Aquilaria crassna Extract Exerts Neuroprotective Effect against Benzo[a]pyrene-Induced Toxicity in Human SH-SY5Y Cells: An RNA-Seq-Based Transcriptome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Extract Preparation
2.3. Cell Culture
2.4. Drug Treatment
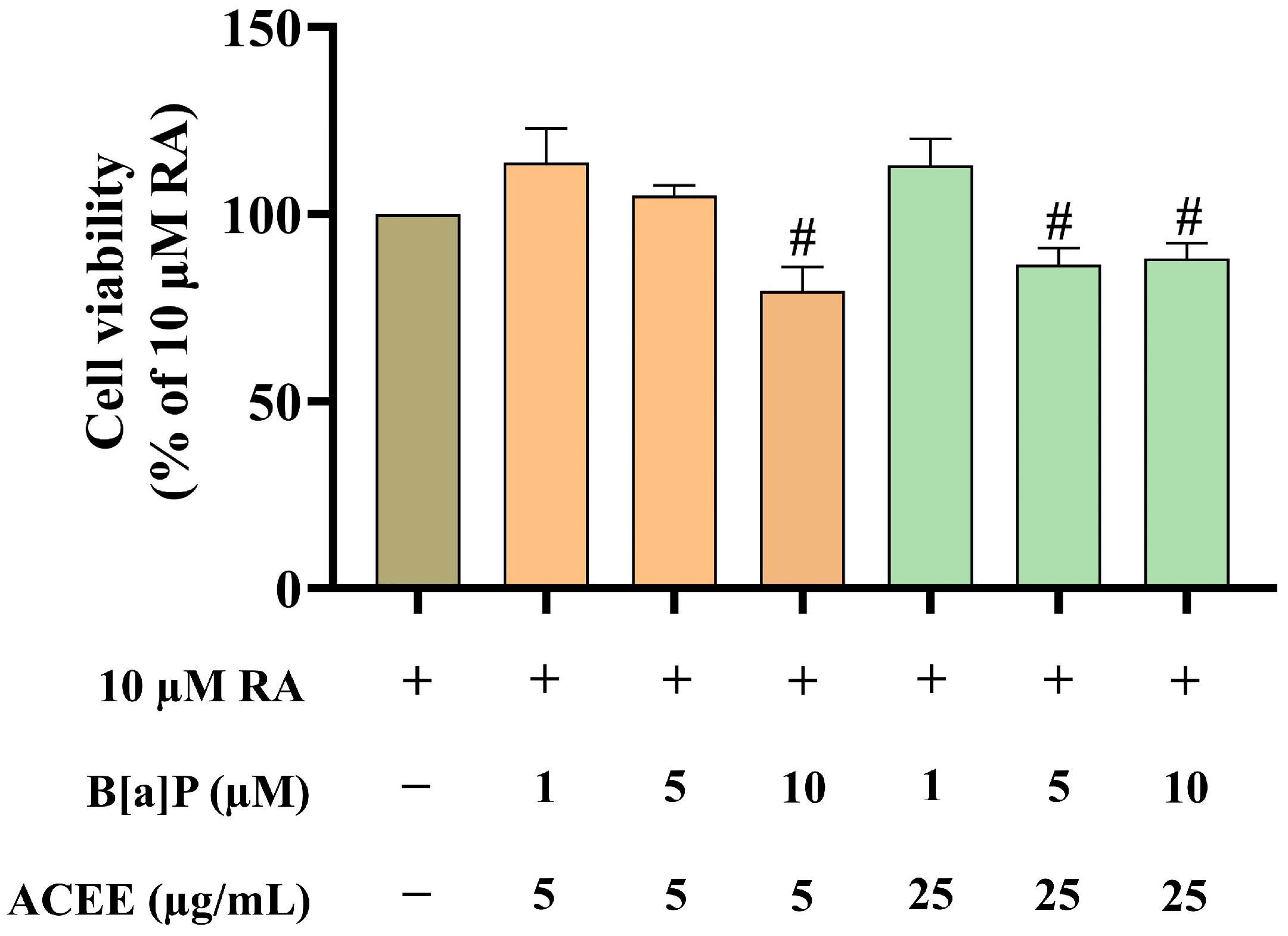
2.5. MTT Viability Assay
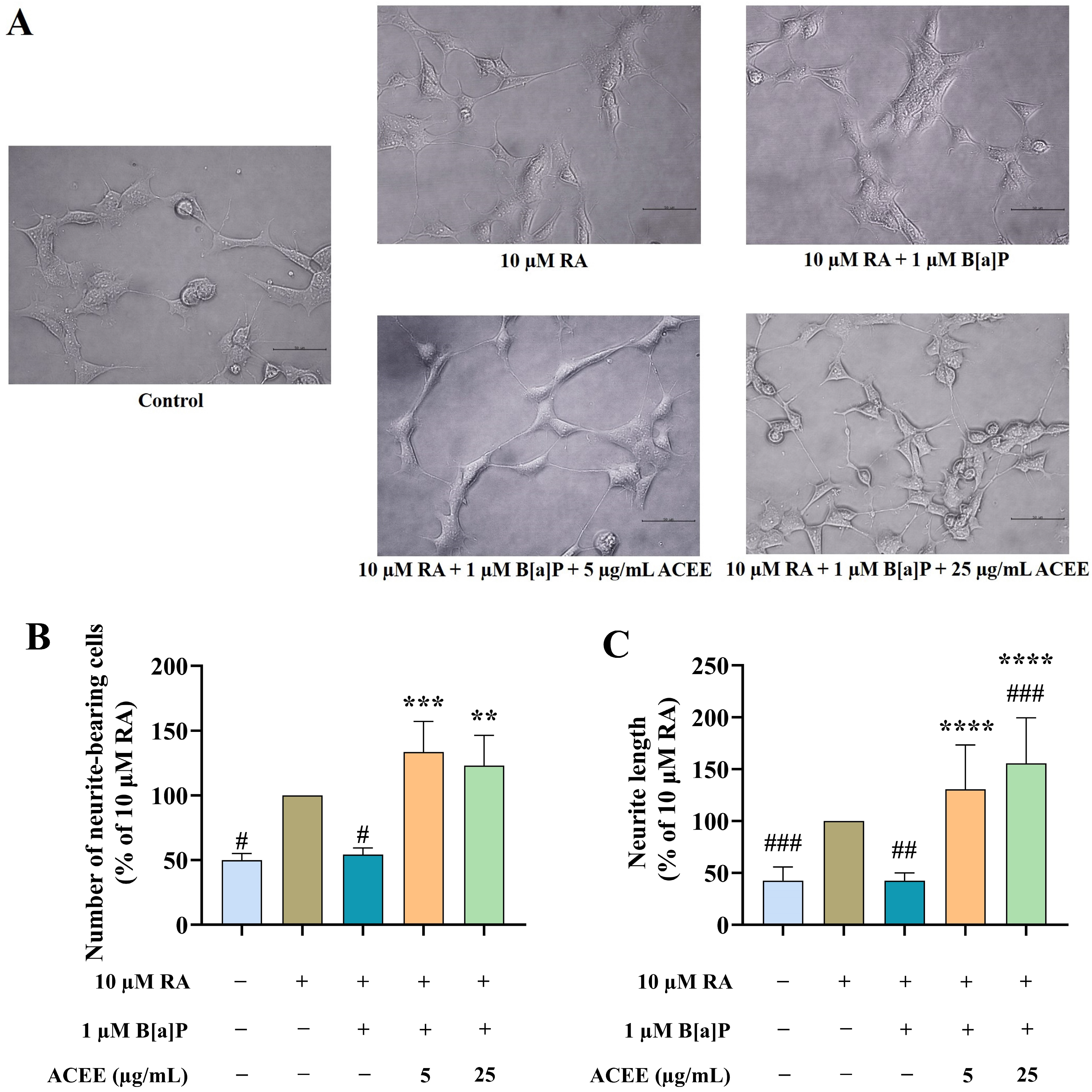
2.6. Neurite Outgrowth Assay
2.7. Analysis of Transcriptome Profiling
2.8. RT-qPCR Analysis
2.9. Western Blot Analysis
2.10. Molecular Docking
2.11. Data Analysis
3. Results
3.1. ACEE Promoted Neuronal Differentiation of B[a]P-Treated SH-SY5Y Cells
3.2. Transcriptome Profiling of SH-SY5Y Cells among Treatment Conditions
3.2.1. The Quality of RNA Sequencing Data
3.2.2. DEGs under Different Treatment Conditions
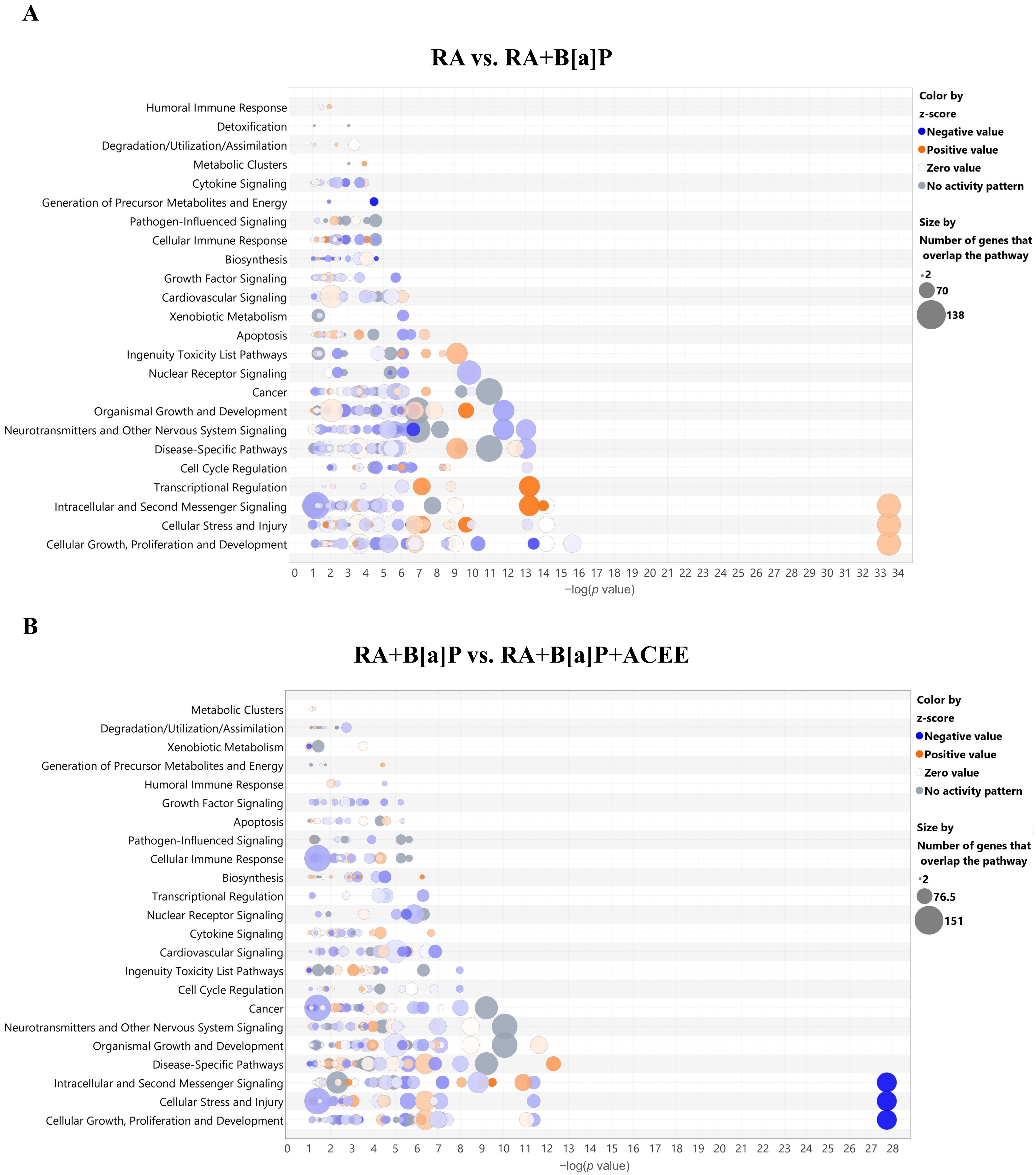
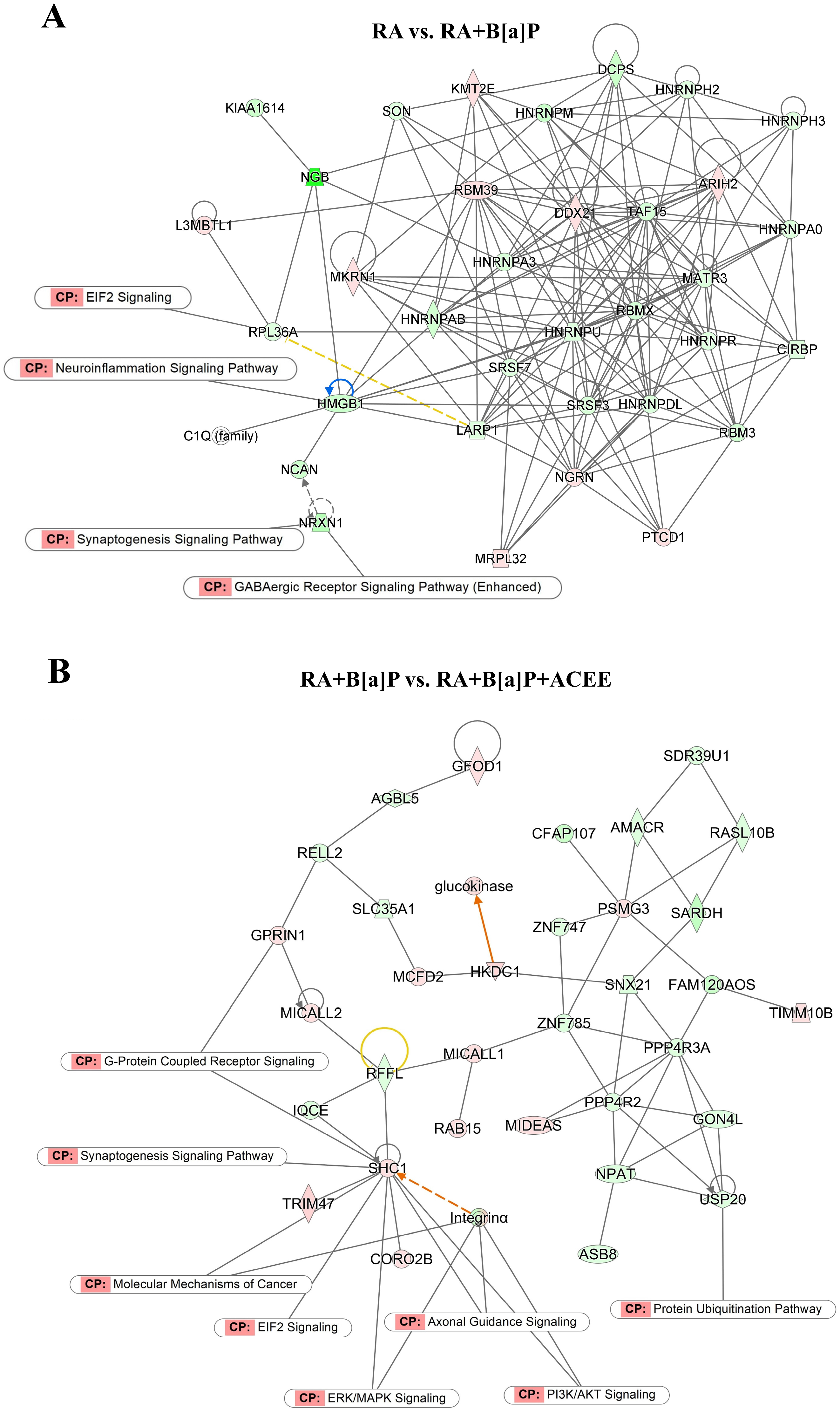
3.2.3. Biological Pathways and Network Analyses of DEGs
3.3. RT-qPCR Validation of Selected DEGs
3.4. Neuroprotective Effect of ACEE Is Mediated through Neuroglobin Up-Regulation via Akt- and ERK-Dependent Signaling Pathways
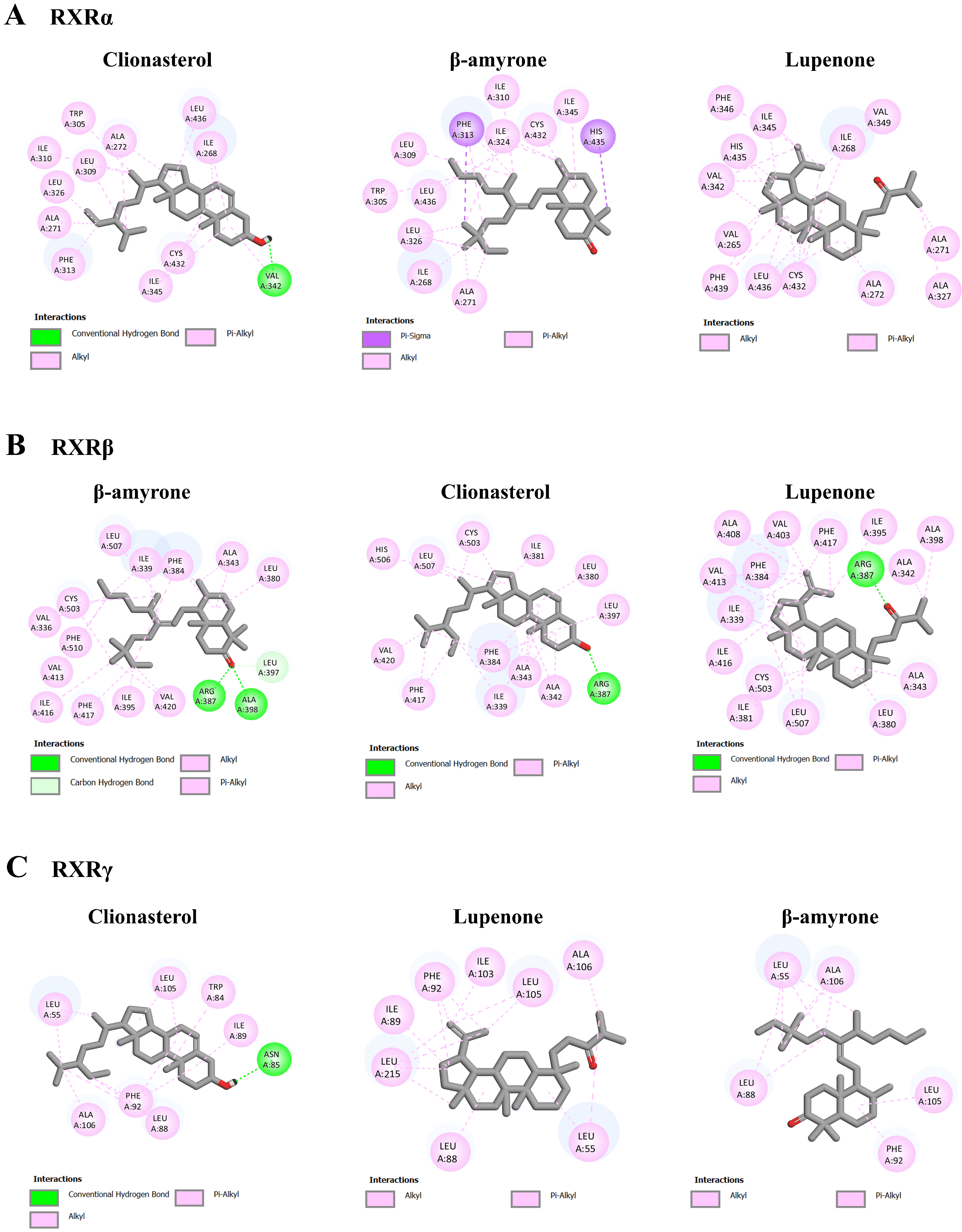
3.5. Molecular Interaction among ACEE-Derived Phytochemical Constituents and CXCR4, GDNF Family Receptor Alpha (GFRA), and Retinoid X Receptor (RXR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendt, T. Dysregulation of neuronal differentiation and cell cycle control in Alzheimer’s disease. In Ageing and Dementia Current and Future Concepts; Springer: Vienna, Austria, 2002; pp. 77–85. [Google Scholar]
- Katta, M.; Mathew, B.A.; Chaturvedi, P.; Ludhiadch, A.; Munshi, A. Advanced molecular therapies for neurological diseases: Focus on stroke, alzheimer’s disease, and parkinson’s disease. Neurol. Sci. 2023, 44, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, L.; Benfeito, S.; Fernandes, C.; Lima, I.; Peixoto, J.; Alves, C.; Machado, C.S.; Gaspar, A.; Borges, F.; Chavarria, D. Drug Development for Alzheimer’s and Parkinson’s Disease: Where Do We Go Now? Pharmaceutics 2024, 16, 708. [Google Scholar] [CrossRef] [PubMed]
- Lengyel-Zhand, Z.; Puentes, L.N.; Mach, R.H. PARkinson’s: From cellular mechanisms to potential therapeutics. Pharmacol. Ther. 2022, 230, 107968. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhoven, P.; de Wilde, A.; Vermunt, L.; Leferink, P.S.; Heetveld, S.; Cummings, J.; Scheltens, P.; Vijverberg, E.G. The Alzheimer’s disease drug development landscape. Alzheimer’s Res. Ther. 2021, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.K.; Wójcik, P.; Stępnicki, P.; Kaczor, A.A. Effects of small molecules on neurogenesis: Neuronal proliferation and differentiation. Acta Pharm. Sin. B 2023, 14, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Rosety, I.; Zagare, A.; Saraiva, C.; Nickels, S.; Antony, P.; Almeida, C.; Glaab, E.; Halder, R.; Velychko, S.; Rauen, T. Impaired neuron differentiation in GBA-associated Parkinson’s disease is linked to cell cycle defects in organoids. Npj Park. Dis. 2023, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Cory-Slechta, D.A.; Merrill, A.; Sobolewski, M. Air pollution–related neurotoxicity across the life span. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O. Neurotoxicity of polycyclic aromatic hydrocarbons: A systematic mapping and review of neuropathological mechanisms. Toxics 2022, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and male fertility: Effects of benzo-α-pyrene and resveratrol on human sperm function in vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene—Environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Guarnieri, G.; Becatti, M.; Squecco, R.; Comeglio, P.; Garella, R.; Tamburrino, L.; Marchiani, S.; Vignozzi, L.; Vannelli, G.B.; Maggi, M. Effects of benzo[a]pyrene on the reproductive axis: Impairment of kisspeptin signaling in human gonadotropin-releasing hormone primary neurons. Environ. Pollut. 