alzheimer Res. 2018, 15, 89–99. [Google Scholar] [CrossRef]
- Koz, S.T.; Baydas, G.; Koz, S.; Demir, N.; Nedzvetsky, V.S. Gingko biloba extract inhibits oxidative stress and ameliorates impaired glial fibrillary acidic protein expression, but can not improve spatial learning in offspring from hyperhomocysteinemic rat dams. Phytother. Res. 2012, 26, 949–955. [Google Scholar] [CrossRef]
- EMA. European Union Herbal Monograph on Ginkgo biloba L., folium. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-ginkgo-biloba-l-folium_en.pdf (accessed on 13 December 2023).
- Dong, W.; Gong, T.; Zhao, S.; Wen, S.; Chen, Q.; Jiang, M.; Ye, W.; Huang, Q.; Wang, C.; Yang, C.; et al. A Novel Extract From Ginkgo biloba Inhibits Neuroinflammation and Maintains White Matter Integrity in Experimental Stroke. Neuroscience 2023, 523, 7–19. [Google Scholar] [CrossRef]
- Kwak, P.A.; Lim, S.C.; Han, S.R.; Shon, Y.M.; Kim, Y.I. Supra-additive neuroprotection by renexin, a mixed compound of Ginkgo biloba extract and cilostazol, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. J. Clin. Neurol. 2012, 8, 284–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaghef, L.; Bafandeh Gharamaleki, H. Effects of Physical Activity and Ginkgo biloba on Cognitive Function and Oxidative Stress Modulation in Ischemic Rats. Int. J. Angiol. 2017, 26, 158–164. [Google Scholar] [CrossRef]
- Yao, Z.H.; Wang, J.; Yuan, J.P.; Xiao, K.; Zhang, S.F.; Xie, Y.C.; Mei, J.H. EGB761 ameliorates chronic cerebral hypoperfusion-induced cognitive dysfunction and synaptic plasticity impairment. Aging 2021, 13, 9522–9541. [Google Scholar] [CrossRef]
- Harada, S.; Tsujita, T.; Ono, A.; Miyagi, K.; Mori, T.; Tokuyama, S. Stachys sieboldii (Labiatae, Chorogi) Protects against Learning and Memory Dysfunction Associated with Ischemic Brain Injury. J. Nutr. Sci. Vitaminol. 2015, 61, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Wang, Y. A Ginkgo biloba extract promotes proliferation of endogenous neural stem cells in vascular dementia rats. Neural Regen. Res. 2013, 8, 1655–1662. [Google Scholar] [CrossRef]
- Paganelli, R.A.; Benetoli, A.; Milani, H. Sustained neuroprotection and facilitation of behavioral recovery by the Ginkgo biloba extract, EGb 761, after transient forebrain ischemia in rats. Behav. Brain Res. 2006, 174, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Domorakova, I.; Mechirova, E.; Dankova, M.; Danielisova, V.; Burda, J. Effect of antioxidant treatment in global ischemia and ischemic postconditioning in the rat hippocampus. Cell Mol. Neurobiol. 2009, 29, 837–844. [Google Scholar] [CrossRef]
- Rapin, J.R.; Lamproglou, I.; Drieu, K.; DeFeudis, F.V. Demonstration of the “anti-stress” activity of an extract of Ginkgo biloba (EGb 761) using a discrimination learning task. Gen. Pharmacol. 1994, 25, 1009–1016. [Google Scholar] [CrossRef]
- Stoll, S.; Scheuer, K.; Pohl, O.; Muller, W.E. Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry 1996, 29, 144–149. [Google Scholar] [CrossRef]
- Winter, J.C. The effects of an extract of Ginkgo biloba, EGb 761, on cognitive behavior and longevity in the rat. Physiol. Behav. 1998, 63, 425–433. [Google Scholar] [CrossRef]
- Wirth, S.; Stemmelin, J.; Will, B.; Christen, Y.; Di Scala, G. Facilitative effects of EGb 761 on olfactory recognition in young and aged rats. Pharmacol. Biochem. Behav. 2000, 65, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wu, J.; Cai, J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br. J. Pharmacol. 2006, 148, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N. The effects of Ginkgo biloba extract on cognitive functions in aged female rats: The role of oxidative stress and brain-derived neurotrophic factor. Behav. Brain Res. 2015, 278, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; Redd, K.; Williams, B.M.; Caler, J.R.; Luo, Y.; McCoy, J.G. Ginkgo biloba extract: Cognitive enhancer or antistress buffer. Pharmacol. Biochem. Behav. 2002, 72, 913–922. [Google Scholar] [CrossRef]
- Pardon, M.C.; Hanoun, N.; Perez-Diaz, F.; Joubert, C.; Launay, J.M.; Christen, Y.; Hamon, M.; Cohen-Salmon, C. Long-term treatment with the antioxidant drug EGb 761 at senescence restored some neurobehavioral effects of chronic ultramild stress exposure seen in young mice. Neurobiol. Aging 2004, 25, 1067–1083. [Google Scholar] [CrossRef]
- Chopin, P.; Briley, M. Effects of four non-cholinergic cognitive enhancers in comparison with tacrine and galanthamine on scopolamine-induced amnesia in rats. Psychopharmacology 1992, 106, 26–30. [Google Scholar] [CrossRef]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, G.S.; Tan, Y.J.; Tao, H.J.; Chen, J.Q.; Pu, Z.J.; Ma, J.Y.; She, W.; Kang, A.; et al. Studies of the Anti-amnesic Effects and Mechanisms of Single and Combined Use of Donepezil and Ginkgo Ketoester Tablet on Scopolamine-Induced Memory Impairment in Mice. Oxid. Med. Cell Longev. 2019, 2019, 8636835. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Wang, Y.; Li, D.; Wang, Q.; Xie, S.; Wang, J.; Zuo, Z. Enhanced anti-amnestic effect of donepezil by Ginkgo biloba extract (EGb 761) via further improvement in pro-cholinergic and antioxidative activities. J. Ethnopharmacol. 2021, 269, 113711. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zheng, D.; Yu, H.; Luo, X.P.; Wu, W. Ginkgo biloba Extract Ameliorates Scopolamine-induced Memory Deficits via Rescuing Synaptic Damage. Curr. Med. Sci. 2022, 42, 474–482. [Google Scholar] [CrossRef]
