Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Cooking of Retail Cuts
2.3. Cut Dissections
2.4. Homogenization
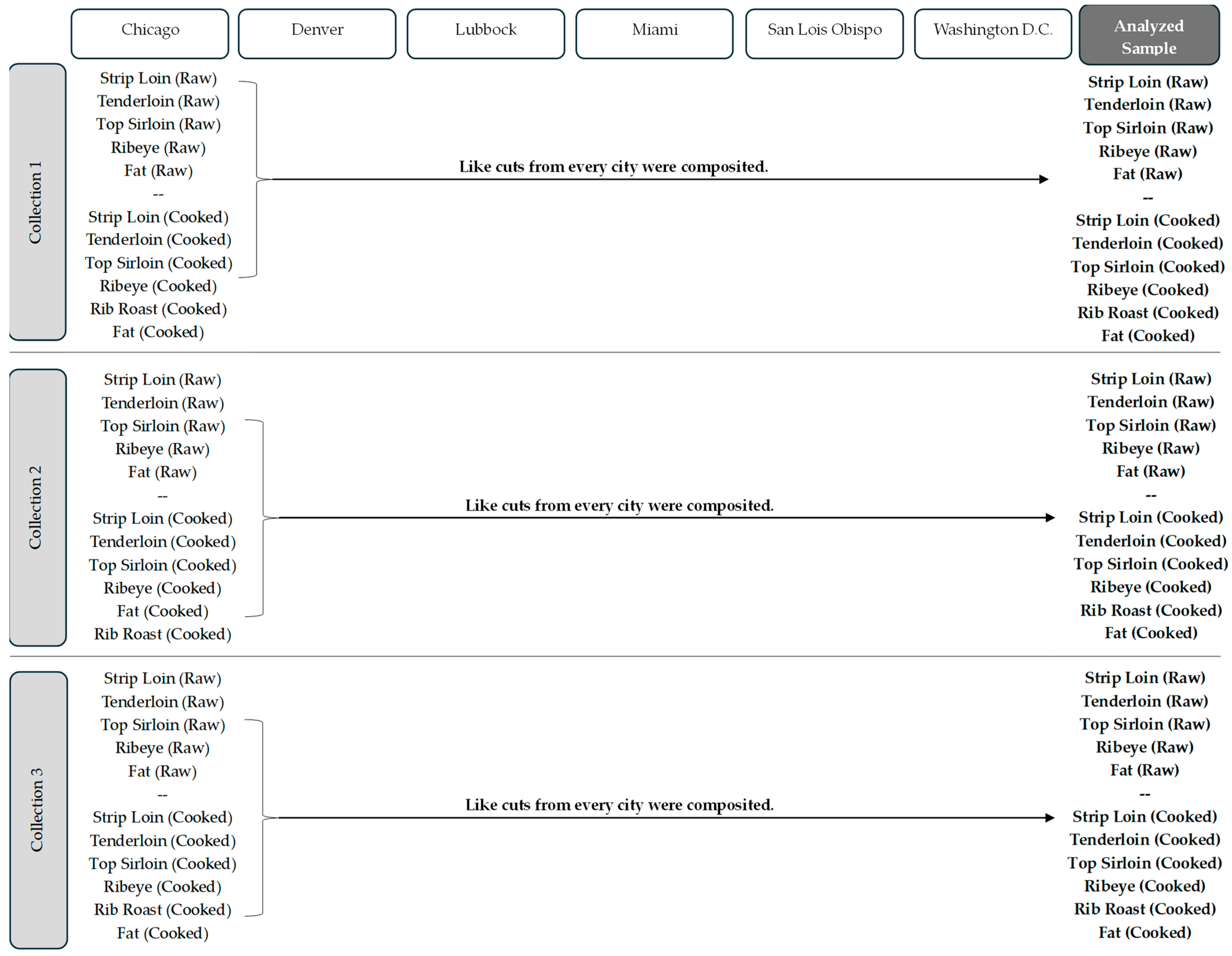
2.5. Compositing Scheme
2.6. Nutrient Analysis
2.6.1. Protein Analysis
2.6.2. Moisture Analysis
2.6.3. Ash Analysis
2.6.4. Lipid Analysis
2.6.5. Fatty Acid Analysis
2.6.6. Cholesterol Analysis
2.6.7. Mineral Analysis
2.6.8. Vitamin E
2.6.9. Vitamin D
2.6.10. Vitamin A
2.6.11. Vitamin K
2.6.12. Amino Acid Analysis
2.7. Total Caloric Value
2.8. Statistical Analysis
3. Results and Discussion
3.1. Separable Components
3.2. Nutrient Analysis of Separable Lean
3.3. Nutrient Analysis of Composited Fat Samples
3.4. Nutrient Labeling Claims
3.5. Fatty Acid Profile
3.6. Amino Acid Profile
3.7. Comparison to USDA Choice Beef Cuts
3.8. Total Caloric Valuee
3.9. Nutrient Loss and Retention Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, K.; Jensen, J.; Ryan, K.J.; Homco-Ryan, C.; McKeith, F.K.; Brewer, M.S. Consumer attitudes towards beef and acceptability of enhanced beef. Meat Sci. 2003, 65, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Behrends, J.M.; Goodson, K.J.; Koohmaraie, M.; Shackelford, S.D.; Wheeler, T.L.; Morgan, W.W.; Reagan, J.O.; Gwartney, B.L.; Wise, J.W.; Savell, J.W. Beef customer satisfaction: Factors affecting consumer evaluations of calcium chloride-injected top sirloin steaks when given instructions for preparation. J. Anim. Sci. 2005, 83, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Goodson, K.J.; Morgan, W.W.; Reagan, J.O.; Gwartney, B.L.; Courington, S.M.; Wise, J.W.; Savell, J.W. Beef customer satisfaction: Factors affecting consumer evaluations of clod steaks. J. Anim. Sci. 2002, 80, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Killinger, K.M.; Calkins, C.R.; Umberger, W.J.; Feuz, D.M.; Eskridge, K.M. Consumer sensory acceptance and value for beef steaks of similar tenderness, but differing in marbling level. J. Anim. Sci. 2004, 82, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.H.; O’Quinn, T.G.; Garmyn, A.J.; Legako, J.F.; Hunt, M.R.; Dinh, T.T.N.