2023, 317, 120766. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Eom, Y.; Yoon, H.; Lee, Y.; Lee, S.H. Benzo[a]pyrene represses synaptic vesicle exocytosis by inhibiting P/Q-type calcium channels in hippocampal neurons. Ecotoxicol. Environ. Saf. 2023, 263, 115301. [Google Scholar] [CrossRef] [PubMed]
- Do, E.; Byung, S. Benzopyrene inhibits differentiation of human SH-SY5Y neuroblastoma cells in culture. Clin. Toxicol. 2017, 7, 2161-0495. [Google Scholar]
- Dutta, K.; Ghosh, D.; Nazmi, A.; Kumawat, K.L.; Basu, A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS ONE 2010, 5, e9984. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.B.; Nayyar, T.; Ramesh, A.; Greenwood, M.; Inyang, F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal. Toxicol. 2000, 12, 511–535. [Google Scholar]
- Liu, D.; Zhao, Y.; Qi, Y.; Gao, Y.; Tu, D.; Wang, Y.; Gao, H.-M.; Zhou, H. Benzo(a)pyrene exposure induced neuronal loss, plaque deposition, and cognitive decline in APP/PS1 mice. J. Neuroinflamm. 2020, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Seidler, F.J. Benzo[a]pyrene impairs neurodifferentiation in PC12 cells. Brain Res. Bull. 2009, 80, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.H.-Y.; Kerr, P.G.; Abbas, P.; Salleh, H.M. Aquilaria spp.(agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Persoon, G.A.; van Beek, H.H. Growing ‘the wood of the gods’: Agarwood production in southeast Asia. In Smallholder Tree Growing for Rural Development and Environmental Services: Lessons from Asia; Springer: Dordrecht, The Netherlands, 2008; pp. 245–262. [Google Scholar]
- Wang, C.; Peng, D.; Liu, Y.; Yu, Z.; Guo, P.; Wei, J. Agarwood alcohol extract ameliorates isoproterenol-induced myocardial ischemia by inhibiting oxidation and apoptosis. Cardiol. Res. Pract. 2020, 2020, 3640815. [Google Scholar] [CrossRef]
- Kankaynar, M.; Ceyhun, H.A.; Baran, A.; Sulukan, E.; Yildirim, S.; Bolat, İ.; Toraman, E.; Nadaroglu, H.; Arslan, M.; Ceyhun, S.B. The anxiolytic and circadian regulatory effect of agarwood water extract and its effects on the next generation; zebrafish modelling. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 269, 109621. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J. Agarwood essential oil displays sedative-hypnotic effects through the GABAergic system. Molecules 2017, 22, 2190. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Hu, D.-B.; Zhang, L.; Xia, M.-Y.; Yan, H.; Li, X.-N.; Luo, J.-F.; Wang, Y.-S.; Yang, J.-H.; Wang, Y.-H. Neuroprotective compounds from the resinous heartwood of Aquilaria sinensis. Phytochemistry 2021, 181, 112554. [Google Scholar] [CrossRef]
- Supasuteekul, C.; Tadtong, S.; Putalun, W.; Tanaka, H.; Likhitwitayawuid, K.; Tengamnuay, P.; Sritularak, B. Neuritogenic and neuroprotective constituents from Aquilaria crassna leaves. J. Food Biochem. 2017, 41, e12365. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Sornkaew, N.; Warayanon, W.; Rangsinth, P.; Sillapachaiyaporn, C.; Vongthip, W.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Aquilaria crassna leaf extract ameliorates glucose-induced neurotoxicity in vitro and improves lifespan in caenorhabditis elegans. Nutrients 2022, 14, 3668. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Rangsinth, P.; Warayanon, W.; Leung, G.P.-H.; Chuchawankul, S.; Prasansuklab, A.; Tencomnao, T. Protective effect of Aquilaria crassna leaf extract against benzo[a]pyrene-induced toxicity in neuronal cells and Caenorhabditis elegans: Possible active constituent includes clionasterol. Nutrients 2023, 15, 3985. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Sukjamnong, S.; Theerasri, A.; Hu, V.W.; Sarachana, T.; Tencomnao, T. Transcriptomic analysis of glutamate-induced HT22 neurotoxicity as a model for screening anti-Alzheimer’s drugs. Sci. Rep. 2023, 13, 7225. [Google Scholar] [CrossRef]
- Wu, B.; Chien, E.Y.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef]
- Goodman, K.M.; Kjær, S.; Beuron, F.; Knowles, P.P.; Nawrotek, A.; Burns, E.M.; Purkiss, A.G.; George, R.; Santoro, M.; Morris, E.P. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 2014, 8, 1894–1904. [Google Scholar] [CrossRef]
- Sandmark, J.; Dahl, G.; Öster, L.; Xu, B.; Johansson, P.; Akerud, T.; Aagaard, A.; Davidsson, P.; Bigalke, J.M.; Winzell, M.S. Structure and biophysical characterization of the human full-length neurturin–GFRa2 complex: A role for heparan sulfate in signaling. J. Biol. Chem. 2018, 293, 5492–5508. [Google Scholar] [CrossRef]
- Gampe, R.T.; Montana, V.G.; Lambert, M.H.; Miller, A.B.; Bledsoe, R.K.; Milburn, M.V.; Kliewer, S.A.; Willson, T.M.; Xu, H.E. Asymmetry in the PPARγ/RXRα crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol. Cell 2000, 5, 545–555. [Google Scholar] [CrossRef]
- Svensson, S.; Östberg, T.; Jacobsson, M.; Norström, C.; Stefansson, K.; Hallén, D.; Johansson, I.C.; Zachrisson, K.; Ogg, D.; Jendeberg, L. Crystal structure of the heterodimeric complex of LXRα and RXRβ ligand-binding domains in a fully agonistic conformation. EMBO J. 2003, 22, 4625–4633. [Google Scholar] [CrossRef]
- Schuetz, A.; Min, J.R.; Loppnau, P.; Weigelt, J.; Sundstrom, M.; Edwards, A.M.; Arrowsmith, C.H.; Bochkarev, A.; Plotnikov, A.N. The Crystal Structure of Human Retinoic Acid Receptor RXR-Gamma Ligand-Binding Domain. Available online: https://www.rcsb.org/structure/2gl8 (accessed on 10 October 2023).
- Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. [Google Scholar] [CrossRef]
- Zhang, L.; Hua, Q.; Tang, K.; Shi, C.; Xie, X.; Zhang, R. CXCR4 activation promotes differentiation of human embryonic stem cells to neural stem cells. Neuroscience 2016, 337, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, H.; Zhang, X.; Zhao, S.; Zhou, Z.; Mu, X.; Zhao, C.; Teng, W. The role of SDF-1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front. Neurosci. 2017, 11, 590. [Google Scholar] [CrossRef]
- Yuelling, L.W.; Waggener, C.T.; Afshari, F.S.; Lister, J.A.; Fuss, B. Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain. Glia 2012, 60, 1605–1618. [Google Scholar] [CrossRef]
- Zhao, J.c.; Zhang, L.x.; Zhang, Y.; Shen, Y.f. The differential regulation of Gap43 gene in the neuronal differentiation of P19 cells. J. Cell. Physiol. 2012, 227, 2645–2653. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in axon regeneration: Implications for the treatment of neurodegenerative diseases. Front. Cell Dev. Biol. 2020, 8, 567537. [Google Scholar] [CrossRef]
- Li, Z.; Xie, J.; Fei, Y.; Gao, P.; Xie, Q.; Gao, W.; Xu, Z. GDNF family receptor alpha 2 promotes neuroblastoma cell proliferation by interacting with PTEN. Biochem. Biophys. Res. Commun. 2019, 510, 339–344. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, D.H.; An, J.Y.; Kang, D.; Park, J.W.; Hwang, E.M.; Seo, E.J.; Jang, I.H.; Ha, C.M.; Lee, B.J. NELL2 function in axon development of hippocampal neurons. Mol. Cells 2020, 43, 581–589. [Google Scholar] [PubMed]