- Hong, S.M.; Yoon, D.H.; Lee, M.K.; Lee, J.K.; Kim, S.Y. A Mixture of Ginkgo biloba L. Leaf and Hericium erinaceus (Bull.) Pers. Fruit Extract Attenuates Scopolamine-Induced Memory Impairments in Mice. Oxid. Med. Cell Longev. 2022, 2022, 9973678. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, X.; Geng, D.; Huang, H.; Zhou, H. Ginkgo biloba leaf extract improves the cognitive abilities of rats with D-galactose induced dementia. J. Biomed. Res. 2013, 27, 29–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Zhang, Y.C.; Chen, G. Effect of Ginkgo biloba Extract EGb761 on Hippocampal Neuronal Injury and Carbonyl Stress of D-Gal-Induced Aging Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 5165910. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, P.; Li, J.; Liu, T.; Zhang, Y.; Wang, Q.; Zhang, J.; Lu, X.; Fan, X. Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease. J. Pharm. Anal. 2021, 11, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.G.; Aduema, W.; Emmanuel, M.U.; Ben-Azu, B.; Orji, B.O.; Akpakpan, E.; Adebayo, O.R.; Onuoha, O.G.; Ajayi, A.M. The Anti-Parkinson Potential of Gingko biloba-Supplement Mitigates Cortico-Cerebellar Degeneration and Neuropathobiological Alterations via Inflammatory and Apoptotic Mediators in Mice. Neurochem. Res. 2022, 47, 2211–2229. [Google Scholar] [CrossRef]
- Walesiuk, A.; Trofimiuk, E.; Braszko, J.J. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacol. Rep. 2005, 57, 176–187. [Google Scholar]
- Walesiuk, A.; Trofimiuk, E.; Braszko, J.J. Ginkgo biloba normalizes stress- and corticosterone-induced impairment of recall in rats. Pharmacol. Res. 2006, 53, 123–128. [Google Scholar] [CrossRef]
- Walesiuk, A.; Braszko, J.J. Preventive action of Ginkgo biloba in stress- and corticosterone-induced impairment of spatial memory in rats. Phytomedicine 2009, 16, 40–46. [Google Scholar] [CrossRef]
- Walesiuk, A.; Braszko, J.J. Gingkoselect alleviates chronic corticosterone-induced spatial memory deficits in rats. Fitoterapia 2010, 81, 25–29. [Google Scholar] [CrossRef]
- Takuma, K.; Hoshina, Y.; Arai, S.; Himeno, Y.; Matsuo, A.; Funatsu, Y.; Kitahara, Y.; Ibi, D.; Hayase, M.; Kamei, H.; et al. Ginkgo biloba extract EGb 761 attenuates hippocampal neuronal loss and cognitive dysfunction resulting from chronic restraint stress in ovariectomized rats. Neuroscience 2007, 149, 256–262. [Google Scholar] [CrossRef]
- Markus, C.R.; Lammers, J.H. Effects of Ginkgo biloba on corticosterone stress responses after inescapable shock exposure in the rat. Pharmacol. Biochem. Behav. 2003, 76, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.G.; Sharma, P.; Patial, V.; Singh, D. Ginkgo biloba L. attenuates spontaneous recurrent seizures and associated neurological conditions in lithium-pilocarpine rat model of temporal lobe epilepsy through inhibition of mammalian target of rapamycin pathway hyperactivation. J. Ethnopharmacol. 2017, 204, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, Z.; Shen, M.; Lin, W.; Wang, Y.; Wang, S.; Li, C.; Wang, S.; Chen, M.; Shan, W.; et al. Insight into Ginkgo biloba L. Extract on the Improved Spatial Learning and Memory by Chemogenomics Knowledgebase, Molecular Docking, Molecular Dynamics Simulation, and Bioassay Validations. ACS Omega 2020, 5, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Chhabra, R.; Nehru, B. Ginkgo biloba extract attenuates hippocampal neuronal loss and cognitive dysfunction resulting from trimethyltin in mice. Phytomedicine 2013, 20, 178–186. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Nehru, B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology 2018, 26, 87–104. [Google Scholar] [CrossRef]
- El Tabaa, M.M.; Sokkar, S.S.; Ramadan, E.S.; Abd El Salam, I.Z.; Zaid, A. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: An animal model study. Neurochem. Int. 2017, 108, 199–212. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, C.; Chen, H.; Geng, R.; Fan, H.; Zhao, H.; Guo, K.; Geng, D. The analog of Ginkgo biloba extract 761 is a protective factor of cognitive impairment induced by chronic fluorosis. Biol. Trace Elem. Res. 2013, 153, 229–236. [Google Scholar] [CrossRef]
- Luo, C.; Fan, L.H.; Zhang, H.; Zhao, J.; Li, L.; Zhang, L.; Zhang, H.X.; Ma, M.M. Effects of ginkgo biloba extract on the cognitive function and expression profile of inflammatory factors in a rat model of hemorrhagic stroke. Neuroreport 2018, 29, 1239–1243. [Google Scholar] [CrossRef]
- Zamberlam, C.R.; Tilger, M.A.S.; Moraes, L.; Cerutti, J.M.; Cerutti, S.M. Ginkgo biloba treatments reverse the impairment of conditioned suppression acquisition induced by GluN2B-NMDA and 5-HT(1A) receptor blockade: Modulatory effects of the circuitry of the dorsal hippocampal formation. Physiol. Behav. 2019, 209, 112534. [Google Scholar] [CrossRef]
- Petkov, V.D.; Belcheva, S.; Petkov, V.V. Behavioral effects of Ginkgo biloba L., Panax ginseng C.A. Mey. and Gincosan. Am. J. Chin. Med. 2003, 31, 841–855. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, J. Extract of Ginkgo biloba leaves reverses yohimbine-induced spatial working memory deficit in rats. Behav. Pharmacol. 2005, 16, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Salem, N.A.; El-Sayed El-Shamarka, M.; Al-Said Ahmed, N.; Seid Hussein, J.; El-Khyat, Z.A. Cannabis-induced impairment of learning and memory: Effect of different nootropic drugs. EXCLI J. 2013, 12, 193–214. [Google Scholar]