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Sensory evaluation of tender beef strip loin steaks of varying marbling levels and quality treatments. Meat Sci. 2015, 100, 24–31. [Google Scholar] [CrossRef] [PubMed]
- O’Quinn, T.G.; Brooks, J.C.; Polkinghorne, R.J.; Garmyn, A.J.; Johnson, B.J.; Starkey, J.D.; Rathmann, R.J.; Miller, M.F. Consumer assessment of beef strip loin steaks of varying fat levels. J. Anim. Sci. 2012, 90, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Savell, J.W.; Cross, H.R.; Carpenter, Z.L.; Murphey, C.E.; Davis, G.W.; Abraham, H.C.; Parrish, F.C.; Berry, B.W. Relationship of USDA quality grades to palatability of cooked beef. J. Food Qual. 1986, 10, 269–286. [Google Scholar] [CrossRef]
- Romans, J.R.; Costello, W.J.; Carlson, C.W.; Greaser, M.L.; Jones, K.W. The Meat We Eat, 13th ed.; Interstate Publishers Inc.: Prairie Village, KS, USA, 1994. [Google Scholar]
- Astrup, A.; Teicholz, N.; Magkos, F.; Bier, D.M.; Brenna, J.T.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; Yusuf, S.; et al. Dietary Saturated Fats and Health: Are the U.S. Guidelines Evidence-Based? Nutrients 2021, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, N.K.; McKillop, K.; Pehrsson, P.R.; Moshfegh, A.; Harnly, J.; Finley, J. USDA’s FoodData Central: What is it and why is it needed today? Am. J. Clin. Nutr. 2022, 115, 619–624. [Google Scholar] [CrossRef] [PubMed]
- FY23 Grade Volume Report. Meat Grading Reports Agricultural Marketing Service. Available online: https://www.ams.usda.gov/reports/meat-grading (accessed on 25 July 2024).
- Meadows, L. What’s Your Beef—Prime, Choice or Select? USDA. 28 January 2013. Available online: https://usda.gov/media/blog/2013/01/28/whats-your-beef-prime-choice-or-select#:~:text=Prime%20beef%20is%20produced%20from,as%20broiling%2C%20roasting%20or%20grilling (accessed on 25 July 2024).