- Shaker, M.R.; Kahtan, A.; Prasad, R.; Lee, J.-H.; Pietrogrande, G.; Leeson, H.C.; Sun, W.; Wolvetang, E.J.; Slonchak, A. Neural epidermal growth factor-like like protein 2 Is expressed in human oligodendroglial cell types. Front. Cell Dev. Biol. 2022, 10, 803061. [Google Scholar] [CrossRef] [PubMed]
- Monfrini, E.; Straniero, L.; Bonato, S.; Compagnoni, G.M.; Bordoni, A.; Dilena, R.; Rinchetti, P.; Silipigni, R.; Ronchi, D.; Corti, S. Neurofascin (NFASC) gene mutation causes autosomal recessive ataxia with demyelinating neuropathy. Park. Relat. Disord. 2019, 63, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chander, P.; Kennedy, M.J.; Winckler, B.; Weick, J.P. Neuron-specific gene 2 (NSG2) encodes an AMPA receptor interacting protein that modulates excitatory neurotransmission. Eneuro 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Haines, B.; Mao, X.; Xie, L.; Spusta, S.; Zeng, X.; Jin, K.; Greenberg, D.A. Neuroglobin expression in neurogenesis. Neurosci. Lett. 2013, 549, 3–6. [Google Scholar] [CrossRef]
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Hardt, O.; De Chevigny, A.; Coré, N.; Goebbels, S.; Seidenfaden, R.; Bosio, A.; Cremer, H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 1201–1206. [Google Scholar] [CrossRef]
- Tutukova, S.; Tarabykin, V.; Hernandez-Miranda, L.R. The role of neurod genes in brain development, function, and disease. Front. Mol. Neurosci. 2021, 14, 662774. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q.R.; Xiong, X.X.; Liu, J.M.; Lai, X.J.; Cheng, C.; Pan, F.; Chen, Y.; Yu, S.B.; Yu, A.C.H. Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol. Neurobiol. 2014, 49, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.J.; Buss, T.N. Accelerated differentiation in response to retinoic acid after retrovirally mediated gene transfer of GAP-43 into mouse neuroblastoma cells. Eur. J. Neurosci. 1992, 4, 910–916. [Google Scholar] [CrossRef]
- Rosskothen-Kuhl, N.; Illing, R.-B. G ap43 transcription modulation in the adult brain depends on sensory activity and synaptic cooperation. PLoS ONE 2014, 9, e92624. [Google Scholar] [CrossRef]
- Takahashi, N.; Onozuka, W.; Watanabe, S.; Wakasugi, K. Chimeric ZHHH neuroglobin acts as a cell membrane-penetrating inducer of neurite outgrowth. FEBS Open Bio 2017, 7, 1338–1349. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shum, A.K.; Platanias, L.C.; Miller, R.J.; Schiltz, G.E. Discovery and characterization of novel small-molecule CXCR4 receptor agonists and antagonists. Sci. Rep. 2016, 6, 30155. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Bespalov, M.M.; Wong, A.W.; Kambur, O.; Jokinen, V.; Lilius, T.O.; Suleymanova, I.; Karelson, G.; Rauhala, P.V.; Karelson, M. A novel small molecule GDNF receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front. Pharmacol. 2017, 8, 365. [Google Scholar] [CrossRef]
- Chitranshi, N.; Dheer, Y.; Kumar, S.; Graham, S.L.; Gupta, V. Molecular docking, dynamics, and pharmacology studies on bexarotene as an agonist of ligand-activated transcription factors, retinoid X receptors. J. Cell. Biochem. 2019, 120, 11745–11760. [Google Scholar] [CrossRef]
- Erain, R. Distribution of Benzo(a)pyrene in Discrete Regions of. Bull. Environ. Contam. Toxicol. 1985, 35, 500–504. [Google Scholar]