- Gaiardo, R.B.; Abreu, T.F.; Tashima, A.K.; Telles, M.M.; Cerutti, S.M. Target Proteins in the Dorsal Hippocampal Formation Sustain the Memory-Enhancing and Neuroprotective Effects of Ginkgo biloba. Front. Pharmacol. 2018, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Su, Y.W.; Ng, M.C.; Chang, C.L.; Lu, K.T. Extract of Ginkgo biloba EGb 761 facilitates fear conditioning measured by fear-potentiated startle. Neurosci. Lett. 2005, 383, 145–150. [Google Scholar] [CrossRef]
- Chen, L.E.; Wu, F.; Zhao, A.; Ge, H.; Zhan, H. Protection Efficacy of the Extract of Ginkgo biloba against the Learning and Memory Damage of Rats under Repeated High Sustained +Gz Exposure. Evid. Based Complement. Altern. Med. 2016, 2016, 6320586. [Google Scholar] [CrossRef]
- Guan, Z.F.; Zhang, X.M.; Tao, Y.H.; Zhang, Y.; Huang, Y.Y.; Chen, G.; Tang, W.J.; Ji, G.; Guo, Q.L.; Liu, M.; et al. EGb761 improves the cognitive function of elderly db/db(-/-) diabetic mice by regulating the beclin-1 and NF-kappaB signaling pathways. Metab. Brain Dis. 2018, 33, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Jain, V.; Kadam, M.; Kumar, R.; Dheer, A.; Prasad, D.; Kumar, B.; Khan, N. Ginkgo biloba L. Prevents Hypobaric Hypoxia-Induced Spatial Memory Deficit Through Small Conductance Calcium-Activated Potassium Channel Inhibition: The Role of ERK/CaMKII/CREB Signaling. Front. Pharmacol. 2021, 12, 669701. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Piasecka, A.; Kachlicki, P.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Szulc, M.; Kaminska, E.; Kujawska, M.; et al. Influence of the Melissa officinalis Leaf Extract on Long-Term Memory in Scopolamine Animal Model with Assessment of Mechanism of Action. Evid. Based Complement. Altern. Med. 2016, 2016, 9729818. [Google Scholar] [CrossRef]
- Kandilarov, I.K.; Zlatanova, H.I.; Georgieva-Kotetarova, M.T.; Kostadinova, I.I.; Katsarova, M.N.; Dimitrova, S.Z.; Lukanov, L.K.; Sadakov, F. Antidepressant Effect and Recognition Memory Improvement of Two Novel Plant Extract Combinations—Antistress I and Anti-stress II on Rats Subjected to a Model of Mild Chronic Stress. Folia Med. 2018, 60, 110–116. [Google Scholar] [CrossRef]
- Chindo, B.A.; Howes, M.R.; Abuhamdah, S.; Yakubu, M.I.; Ayuba, G.I.; Battison, A.; Chazot, P.L. New Insights Into the Anticonvulsant Effects of Essential Oil From Melissa officinalis L. (Lemon balm). Front. Pharmacol. 2021, 12, 760674. [Google Scholar] [CrossRef]
- Naseri, M.; Arabi Mianroodi, R.; Pakzad, Z.; Falahati, P.; Borbor, M.; Azizi, H.; Nasri, S. The effect of Melissa officinalis L. extract on learning and memory: Involvement of hippocampal expression of nitric oxide synthase and brain-derived neurotrophic factor in diabetic rats. J. Ethnopharmacol. 2021, 276, 114210. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Badary, O.A. Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: Biochemical, histological and behavioral changes. Neurol. Res. 2018, 40, 324–333. [Google Scholar] [CrossRef]
- Azzubaidi, M.S.; Saxena, A.K.; Talib, N.A.; Ahmed, Q.U.; Dogarai, B.B. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiol. Exp. 2012, 72, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Fanoudi, S.; Alavi, M.S.; Hosseini, M.; Sadeghnia, H.R. Nigella sativa and thymoquinone attenuate oxidative stress and cognitive impairment following cerebral hypoperfusion in rats. Metab. Brain Dis. 2019, 34, 1001–1010. [Google Scholar] [CrossRef]
- Abdelghany, A.K.; El-Nahass, E.S.; Ibrahim, M.A.; El-Kashlan, A.M.; Emeash, H.H.; Khalil, F. Neuroprotective role of medicinal plant extracts evaluated in a scopolamine-induced rat model of Alzheimer’s disease. Biomarkers 2022, 27, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Poorgholam, P.; Yaghmaei, P.; Hajebrahimi, Z. Thymoquinone recovers learning function in a rat model of Alzheimer’s disease. Avicenna J. Phytomed 2018, 8, 188–197. [Google Scholar]
- Tahmasebi, S.; Oryan, S.; Mohajerani, H.R.; Akbari, N.; Palizvan, M.R. Probiotics and Nigella sativa extract supplementation improved behavioral and electrophysiological effects of PTZ-induced chemical kindling in rats. Epilepsy Behav. 2020, 104, 106897. [Google Scholar] [CrossRef]
- Vafaee, F.; Hosseini, M.; Hassanzadeh, Z.; Edalatmanesh, M.A.; Sadeghnia, H.R.; Seghatoleslam, M.; Mousavi, S.M.; Amani, A.; Shafei, M.N. The Effects of Nigella sativa Hydro-alcoholic Extract on Memory and Brain Tissues Oxidative Damage after Repeated Seizures in Rats. Iran. J. Pharm. Res. 2015, 14, 547–557. [Google Scholar]
- Fouad, I.A.; Sharaf, N.M.; Abdelghany, R.M.; El Sayed, N. Neuromodulatory Effect of Thymoquinone in Attenuating Glutamate-Mediated Neurotoxicity Targeting the Amyloidogenic and Apoptotic Pathways. Front. Neurol. 2018, 9, 236. [Google Scholar] [CrossRef]
- Imam, A.; Ajao, M.S.; Amin, A.; Abdulmajeed, W.I.; Ibrahim, A.; Olajide, O.J.; Ajibola, M.I.; Alli-Oluwafuyi, A.; Balogun, W.G. Cannabis-induced Moto-Cognitive Dysfunction in Wistar Rats: Ameliorative Efficacy of Nigella sativa. Malays. J. Med. Sci. 2016, 23, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Kazemi, S.; Ebrahimpour, A.; Pourabdolhossein, F.; Satarian, L.; Eghbali, A.; Moghadamnia, A.A. Thymoquinone Improved Nonylphenol-Induced Memory Deficit and Neurotoxicity Through Its Antioxidant and Neuroprotective Effects. Mol. Neurobiol. 2022, 59, 3600–3616. [Google Scholar] [CrossRef]