- North American Meat Institute. The Meat Buyer’s Guide, 8th ed.; North American Meat Association: Washington, DC, USA, 2014. [Google Scholar]
- Acheson, R.; Woerner, D.; Martin, J.; Belk, K.; Engle, T.; Brown, T.; Brooks, J.; Luna, A.; Thompson, L.; Grimes, H.; et al. Nutrient database improvement project: Separable components and proximate composition of raw and cooked retail cuts from the beef loin and round. Meat Sci. 2015, 110, 236–244. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis; Official method 992.15; AOAC: Arlington, VA, USA, 1996.
- AOAC Official Methods of Analysis; Official method 950.46; AOAC: Arlington, VA, USA, 1950.
- AOAC Official Methods of Analysis; Official method 923.03; AOAC: Arlington, VA, USA, 2005.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis, 17th ed.; Official method 983.23; AOAC: Arlington, VA, USA, 1984.
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester (fame) synthesis: Application to wet meat, tissues, oils and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis; Official method 994.10; AOAC: Arlington, VA, USA, 2010.
- AOAC Official Methods of Analysis; Official method 985.35; AOAC: Arlington, VA, USA, 1988.
- AOAC Official Methods of Analysis; Official method 985.01; AOAC: Arlington, VA, USA, 1996.
- AOAC Official Methods of Analysis; Official method 999.10; AOAC: Arlington, VA, USA, 2005.
- AOAC Official Methods of Analysis; Official method 993.14; AOAC: Arlington, VA, USA, 1993.
- AOAC Official Methods of Analysis; Official method 2001.13; AOAC: Arlington, VA, USA, 2011.
- AOAC Official Methods of Analysis; Official method 2016.13; AOAC: Arlington, VA, USA, 2016.
- AOAC Official Methods of Analysis; Official method 999.15; AOAC: Arlington, VA, USA, 2003.
- Martin, J.; Brooks, J.; Thompson, L.; Savell, J.; Harris, K.; May, L.; Haneklaus, A.; Schutz, J.; Belk, K.; Engle, T.; et al. Nutrient database improvement project: The influence of USDA. Quality and yield grade on the separable components and proximate composition of raw and cooked retail cuts from the beef rib and plate. Meat Sci. 2013, 95, 486–494. [Google Scholar] [CrossRef] [PubMed]
- USDHHS. 2019. Nutrient Recommendations: Dietary Reference Intakes (DRI). National Institutes of Health. 2019. Available online: https://ods.od.nih.gov/HealthInformation/Dietary_Reference_Intakes.aspx (accessed on 20 September 2021).
- Hunt, M.; Legako, J.; Dinh, T.; Garmyn, A.; O’Quinn, T.; Corbin, C.; Rathmann, R.; Brooks, J.; Miller, M. Assessment of Volatile Compounds, Neutral and Polar Lipid Fatty Acids of Four Beef Muscles from USDA Choice and Select Graded Carcasses and Their Relationships with Consumer Palatability Scores and Intramuscular Fat Content. Meat Sci. 2016, 116, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E. Health effects of oleic acid and long chain n-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- McNeill, S.H.; Harris, K.B.; Field, T.G.; Van Elswyk, M.E. The evolution of lean beef: Identifying lean beef in today’s U.S. marketplace. Meat Sci. 2012, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Monounsaturated Fat; American Heart Association: Dallas, TX, USA, 2015; Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/monounsaturated-fats (accessed on 21 September 2021).
- U.S. Department of Agriculture, Agricultural Research Service. FoodData. Central, 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 15 May 2021).