- Ramesh, A.; Inyang, F.; Hood, D.B.; Archibong, A.E.; Knuckles, M.E.; Nyanda, A.M. Metabolism, bioavailability, and toxicokinetics of Benzo(α)pyrenein F-344 rats following oral administration. Exp. Toxicol. Pathol. 2001, 53, 275–290. [Google Scholar] [CrossRef]
- Stephanou, P.; Konstandi, M.; Pappas, P.; Marselos, M. Alterations in central monoaminergic neurotrasmission induced by polycyclic aromatic hydrocarbons in rats. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 475–481. [Google Scholar] [CrossRef]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The role of polycyclic aromatic hydrocarbon–DNA adducts in inducing mutations in mouse skin. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2008, 649, 161–178. [Google Scholar] [CrossRef]
- Gao, D.; Wu, M.; Wang, C.; Wang, Y.; Zuo, Z. Chronic exposure to low benzo[a]pyrene level causes neurodegenerative disease-like syndromes in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 167, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Au, D.W.-T.; Mo, J.; Chen, L.; Cheung, K.-M.; Kong, R.Y.-C.; Seemann, F. Assessment of parental benzo[a]pyrene exposure-induced cross-generational neurotoxicity and changes in offspring sperm DNA methylome in medaka fish. Environ. Epigenetics 2022, 8, dvac013. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.-Y.; Tang, D.; Diaz, D.; Hoepner, L.; Barr, D.; Tu, Y.-H.; Camann, D. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect. 2006, 114, 1287–1292. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Targett, I.L.; Crompton, L.A.; Conway, M.E.; Craig, T.J. Differentiation of SH-SY5Y neuroblastoma cells using retinoic acid and BDNF: A model for neuronal and synaptic differentiation in neurodegeneration. Vitr. Cell. Dev. Biol.-Anim. 2024, 1–10. [Google Scholar] [CrossRef]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinäniemi, M.; Glusman, G.; Köglsberger, S.; Boyd, O. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Ong, J.H.-J.; Ong, W.-Y. Potential therapeutic applications for inhibitors of autotaxin, a bioactive lipid-producing lysophospholipase D, in disorders affecting the nervous system. ACS Chem. Neurosci. 2018, 9, 398–400. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Latchney, S.E.; Masiulis, I.; Zaccaria, K.J.; Lagace, D.C.; Powell, C.M.; McCasland, J.S.; Eisch, A.J. Developmental and adult GAP-43 deficiency in mice dynamically alters hippocampal neurogenesis and mossy fiber volume. Dev. Neurosci. 2014, 36, 44–63. [Google Scholar] [CrossRef]
- Zaccaria, K.J.; Lagace, D.C.; Eisch, A.J.; McCasland, J.S. Resistance to change and vulnerability to stress: Autistic-like features of GAP43-deficient mice. Genes Brain Behav. 2010, 9, 985–996. [Google Scholar] [CrossRef]
- Mehrotra, S.; Pierce, M.L.; Cao, Z.; Jabba, S.V.; Gerwick, W.H.; Murray, T.F. Antillatoxin-stimulated neurite outgrowth involves the brain-derived neurotrophic factor (BDNF)-tropomyosin related kinase B (TrkB) signaling pathway. J. Nat. Prod. 2022, 85, 562–571. [Google Scholar] [CrossRef]
- Lee, T.-y.; Cho, I.-S.; Bashyal, N.; Naya, F.J.; Tsai, M.-J.; Yoon, J.S.; Choi, J.-M.; Park, C.-H.; Kim, S.-S.; Suh-Kim, H. ERK regulates NeuroD1-mediated neurite outgrowth via proteasomal degradation. Exp. Neurobiol. 2020, 29, 189–206. [Google Scholar] [CrossRef]
- Pesce, A.; Bolognesi, M.; Bocedi, A.; Ascenzi, P.; Dewilde, S.; Moens, L.; Hankeln, T.; Burmester, T. Neuroglobin and cytoglobin. EMBO Rep. 2002, 3, 1146–1151. [Google Scholar] [CrossRef]
- Luyckx, E.; Van Acker, Z.P.; Ponsaerts, P.; Dewilde, S. Neuroglobin expression models as a tool to study its function. Oxidative Med. Cell. Longev. 2019, 2019, 5728129. [Google Scholar] [CrossRef]