- Kulkarni, R.; Mehta, R.; Goswami, S.K.; Hammock, B.D.; Morisseau, C.; Hwang, S.H.; Mallappa, O.; Azeemuddin, M.M.; Rafiq, M.; Manjula, S.N. Neuroprotective effect of herbal extracts inhibiting soluble epoxide hydrolase (sEH) and cyclooxygenase (COX) against chemotherapy-induced cognitive impairment in mice. Biochem. Biophys. Res. Commun. 2023, 667, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.A.R.; Nawaz, S.; Gul, S.; Salman, T.; Naqvi, S.; Dar, A.; Haleem, D.J. Neurochemical and behavioral effects of Nigella sativa and Olea europaea oil in rats. Nutr. Neurosci. 2018, 21, 185–194. [Google Scholar] [CrossRef]
- Beheshti, F.; Hosseini, M.; Vafaee, F.; Shafei, M.N.; Soukhtanloo, M. Feeding of Nigella sativa during neonatal and juvenile growth improves learning and memory of rats. J. Tradit. Complement. Med. 2016, 6, 146–152. [Google Scholar] [CrossRef]
- Asghari, A.A.; Hosseini, M.; Bafadam, S.; Rakhshandeh, H.; Farazandeh, M.; Mahmoudabady, M. Olea europaea L. (Olive) leaf extract ameliorates learning and memory deficits in streptozotocin-induced diabetic rats. Avicenna J. Phytomed. 2022, 12, 163–174. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.S.; Park, H.W.; Ahn, N.G.; Cho, B.G.; Cho, Y.L.; Kwak, Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Nitta, H.; Matsumoto, K.; Shimizu, M.; Ni, X.H.; Watanabe, H. Panax ginseng extract improves the scopolamine-induced disruption of 8-arm radial maze performance in rats. Biol. Pharm. Bull. 1995, 18, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shen, X.; Yu, H.; Sun, L.; Lin, W.; Zhang, C. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J. Ginseng Res. 2016, 40, 211–219. [Google Scholar] [CrossRef]
- Hsieh, M.T.; Peng, W.H.; Wu, C.R.; Wang, W.H. The ameliorating effects of the cognitive-enhancing Chinese herbs on scopolamine-induced amnesia in rats. Phytother. Res. 2000, 14, 375–377. [Google Scholar] [CrossRef]
- Al-Hazmi, M.A.; Rawi, S.M.; Arafa, N.M.; Wagas, A.; Montasser, A.O. The potent effects of ginseng root extract and memantine on cognitive dysfunction in male albino rats. Toxicol. Ind. Health 2015, 31, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Seo, J.Y.; Lee, S.K.; Oh, J.; Kim, J.S. Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism. J. Ginseng Res. 2021, 45, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lee, S.; Lee, T.; Yun, S.; Kim, Y.; Yang, H. Smart Farming Enhances Bioactive Compounds Content of Panax ginseng on Moderating Scopolamine-Induced Memory Deficits and Neuroinflammation. Plants 2023, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Pena, I.D.; Yoon, S.Y.; Kim, H.J.; Park, S.; Hong, E.Y.; Ryu, J.H.; Park, I.H.; Cheong, J.H. Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J. Ginseng Res. 2014, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lv, J.; Dong, L.; Jiang, N.; Wang, Y.; Wang, Q.; Li, Y.; Chen, S.; Fan, B.; Wang, F.; et al. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother. Res. 2018, 32, 1056–1063. [Google Scholar] [CrossRef]
- Peng, X.; Hao, M.; Zhao, Y.; Cai, Y.; Chen, X.; Chen, H.; Zhang, Y.; Dong, L.; Liu, X.; Ding, C.; et al. Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine 2021, 93, 153772. [Google Scholar] [CrossRef]
- Li, H.; Kang, T.; Qi, B.; Kong, L.; Jiao, Y.; Cao, Y.; Zhang, J.; Yang, J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 179, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, J.; Zhang, J.; Wang, T.; Yan, Y.; Tao, Z.; Li, S.; Zhang, H.; Kang, T.; Yang, J. Ginseng Protein Reverses Amyloid Beta Peptide and H2O2 Cytotoxicity in Neurons, and Ameliorates Cognitive Impairment in AD Rats Induced by a Combination of D-Galactose and AlCl3. Phytother. Res. 2017, 31, 284–295. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, X.; Zeng, J.; Xu, C.; Liu, J.; Zhang, M.; Li, C.; Chen, J.; Li, T.; Wang, Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS ONE 2014, 9, e101291. [Google Scholar] [CrossRef]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by D-Galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef]
- Zhong, S.J.; Wang, L.; Gu, R.Z.; Zhang, W.H.; Lan, R.; Qin, X.Y. Ginsenoside Rg1 ameliorates the cognitive deficits in D-galactose and AlCl3-induced aging mice by restoring FGF2-Akt and BDNF-TrkB signaling axis to inhibit apoptosis. Int. J. Med. Sci. 2020, 17, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chen, K.C.; Zhou, Y.; Wei, H.; Qi, M.H.; Wang, Z.; Zheng, Y.N.; Chen, R.X.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomedicine 2022, 104, 154341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 Prevents Cognitive Impairment by Improving Mitochondrial Dysfunction in the Rat Model of Alzheimer’s Disease. J. Agric. Food Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Gu, J.; Zhao, B.; Wang, S.; Yuan, J.; Wang, C.; Chen, J.; Liu, J.; Feng, L.; Jia, X. Ginseng improves cognitive deficit via the RAGE/NF-kappaB pathway in advanced glycation end product-induced rats. J. Ginseng Res. 2015, 39, 116–124. [Google Scholar] [CrossRef]
- Yoon, E.J.; Ahn, J.W.; Kim, H.S.; Choi, Y.; Jeong, J.; Joo, S.S.; Park, D. Improvement of Cognitive Function by Fermented Panax ginseng C.A. Meyer Berries Extracts in an AF64A-Induced Memory Deficit Model. Nutrients 2023, 15, 3389. [Google Scholar] [CrossRef]
- Luo, H.; Hu, J.; Wang, Y.; Chen, Y.; Zhu, D.; Jiang, R.; Qiu, Z. In vivo and in vitro neuroprotective effects of Panax ginseng glycoproteins. Int. J. Biol. Macromol. 2018, 113, 607–615. [Google Scholar] [CrossRef]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Abeta25-35 in rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef]