- Ciccone, L.; Nencetti, S.; Socci, S.; Orlandini, E. Neuroglobin and neuroprotection: The role of natural and synthetic compounds in neuroglobin pharmacological induction. Neural Regen. Res. 2021, 16, 2353–2358. [Google Scholar] [PubMed]
- Li, S.; Deng, L.; Gong, L.; Bian, H.; Dai, Y.; Wang, Y. Upregulation of CXCR4 favoring neural-like cells migration via AKT activation. Neurosci. Res. 2010, 67, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Theus, M.H.; Wei, L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev. Growth Differ. 2006, 48, 513–523. [Google Scholar] [CrossRef]
- Odaka, H.; Numakawa, T.; Yoshimura, A.; Nakajima, S.; Adachi, N.; Ooshima, Y.; Inoue, T.; Kunugi, H. Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: Possible involvement of ERK and PI3K/Akt signaling in the neuronal differentiation. Neurosci. Res. 2016, 113, 28–36. [Google Scholar] [CrossRef]
- Semprich, C.I.; Davidson, L.; Amorim Torres, A.; Patel, H.; Briscoe, J.; Metzis, V.; Storey, K.G. ERK1/2 signalling dynamics promote neural differentiation by regulating chromatin accessibility and the polycomb repressive complex. PLoS Biol. 2022, 20, e3000221. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, T.; Li, L.; Wang, H.; Zhang, D.; Yang, H. Benzo(a)pyrene promotes Hep-G2 cell migration and invasion by upregulating phosphorylated extracellular signal-regulated kinase expression. Oncol. Lett. 2018, 15, 8325–8332. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, L.; He, W.; Yang, J.; Geng, C.; Chen, Y.; Liu, T.; Chen, H.; Li, Y. Benzo[a]pyrene promotes gastric cancer cell proliferation and metastasis likely through the Aryl hydrocarbon receptor and ERK-dependent induction of MMP9 and c-myc. Int. J. Oncol. 2016, 49, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, A.; Schulz, S.; Guyon, A.; Wu, D.-F.; Pfeiffer, M.; Odemis, V.; Höllt, V.; Stumm, R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J. Neurosci. 2008, 28, 4488–4500. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.T.; Hu, S.; Sheng, W.S.; Olson, J.M.; Cheeran, M.C.-J.; Chan, A.S.; Lokensgard, J.R.; Peterson, P.K. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Dev. Brain Res. 2004, 152, 159–169. [Google Scholar] [CrossRef]
- Lu, M.; Grove, E.A.; Miller, R.J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Pozas, E.; Ibáñez, C.F. GDNF and GFRα1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron 2005, 45, 701–713. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Alvarez, S.; Shankaranarayanan, P.; de Lera, A.R.; Bourguet, W.; Gronemeyer, H. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr. Top. Med. Chem. 2012, 12, 505–527. [Google Scholar] [CrossRef] [PubMed]


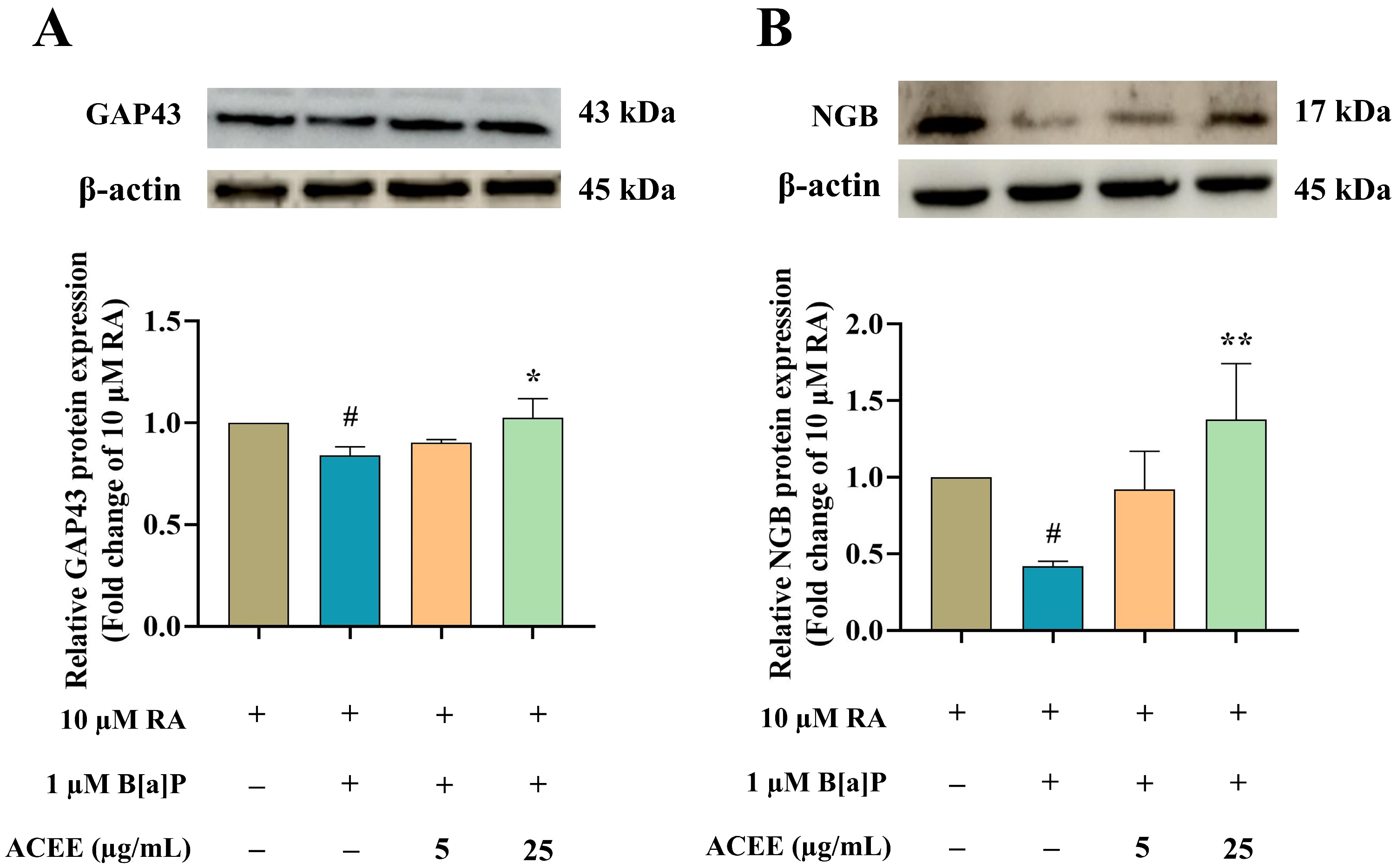
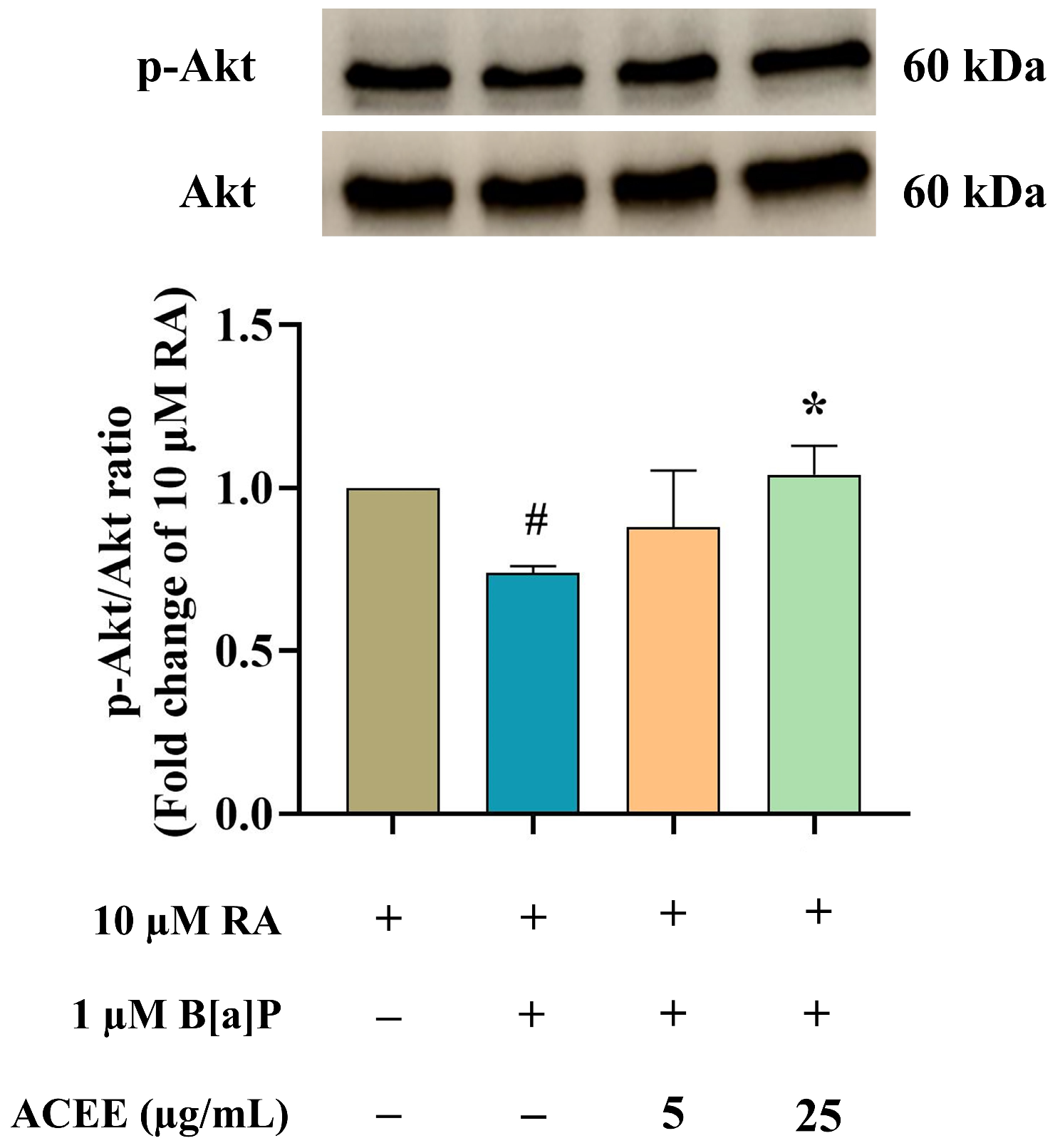

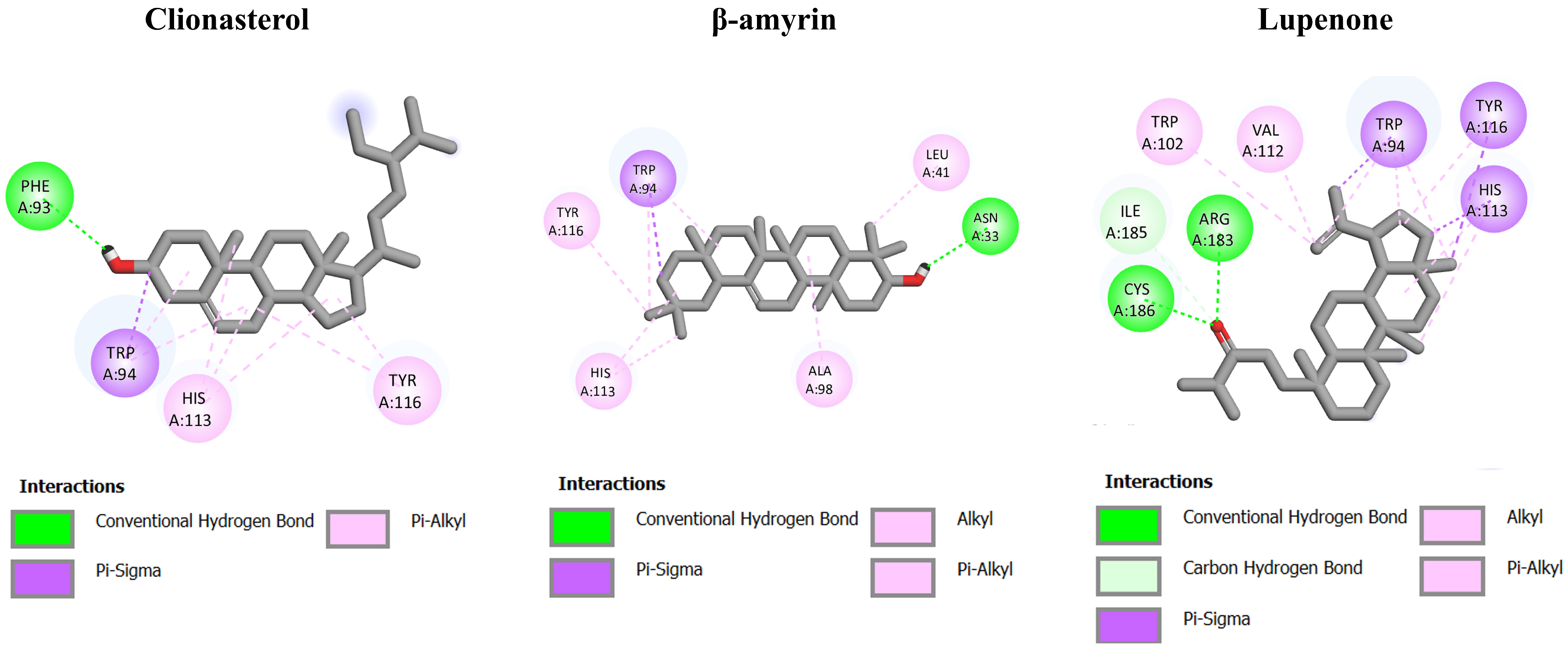
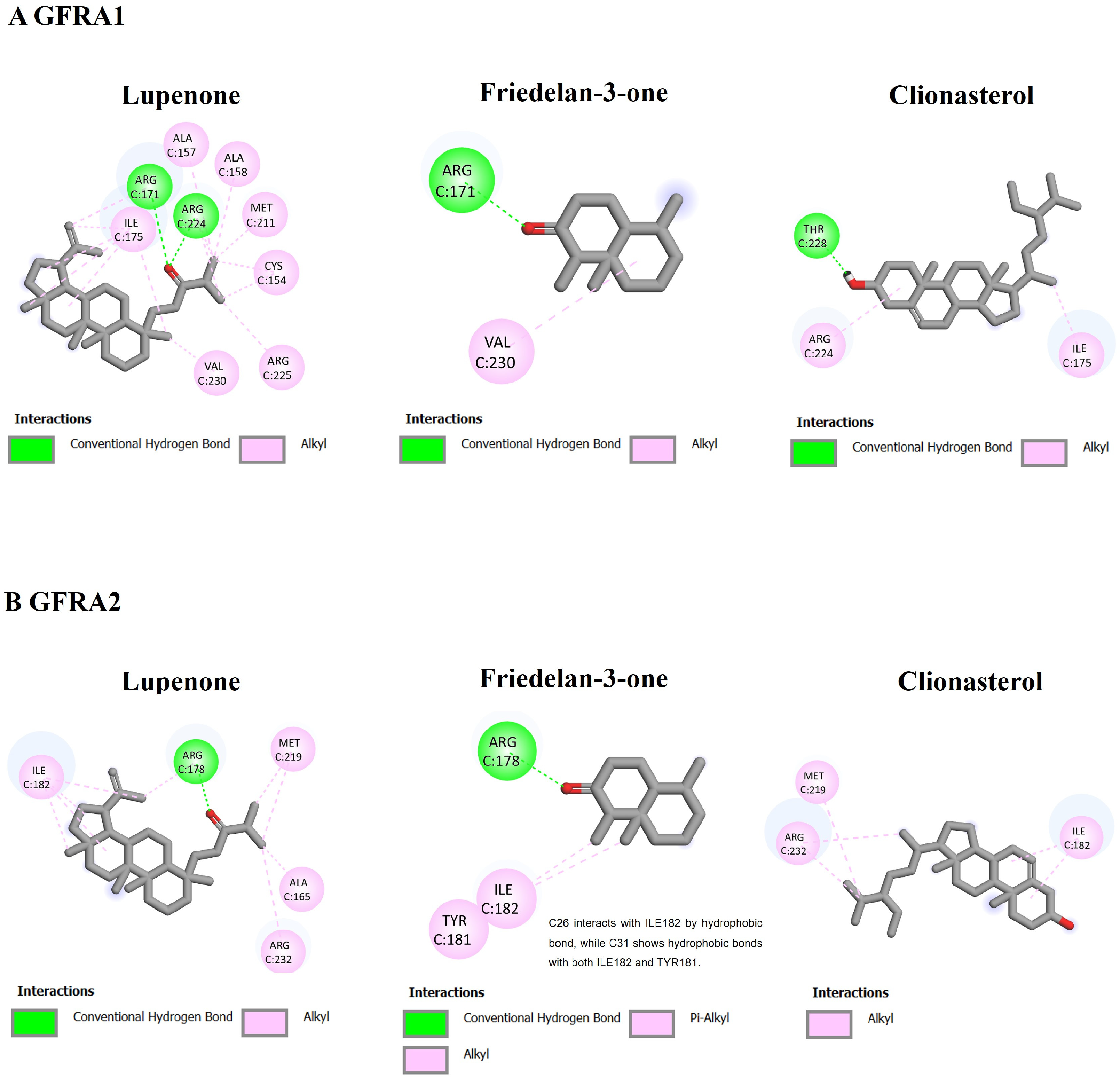
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | GGCATCCTCACCCTGAAGTA | AGCCTGGATAGCAACGTACA |
| GAP43 | AGCCTAAACAAGCCGATGTG | TCAGGCATGTTCTTGGTCAG |
| ENPP2 | CACTTTTCATCTGCGAGGGC | CAGCTTTCACCCCTTGCTTG |
| CXCR4 | TGGAGGGGATCAATATACACTTC | AGCGTGATGACAAAGAGGAGG |
| NGB | CCAGTTCTCCAGCCCAGAG | CTTCACACCCACTGCCCG |
| NELL2 | CAACATTTTGGCTAGGACAGAGA | CTGAGCAATAAATCCCTGGGG |
| NEUROD1 | AGAGGTCTGGAATGTGGCAG | GCGTAGGGATGGCTTATGGA |
| NSG2 | GTGAAGCTGAACAGTAACCCC | TCTGTTCCGGCTGATATTCC |
| GFRA2 | CCTTGGAGGTCTTGCAGGAG | GAGGTCACCGGCTCATAGG |
| BASP1 | ATCTTGGGGAAGGAGAAGGC | CCATGGGGTTGCTCTGTCTA |
| NFASC | CTTCAACATCGCCAAGGACC | GTTTTCCTTGGGCCACAGAG |
| Comparisons | Up-Regulated DEGs | Down-Regulated DEGs | Total DEGs |
|---|---|---|---|
| RA vs. RA + B[a]P | 2182 | 2007 | 4189 |
| RA + B[a]P vs. RA + B[a]P + ACEE | 1918 | 2195 | 4113 |
| Categories | p Value | No. of Genes |
|---|---|---|
| RA vs. RA + B[a]P | ||
| Canonical Pathways | ||
| EIF2 signaling | 3.31 × 10−34 | |
| mTOR signaling | 2.32 × 10−16 | |
| Regulation of eIF4 and p70S6K signaling | 6.57 × 10−15 | |
| Unfolded protein response | 9.93 × 10−15 | |
| Kinetochore metaphase signaling pathway | 3.54 × 10−14 | |
| Diseases/Disorders | ||
| Cancer | 8.21 × 10−17–5.25 × 10−277 | 4022 |
| Organismal injury and abnormalities | 9.07 × 10−17–5.25 × 10−277 | 4064 |
| Endocrine system disorders | 1.27 × 10−18–2.51 × 10−198 | 3502 |
| Gastrointestinal disease | 4.48 × 10−17–2.56 × 10−175 | 3624 |
| Neurological disease | 3.40 × 10−18–8.18 × 10−106 | 3014 |
| Biological Functions | ||
| Organismal survival | 6.09 × 10−18–8.13 × 10−80 | 1220 |
| Nervous system development and function | 5.33 × 10−17–1.04 × 10−40 | 868 |
| Organismal development | 9.52 × 10−17–1.04 × 10−40 | 1313 |
| Tissue development | 8.50 × 10−17–1.04 × 10−40 | 760 |
| Tissue morphology | 3.89 × 10−17–2.94 × 10−25 | 882 |
| RA + B[a]P vs. RA + B[a]P + ACEE | ||
| Canonical Pathways | ||
| EIF2 signaling | 1.89 × 10−28 | |
| Coronavirus pathogenesis pathway | 4.95 × 10−13 | |
| Insulin secretion signaling pathway | 2.34 × 10−12 | |
| Regulation of eIF4 and p70S6K signaling | 4.06 × 10−12 | |
| mTOR signaling | 8.47 × 10−12 | |
| Diseases/Disorders | ||
| Cancer | 1.09 × 10−17–6.11 × 10−283 | 3971 |
| Organismal injury and abnormalities | 3.84 × 10−17–6.11 × 10−283 | 4011 |
| Endocrine system disorders | 2.82 × 10−19–2.00 × 10−207 | 3496 |
| Gastrointestinal disease | 5.17 x10−21–1.42 × 10−189 | 3596 |
| Neurological disease | 3.84 × 10−17–2.26 × 10−116 | 2969 |
| Biological Functions | ||
| Organismal survival | 2.10 × 10−17–1.30 × 10−71 | 1181 |
| Nervous system development and function | 2.76 × 10−17–1.44 × 10−54 | 904 |
| Organismal development | 2.76 × 10−17–1.44 × 10−54 | 1582 |
| Tissue development | 8.08 × 10−18–1.44 × 10−54 | 1146 |
| Embryonic development | 1.42 × 10−17–7.73 × 10−36 | 1046 |
| Name | p Value | No. of Genes |
|---|---|---|
| RA vs. RA + B[a]P | ||
| Neurological Disease | ||
| Brain tumor | 8.63 × 10−95 | 2379 |
| Motor dysfunction or movement disorder | 6.52 × 10−48 | 637 |
| Neuromuscular disease | 7.54 × 10−34 | 526 |
| Cognitive impairment | 1.83 × 10−33 | 446 |
| Progressive neurological disorder | 6.37 × 10−21 | 485 |
| Nervous System Development and Function | ||
| Neuritogenesis | 2.04 × 10−40 | 407 |
| Development of neurons | 9.13 × 10−37 | 480 |
| Morphology of nervous system | 1.2 × 10−33 | 478 |
| Proliferation of neuronal cells | 3.56 × 10−30 | 276 |
| Dendritic growth/branching | 8.16 × 10−20 | 170 |
| RA + B[a]P vs. RA + B[a]P + ACEE | ||
| Neurological Disease | ||
| Brain tumor | 1.6 × 10−99 | 2364 |
| Cognitive impairment | 8.76 × 10−38 | 454 |
| Neuromuscular disease | 1.82 × 10−27 | 498 |
| Dementia | 2.57 × 10−17 | 287 |
| Tauopathy | 3.63 × 10−17 | 273 |
| Nervous System Development and Function | ||
| Neuritogenesis | 1.44 × 10−54 | 437 |
| Development of neurons | 4.84 × 10−54 | 522 |
| Proliferation of neuronal cells | 1.14 × 10−37 | 291 |
| Morphology of nervous system | 1.26 × 10−37 | 485 |
| Dendritic growth/branching | 4.82 × 10−23 | 176 |
| Genes | Changes Observed in Neuronal Differentiation | |
|---|---|---|