- Song, X.Y.; Hu, J.F.; Chu, S.F.; Zhang, Z.; Xu, S.; Yuan, Y.H.; Han, N.; Liu, Y.; Niu, F.; He, X.; et al. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3beta/tau signaling pathway and the Abeta formation prevention in rats. Eur. J. Pharmacol. 2013, 710, 29–38. [Google Scholar] [CrossRef]
- Chu, S.; Gu, J.; Feng, L.; Liu, J.; Zhang, M.; Jia, X.; Liu, M.; Yao, D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M.; Ye, R.; Wang, W.; Liu, X.; Zhang, G.; Han, J.; Zhang, Y.; Wang, B.; Zhao, J.; et al. Ginsenoside Rd attenuates tau protein phosphorylation via the PI3K/AKT/GSK-3beta pathway after transient forebrain ischemia. Neurochem. Res. 2014, 39, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Ma, X.; Zhang, Z.J.; Sun, T.; Xia, F.; Zhao, G.; Wu, Y.M. Ginsenoside Reduces Cognitive Impairment During Chronic Cerebral Hypoperfusion Through Brain-Derived Neurotrophic Factor Regulated by Epigenetic Modulation. Mol. Neurobiol. 2017, 54, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Y.J.; Niu, T.Y.; Chen, T.T.; Li, H.B.; Wu, S.H.; Li, G.L. Neuroprotective effect of 20 (S)—Protopanaxadiol (PPD) attenuates NLRP3 inflammasome-mediated microglial pyroptosis in vascular dementia rats. Neurosci. Lett. 2023, 814, 137439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Wang, J.J.; Zhang, X.H.; Yu, Y.; Kang, Z.S. Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen. Res. 2018, 13, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Matsumoto, K.; Shimizu, M.; Ni, X.H.; Watanabe, H. Panax ginseng extract improves the performance of aged Fischer 344 rats in radial maze task but not in operant brightness discrimination task. Biol. Pharm. Bull. 1995, 18, 1286–1288. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.F.; Li, Q.; Li, Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience 2011, 183, 189–202. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, S. Administration of red ginseng ameliorates memory decline in aged mice. J. Ginseng Res. 2015, 39, 250–256. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Bhattacharya, A.; Chakrabarti, A. Adaptogenic activity of Siotone, a polyherbal formulation of Ayurvedic rasayanas. Indian. J. Exp. Biol. 2000, 38, 119–128. [Google Scholar]
- Kumar, V.; Singh, P.N.; Bhattacharya, S.K. Anti-stress activity of Indian Hypericum perforatum L. Indian. J. Exp. Biol. 2001, 39, 344–349. [Google Scholar]
- Bhattacharya, S.K.; Muruganandam, A.V. Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol. Biochem. Behav. 2003, 75, 547–555. [Google Scholar] [CrossRef]
- Kezhu, W.; Pan, X.; Cong, L.; Liming, D.; Beiyue, Z.; Jingwei, L.; Yanyan, Y.; Xinmin, L. Effects of Ginsenoside Rg1 on Learning and Memory in a Reward-directed Instrumental Conditioning Task in Chronic Restraint Stressed Rats. Phytother. Res. 2017, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, N.; Lv, J.; Huang, H.; Liu, X. Ginsenoside Rd reverses cognitive deficits by modulating BDNF-dependent CREB pathway in chronic restraint stress mice. Life Sci. 2020, 258, 118107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lv, J.; Jiang, N.; Huang, H.; Wang, Q.; Liu, X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother. Res. 2021, 5, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.J.; Cen, F.; Fan, Z.Q.; Xu, Y.; Shen, H.Y.; Zhou, M.M. Serum and Brain Metabolomic Variations Reveal Perturbation of Sleep Deprivation on Rats and Ameliorate Effect of Total Ginsenoside Treatment. Int. J. Genom. 2017, 2017, 5179271. [Google Scholar] [CrossRef]
- Lu, C.; Shi, Z.; Dong, L.; Lv, J.; Xu, P.; Li, Y.; Qu, L.; Liu, X. Exploring the Effect of Ginsenoside Rh1 in a Sleep Deprivation-Induced Mouse Memory Impairment Model. Phytother. Res. 2017, 31, 763–770. [Google Scholar] [CrossRef]
- Huang, L.; Peng, Z.; Lu, C.; Chen, Y.; Lv, J.W.; Qin, M.; Liao, D.F.; Liu, X.M.; Shi, Z. Ginsenoside Rg1 alleviates repeated alcohol exposure-induced psychomotor and cognitive deficits. Chin. Med. 2020, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, S.K.; Seung, T.W.; Jin, D.E.; Guo, T.; Heo, H.J. Effect of Ginseng (Panax ginseng) Berry EtOAc Fraction on Cognitive Impairment in C57BL/6 Mice under High-Fat Diet Inducement. Evid. Based Complement. Altern. Med. 2015, 2015, 316527. [Google Scholar] [CrossRef]
- Pak, M.E.; Kim, Y.R.; Kim, H.N.; Ahn, S.M.; Shin, H.K.; Baek, J.U.; Choi, B.T. Studies on medicinal herbs for cognitive enhancement based on the text mining of Dongeuibogam and preliminary evaluation of its effects. J. Ethnopharmacol. 2016, 179, 383–390. [Google Scholar] [CrossRef]
- Tu, T.T.; Sharma, N.; Shin, E.J.; Tran, H.Q.; Lee, Y.J.; Nah, S.Y.; Tran, H.P.; Jeong, J.H.; Jeong, J.H.; Ko, S.K.; et al. Treatment with Mountain-Cultivated Ginseng Alleviates Trimethyltin-Induced Cognitive Impairments in Mice via IL-6-Dependent JAK2/STAT3/ERK Signaling. Planta Med. 2017, 83, 1342–1350. [Google Scholar] [CrossRef]
- Ramya, E.M.; Kumar, G.P.; Chandrasekhar, Y.; Anilakumar, K.R. Adaptogenic potential of ginsenosides against domoic acid-induced toxicity by regulating neuronal stress and kinate receptors: Ex vivo and in silico studies. J. Food Biochem. 2022, 46, e14089. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Lee, H.L.; Go, M.J.; Kim, T.Y.; Joo, S.G.; Lee, H.S.; Heo, H.J. Korean Red Ginseng Prevents the Deterioration of Lung and Brain Function in Chronic PM(2.5)-Exposed Mice by Regulating Systemic Inflammation. Int. J. Mol. Sci. 2023, 24, 13266. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Subaiea, G.M.; Eid, A.; Li, L.; Seeram, N.P.; Zawia, N.H. Pomegranate extract modulates processing of amyloid-beta precursor protein in an aged Alzheimer’s disease animal model. Curr. Alzheimer Res. 2014, 11, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Hosseini, M.; Mirzavi, F.; Amirahmadi, S.; Arab, F.L.; Rajabian, A. Punica granatum peel supplementation attenuates cognitive deficits and brain injury in rat by targeting the Nrf2-HO-1 pathway. Food Sci. Nutr. 2023, 11, 168–180. [Google Scholar] [CrossRef]
- Kumar, S.; Maheshwari, K.K.; Singh, V. Protective effects of Punica granatum seeds extract against aging and scopolamine induced cognitive impairments in mice. Afr. J. Tradit. Complement. Altern. Med. 2008, 6, 49–56. [Google Scholar] [CrossRef]
- Cambay, Z.; Baydas, G.; Tuzcu, M.; Bal, R. Pomegranate (Punica granatum L.) flower improves learning and memory performances impaired by diabetes mellitus in rats. Acta Physiol. Hung. 2011, 98, 409–420. [Google Scholar] [CrossRef]
- Ridzwan, N.; Jumli, M.N.; Baig, A.A.; Rohin, M.A.K. Pomegranate-derived anthocyanin regulates MORs-cAMP/CREB-BDNF pathways in opioid-dependent models and improves cognitive impairments. J. Ayurveda Integr. Med. 2020, 11, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Sreemantula, S.; Nammi, S.; Kolanukonda, R.; Koppula, S.; Boini, K.M. Adaptogenic and nootropic activities of aqueous extract of Vitis vinifera (grape seed): An experimental study in rat model. BMC Complement. Altern. Med. 2005, 5, 1. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Y.; Hong, Y.; Han, Y.E.; Oh, S.J.; Lee, B.; Choi, C.W.; Kim, M.S. The central administration of vitisin a, extracted from Vitis vinifera, improves cognitive function and related signaling pathways in a scopolamine-induced dementia model. Biomed. Pharmacother. 2023, 163, 114812. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Choi, Y.H.; Han, Y.E.; Oh, S.J.; Lee, A.; Lee, B.; Magnan, R.; Ryu, S.Y.; Choi, C.W.; Kim, M.S. Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model. Antioxidants 2021, 10, 835. [Google Scholar] [CrossRef]
- Rapaka, D.; Bitra, V.R.; Vishala, T.C.; Akula, A. Vitis vinifera acts as anti-Alzheimer’s agent by modulating biochemical parameters implicated in cognition and memory. J. Ayurveda Integr. Med. 2019, 10, 241–247. [Google Scholar] [CrossRef]
- Lakshmi, B.V.; Sudhakar, M.; Anisha, M. Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium-induced oxidative stress in rat brain. Neurotoxicology 2014, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Ghafarimoghadam, M.; Mashayekh, R.; Gholami, M.; Fereydani, P.; Shelley-Tremblay, J.; Kandezi, N.; Sabouri, E.; Motaghinejad, M. A review of behavioral methods for the evaluation of cognitive performance in animal models: Current techniques and links to human cognition. Physiol. Behav. 2022, 244, 113652. [Google Scholar] [CrossRef]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Miller, L.G. Herbal medicinals: Selected clinical considerations focusing on known or potential drug-herb interactions. Arch. Intern. Med. 1998, 158, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, P.L.; Katz, M.M.; Berman, N.; Itil, T.M.; Freedman, A.M.; Schatzberg, A.F. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA 1997, 278, 1327–1332. [Google Scholar] [CrossRef]
- Kanowski, S.; Herrmann, W.M.; Stephan, K.; Wierich, W.; Horr, R. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry 1996, 29, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, C.W.; Shi, J.; Lin, Z.X. Effects of Ginkgo biloba on dementia: An overview of systematic reviews. J. Ethnopharmacol. 2017, 195, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2019, 10, 1688. [Google Scholar] [CrossRef]
- Olson, I.R.; Jiang, Y.; Moore, K.S. Associative learning improves visual working memory performance. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 889–900. [Google Scholar] [CrossRef]
- Enquist, M.; Lind, J.; Ghirlanda, S. The power of associative learning and the ontogeny of optimal behaviour. R. Soc. Open Sci. 2016, 3, 160734. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr. Spatial Navigation (Water Maze) Tasks. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2009; Chapter 13. [Google Scholar] [PubMed]
- Murphy, G.G.; Rahnama, N.P.; Silva, A.J. Investigation of age-related cognitive decline using mice as a model system: Behavioral correlates. Am. J. Geriatr. Psychiatry 2006, 14, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, V.D.; Jech, R.; Ruzicka, E.; Nadel, L.; Kalina, M.; Stepankova, K.; Bures, J. Rat spatial memory tasks adapted for humans: Characterization in subjects with intact brain and subjects with selective medial temporal lobe thermal lesions. Physiol. Res. 2002, 51 (Suppl. S1), S49–S65. [Google Scholar] [CrossRef]
- Tucker, L.B.; Velosky, A.G.; McCabe, J.T. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci. Biobehav. Rev. 2018, 88, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Konishi, K.; McKenzie, S.; Etchamendy, N.; Roy, S.; Bohbot, V.D. Hippocampus-dependent spatial learning is associated with higher global cognition among healthy older adults. Neuropsychologia 2017, 106, 310–321. [Google Scholar] [CrossRef]
- Commission, E. Herbal Medicinal Products. Available online: https://health.ec.europa.eu/medicinal-products/herbal-medicinal-products_en#list-of-approved-substances-preparations-and-combinations (accessed on 27 May 2024).
- U.S. Food and Drug Administration. What is a Botanical Drug? Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/what-botanical-drug (accessed on 27 May 2024).