| CXCR4 | C-X-C motif chemokine receptor 4 | Up-regulation in neuronal differentiation [36] Up-regulation in neuronal regeneration following cerebral ischemia [37] |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 | Up-regulation in oligodendrocyte differentiation in the zebrafish hindbrain [38] |
| GAP43 | Growth-associated protein 43 | Up-regulation in neuronal differentiation [39] Up-regulation in axon regeneration for the treatment of NDs [40] |
| GFRA2 | GDNF family receptor alpha 2 | Up-regulation in the promotion of neuronal proliferation [41] |
| NELL2 | Neural EGFL-like 2 | Up-regulation in neuronal differentiation, polarization, and axon guidance [42,43] |
| NFASC | Neurofascin | Up-regulation in nervous system development and function of node of Ranvier [44] |
| NSG2 | Neuronal vesicle trafficking associated 2 | Up-regulation required in normal synapse formation and/or maintenance [45] |
| NGB | Neuroglobin | Up-regulation in the promotion of neurogenesis [46,47] |
| BASP1 | Brain-abundant membrane-attached signal protein 1 | Up-regulation in axon regeneration for the treatment of NDDs [40] |
| NEUROD1 | Neuronal differentiation 1 | Up-regulation in neuronal differentiation and brain development [48,49] |
| No. | CXCR4 | GFRA1 | GFRA2 | RXRα | RXRβ | RXRγ |
|---|---|---|---|---|---|---|
| 1 | Clionasterol (−9.20) | Lupenone (−7.66) | Lupenone (−6.86) | Clionasterol (−11.75) | β-amyrone (−12.08) | Clionasterol (−9.13) |
| 2 | β-amyrin (−8.69) | Friedelan-3-one (−5.75) | Friedelan-3-one (−6.52) | β-amyrone (−10.87) | Clionasterol (−11.39) | Lupenone (−8.12) |
| 3 | Lupenone (−8.69) | Clionasterol (−5.43) | Clionasterol (−6.15) | Lupenone (−9.37) | Lupenone (−11.33) | β-amyrone (−6.79) |
| Std * | NUCC-390 (−8.11) | BT13 (−5.31) | BT13 (−5.55) | 9-cis-retinoic acid (−11.46) | 9-cis-retinoic acid (−10.63) | 9-cis-retinoic acid (−7.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarachotanant, N.; Sukjamnong, S.; Rangsinth, P.; Chaikhong, K.; Sillapachaiyaporn, C.; Leung, G.P.-H.; Hu, V.W.; Sarachana, T.; Chuchawankul, S.; Tencomnao, T.; et al. Aquilaria crassna Extract Exerts Neuroprotective Effect against Benzo[a]pyrene-Induced Toxicity in Human SH-SY5Y Cells: An RNA-Seq-Based Transcriptome Analysis. Nutrients 2024, 16, 2727. https://doi.org/10.3390/nu16162727
Pattarachotanant N, Sukjamnong S, Rangsinth P, Chaikhong K, Sillapachaiyaporn C, Leung GP-H, Hu VW, Sarachana T, Chuchawankul S, Tencomnao T, et al. Aquilaria crassna Extract Exerts Neuroprotective Effect against Benzo[a]pyrene-Induced Toxicity in Human SH-SY5Y Cells: An RNA-Seq-Based Transcriptome Analysis. Nutrients. 2024; 16(16):2727. https://doi.org/10.3390/nu16162727
Chicago/Turabian StylePattarachotanant, Nattaporn, Suporn Sukjamnong, Panthakarn Rangsinth, Kamonwan Chaikhong, Chanin Sillapachaiyaporn, George Pak-Heng Leung, Valerie W. Hu, Tewarit Sarachana, Siriporn Chuchawankul, Tewin Tencomnao, and et al. 2024. "Aquilaria crassna Extract Exerts Neuroprotective Effect against Benzo[a]pyrene-Induced Toxicity in Human SH-SY5Y Cells: An RNA-Seq-Based Transcriptome Analysis" Nutrients 16, no. 16: 2727. https://doi.org/10.3390/nu16162727
APA StylePattarachotanant, N., Sukjamnong, S., Rangsinth, P., Chaikhong, K., Sillapachaiyaporn, C., Leung, G. P.-H., Hu, V. W., Sarachana, T., Chuchawankul, S., Tencomnao, T., & Prasansuklab, A. (2024). Aquilaria crassna Extract Exerts Neuroprotective Effect against Benzo[a]pyrene-Induced Toxicity in Human SH-SY5Y Cells: An RNA-Seq-Based Transcriptome Analysis. Nutrients, 16(16), 2727. https://doi.org/10.3390/nu16162727