- Itil, T.M.; Eralp, E.; Ahmed, I.; Kunitz, A.; Itil, K.Z. The pharmacological effects of ginkgo biloba, a plant extract, on the brain of dementia patients in comparison with tacrine. Psychopharmacol. Bull. 1998, 34, 391–397. [Google Scholar]
- Yasui-Furukori, N.; Furukori, H.; Kaneda, A.; Kaneko, S.; Tateishi, T. The effects of Ginkgo biloba extracts on the pharmacokinetics and pharmacodynamics of donepezil. J. Clin. Pharmacol. 2004, 44, 538–542. [Google Scholar] [CrossRef]
- Canevelli, M.; Adali, N.; Kelaiditi, E.; Cantet, C.; Ousset, P.J.; Cesari, M.; Group, I.D. Effects of Gingko biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: Data from the ICTUS study. Phytomedicine 2014, 21, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Rowin, J.; Lewis, S.L. Spontaneous bilateral subdural hematomas associated with chronic Ginkgo biloba ingestion. Neurology 1996, 46, 1775–1776. [Google Scholar] [CrossRef]
- Galluzzi, S.; Zanetti, O.; Binetti, G.; Trabucchi, M.; Frisoni, G.B. Coma in a patient with Alzheimer’s disease taking low dose trazodone and Gingko biloba. J. Neurol. Neurosurg. Psychiatry 2000, 68, 679–680. [Google Scholar] [CrossRef]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: An emerging approach to determine the composition of herbal products. Comput. Struct. Biotechnol. J. 2013, 4, e201301007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, S.; Cai, Y.; Yang, Q.; Wang, Y.; Yu, X.; Sun, W.; Qiu, S.; Li, X.; Guo, Y.; et al. Decoding active compounds and molecular targets of herbal medicine by high-throughput metabolomics technology: A systematic review. Bioorg Chem. 2024, 144, 107090. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tian, Q.; Guo, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules 2024, 29, 2198. [Google Scholar] [CrossRef]
- Commisso, M.; Bianconi, M.; Di Carlo, F.; Poletti, S.; Bulgarini, A.; Munari, F.; Negri, S.; Stocchero, M.; Ceoldo, S.; Avesani, L.; et al. Multi-approach metabolomics analysis and artificial simplified phytocomplexes reveal cultivar-dependent synergy between polyphenols and ascorbic acid in fruits of the sweet cherry (Prunus avium L.). PLoS ONE 2017, 12, e0180889. [Google Scholar] [CrossRef]
- Wieland, L.S.; Feinberg, T.M.; Ludeman, E.; Prasad, N.K.; Amri, H. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, X.; Sun, J.; Su, E.; Cao, F.; Zhao, L. Study on Synergistic Anti-Inflammatory Effect of Typical Functional Components of Extracts of Ginkgo biloba Leaves. Molecules 2023, 28, 1377. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Liu, X.; Su, E.; Cao, F.; Zhao, L. Study on Synergistic Antioxidant Effect of Typical Functional Components of Hydroethanolic Leaf Extract from Ginkgo biloba In Vitro. Molecules 2022, 27, 439. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Fang, X.; Xu, J.; Jiang, Y.; Cao, F.; Zhao, L. Synergistic Effects of Ginkgolide B and Protocatechuic Acid on the Treatment of Parkinson’s Disease. Molecules 2020, 25, 3976. [Google Scholar] [CrossRef]
- Liu, X.G.; Cheng, C.Y.; Wang, J.X.; Luo, H.; Tu, L.F.; Lin, L.; Wu, B.; Wang, H.Y.; Liu, K.; Li, P.; et al. A metabolic exposure-oriented network regulation strategy for the identification of effective combination in the extract of Ginkgo biloba L. J. Pharm. Biomed. Anal. 2018, 149, 151–159. [Google Scholar] [CrossRef]
| Author, Year | Plant and Dosage | Part Used and Preparation | Cognitive Function | Neuropsychological Test | Study Design and Method | Main Results |
|---|---|---|---|---|---|---|
| Tohda et al., 2017 [1] | Dioscorea batatas (50 mg/day) for 12 weeks, followed by 6 weeks washing out, followed by other 12 weeks of treatment | Tuber (yam); The preparation process is not explained (Dioscorea capsules prepared by Shiratori Pharmaceutical, Tokyo, Japan) | LTM, WM, visual-spatial abilities, language, attention | RBANS: List Learning, Story Memory; Figure Copy, Line Orientation, Picture Naming, Semantic Fluency, Digit Span, Digit Symbol Coding, List Recall, List Recognition, Story Recall, Figure Recall; MMSE | Placebo-controlled, randomized, double-blind, crossover study. 28 healthy participants (17 were given yam extract for 12 weeks and, after 6 weeks washout period, placebo for 12 weeks. 11 were given placebo for 12 weeks and, after 6 weeks washout period, yam extract for 12 weeks) were tested before and after treatment | From before to after the 12-week treatment period the diosgenin-rich yam extract consumption led to a significant increase in the RBANS total score.). This increase was age-dependent (47–81 years group significantly increased compared to placebo). Regarding the cognitive subtests, diosgenin-rich yam extract was found to significantly improve semantic fluency |
| Elsabagh et al., 2005 [2] | Ginkgo biloba (120 mg) experiment 1: single dose; experiment 2: 6-week period daily administration | Leaves; The preparation process is not explained (Ginkgo tablets prepared by Lichtwer Pharma, Marlow, UK) | Sustained attention, MLT, WM, executive functions | CANTAB: Word Presentation, Picture Presentation, Intra Dimensional/Extra Dimensional, Stockings of Cambridge, Spatial Working Memory, Pattern Recognition Memory, Spatial Recognition Memory, Word Recall, Picture Recall, PASAT | Placebo-controlled double-blind design. Experiment 1: students (26 exp. group, 26 control group with placebo) were tested 4 h later. Experiment 2: students (20 exp. group, 20 control group with placebo) were tested at baseline and after 6 weeks | Experiment 1: The acute dose of Ginkgo significantly improved performance on PASAT and Pattern-Recognition Memory Task. Experiment 2: No effect of the treatment |
| Mix & Crews, 2000 [3] | Ginkgo biloba (180 mg/d) for 6 weeks | Leaves; The preparation process is not explained (G. biloba extract EGb 761 manifactured by Schwabe, Karlsruhe, Germany Pharmaceuticals, Gmbh, Karlsruhe, Germany) | Verbal and non-verbal LTM and WM, attention, visual discrimination, executive functions | MMSE, Stroop Color and Word Test; Trail Making Test (Parts A and B); WMS-R: Logical Memory I and II, Visual Reproduction I and II | Double-blind, fixed-dose, placebo-controlled, parallel-group experimental design. Elderly healthy participants (24 exp. group, 24 control group with placebo) were tested before and after 6 weeks | Exp. group exhibited significantly more improvement on Stroop Color and Word Test by the end of treatment as compared control group |
| Santos et al., 2003 [4] | Ginkgo biloba (80 mg/day) for 8 months | Leaves; The preparation process is not explained (Ginkgo extract was prepared by Magister Medicamentos Ltd.a, Sao Paulo, Brazil) | Verbal and non-verbal LTM and WM, language, logical reasoning, mathematical processing, visual-spatial abilities, attention, executive functions | WAIS-R: Information, Digit Span forward and backward, Vocabulary, Arithmetic, Comprehension, Similarities, Picture Arrangement, Picture Completion, Block Design, Object Assembly, Digit Symbol; WMS-R: Information, Orientation, Mental Control, Logical Memory, Verbal Paired Associates; Corsi Block-Tapping Test; Rey-Osterrieth Complex Figure Test; WCST; Toulouse Pieron Concentrated Attention; Verbal Free Recall | Double-blind study with placebo. Elderly healthy male participants (23 exp. group, 25 control group with placebo) were tested before and after 8 months | The exp. group compared to control group significantly improved performance on Corsi Block-Tapping Test, Toulouse Pieron Concentrated Attention, Verbal Free Recall (not in number of words but in errors); WAIS-R (Vocabulary, Arithmetic, Comprehension, Similarities, Block Design, Object Assembly, Digit Symbol); WMS-R (Mental Control, Verbal Paired Associates); Rey-Osterrieth Complex Figure Test (only in delayed retrieval); WCST (only mean number of non-perseverative errors) |
| Kennedy et al., 2004 [5] | Melissa officinalis (300 mg, 600 mg), 2 separate single doses | Leaves; dried leaves extracted up to exhaustion in a 30:70 methanol–water mixture. The liquid extract is evaporated and homogenized. Dried glucose syrup and colloidal anhydrous silicon dioxide (to 7% and 3% of the final dried weight, respectively) are added | Mathematical processing, visual discrimination, auditory processing, memory | DISS battery: Mathematical Processing, Visual Monitoring, Auditory Monitoring, and Memory Search tasks | Double-blind, placebo-controlled, randomized, balanced crossover experiment. 18 healthy volunteers received two separate single doses (300 mg, 600 mg) and a placebo, separated by a 7-day washout period. The battery was administered after each dose | Increase in the speed of Mathematical Processing task (no accuracy reduction) in 300-mg dose vs. placebo |
| Sayeed et al., 2013 [6] | Nigella sativa (500 mg) twice daily for 9 weeks | Seeds; seeds were crushed with mortar and pestle (60 min), then they were passed through a stainless steel screen (mesh size #30; 20 min) and filled into empty hard gelatin capsule shells (size #0) using a manual capsule filling machine (20 min) | LTM, verbal WM, visual-spatial abilities, visual discrimination, selective attention, information processing speed, executive functions | WMS-R: Logical Memory Test; Wais-R: Digit Span Test; Rey-Osterrieth Complex Figure Test; Letter Cancellation Test, Trail Making Test; Stroop Color and Word Test | Double-blind, placebo-controlled, randomized experiment. Elderly healthy volunteers (20 exp. group, 20 control group with placebo) were tested before and after 9 weeks | Differences in exp. group vs. control over time in the score of Logical Memory Test-I and II, total score of Digit Span, 30 min delayed-recall, percent score in Rey-Osterrieth Complex Figure Test, time taken to complete Letter Cancellation Test, time taken in Trail Making Test-A and test-B, Stroop Test (except Part W-Time) |
| Mazza et al., 2018 [7] | Olea europaea (extravirgin olive oil 20–30 g/day) for 1 year | Fruits; The preparation process is not explained (oil prepared by Opipari and Torchia Companies, Calabria, Italy) | Orientation, attention, mathematical processing, language, memory | MMSE; ADAS: ADAS-Cog subscale | Randomized study. Elderly healthy participants (55 exp. group with Mediterranean Diet + extravirgin olive oil, 55 control group with Mediterranean Diet alone) were tested at baseline and after 1 year | Exp. group compared to control group showed higher reduction of ADAS-cog scores (improved test) after 1 year (adjusted for food groups which were diferent between groups) |
| Lee et al., 2008 [8] | Panax ginseng (4.5 g/d, 9 g/d) for 12 weeks | Roots; The preparation process is not explained (powdered and encapsulated by Nonghyup Co., Seoul, Republic of Korea) | Memory, visual-spatial abilities, language, and orientation | MMSE; ADAS | Prospective open-label study. Alzheimer patients (58 exp. group, 39 control group, 9 higher dose group) were measured at baseline, after 4 weeks and after 12 weeks | Exp. group improved MMSE (after 12 weeks) and ADAS-cog subscale (after 4 and after 12 weeks) compared to control group. No differences between exp. group and higher dose group at 12 weeks |
| Petrou et al., 2021 [9] | Punica granatum (125 mg/day) | Seeds; The preparation process is not explained (a nano-formulation of pomegranate seed oil, produced by Granalix Bio Technologies Ltd., Jerusalem, Israel, was used) | Information processing speed, mathematical processing, verbal and non-verbal memory | PASAT; BICAMS: Symbol Digit Modalities Test, CVLT-II, Brief Visuospatial Memory Test | Randomized, double-blind, crossover clinical trial. 30 patients with multiple sclerosis (15 -group A- were given Pomenagrade supplemention for the first three months and then placebo pills for 3 months. Group B the opposite. Then the two groups received Pomenagrade supplemention for 6 additional months) were tested at baseline, after 3 months, after 6 months | CVLT-II significantly increased at 3-months in the group of patients who were treated with GranaGard, as compared to baseline |
| Lee et al., 2017 [10] | Vitis vinifera (72 g/day) for 6 months | Fruits; The preparation process is not explained (the dried-grape powder was provided by the California Table Grape Commission, Fresno, CA, USA) | LTM, WM, language, executive functions, information processing speed, visual-spatial abilities, attention | ADAS-Cog; MMSE; Hopkins Verbal Learning Test-Revised; Benton Visual Retention Test; Rey-Osterreith Complex Figure Test copy and delayed; Boston Naming Test; Letter Fluency; Category Fluency (animal naming); Stroop Color and Word Test; Trail Making Test (Part A and B); WCST; WAIS-III: Digital Symbol, Symbol Speed, Block Design, Symbol Search Total, Letter-Number Sequencing, Digital Span; WTAR | Prospective, placebo-controlled, double-blind randomized trial design. Subjects with mild decline in cognition (5 exp. group, 5 control group with placebo) were tested at baseline and after 6 months | No significant differences in scores on the neuropsychological battery of tests between the two groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piva, A.; Benvegnù, G.; Negri, S.; Commisso, M.; Ceccato, S.; Avesani, L.; Guzzo, F.; Chiamulera, C. Whole Plant Extracts for Neurocognitive Disorders: A Narrative Review of Neuropsychological and Preclinical Studies. Nutrients 2024, 16, 3156. https://doi.org/10.3390/nu16183156
Piva A, Benvegnù G, Negri S, Commisso M, Ceccato S, Avesani L, Guzzo F, Chiamulera C. Whole Plant Extracts for Neurocognitive Disorders: A Narrative Review of Neuropsychological and Preclinical Studies. Nutrients. 2024; 16(18):3156. https://doi.org/10.3390/nu16183156
Chicago/Turabian StylePiva, Alessandro, Giulia Benvegnù, Stefano Negri, Mauro Commisso, Sofia Ceccato, Linda Avesani, Flavia Guzzo, and Cristiano Chiamulera. 2024. "Whole Plant Extracts for Neurocognitive Disorders: A Narrative Review of Neuropsychological and Preclinical Studies" Nutrients 16, no. 18: 3156. https://doi.org/10.3390/nu16183156
APA StylePiva, A., Benvegnù, G., Negri, S., Commisso, M., Ceccato, S., Avesani, L., Guzzo, F., & Chiamulera, C. (2024). Whole Plant Extracts for Neurocognitive Disorders: A Narrative Review of Neuropsychological and Preclinical Studies. Nutrients, 16(18), 3156. https://doi.org/10.3390/nu16183156