- He, W.; Connolly, E.D.; Cross, H.R.; Wu, G. Dietary protein and amino acid intakes for mitigating sarcopenia in humans. Crit. Rev. Food Sci. Nutr. 2024, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.N.; To, K.V.; Schilling, M.W. Fatty Acid Composition of Meat Animals as Flavor Precursors. Meat Muscle Biol. 2021, 5, 34. [Google Scholar] [CrossRef]

| Item | Initial Cut Weight 2 (g) | Separable Lean 3 (%) | External Fat 4 (%) | Seam Fat 5 (%) | Refuse 6 (%) |
|---|---|---|---|---|---|
| Raw | |||||
| Strip Loin Steak | 752.0 ± 17.09 | 74.9 ± 4.64 | 12.2 ± 1.06 | 4.0 ± 0.77 | 2.7 ± 0.59 |
| Tenderloin Steak | 461.4 ± 38.15 | 95.9 ± 1.04 | 2.3 ± 0.56 | 1.3 ± 0.50 | - |
| Top Sirloin Steak | 583.1 ± 49.17 | 95.3 ± 0.82 | 3.6 ± 0.81 | 0.5 ± 0.28 | - |
| Ribeye Steak | 904.7 ± 45.39 | 74.9 ± 2.09 | 11.2 ± 1.07 | 13.1 ± 1.26 | - |
| Rib Roast | 1543.6 ± 78.23 | 71.6 ± 1.62 | 15.7 ± 1.68 | 12.2 ± 1.09 | - |
| Cooked | |||||
| Strip Loin Steak | 543.4 ± 22.14 | 81.7 ± 0.85 | 12.2 ± 0.77 | 1.8 ± 0.36 | 3.7 ± 0.60 |
| Tenderloin Steak | 313.1 ± 22.22 | 96.9 ± 1.03 | 0.9 ± 0.36 | 1.6 ± 0.53 | 0.4 ± 0.38 |
| Top Sirloin Steak | 419.1 ± 33.47 | 97.0 ± 0.65 | 1.9 ± 0.43 | 0.4 ± 0.17 | 0.5 ± 0.22 |
| Ribeye Steak | 616.2 ± 20.35 | 76.8 ± 1.22 | 11.2 ± 0.94 | 11.2± 0.97 | 0.2 ± 0.13 |
| Rib Roast | 1130.2 ± 66.71 | 73.7 ± 1.72 | 13.2 ± 1.16 | 12.3 ± 1.44 | 0.1 ± 0.12 |
| Item, Units | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Rib Roast 4 | SEM 5 | p-Value |
|---|---|---|---|---|---|---|
| Proximates | ||||||
| Protein, % | 21.7 | 21.8 | 23.2 | 21.9 | 0.484 | 0.17 |
| Lipid, % | 11.6 a | 9.2 b | 6.5 c | 12.7 a | 0.48 | <0.01 |
| Moisture, % | 65.9 b | 69.2 a | 70.1 a | 64.6 b | 0.39 | <0.01 |
| Ash, % | 0.9 b | 1.0 ab | 1.1 a | 0.8 c | 0.03 | <0.01 |
| Cholesterol, mg/100 g | 55.4 | 57.2 | 58.9 | 58.1 | 1.51 | 0.44 |
| Vitamins | ||||||
| Vitamin A (total), IU/100 g 6 | 0.33 | * | * | * | 16.7 | - |
| Vitamin D (total D2 and D3), IU/100 g 7 | * | * | * | * | - | - |
| Vitamin E, IU/kg 8 | * | * | * | * | - | - |
| Vitamin K1, µg/100 g | 0.93 | 0.50 | 0.13 | 0.83 | 0.314 | 0.33 |
| Vitamin B1 (Thiamin), mg/100 g | 0.08 | 0.14 | 0.12 | 0.15 | 0.021 | 0.23 |
| Vitamin B2 (Riboflavin), mg/100 g | 0.22 | 0.18 | 0.13 | 0.11 | 0.068 | 0.65 |
| Vitamin B3 (Niacin), mg/100 g | 4.89 | 5.85 | 4.42 | 4.37 | 0.753 | 0.51 |
| Vitamin B5 (Pantothenic Acid), mg/100 g | 0.42 | 0.55 | 0.57 | 0.53 | 0.067 | 0.42 |
| Vitamin B6 (Pyridoxine), µg/100 g | 0.10 b | 0.26 a | 0.20 ab | 0.32 a | 0.027 | <0.01 |
| Vitamin B12 (Cyanocobalamin), µg/100 g | 1.48 | 1.51 | 1.76 | 1.69 | 0.181 | 0.65 |
| Minerals | ||||||
| Potassium, mg/100 g | 289 a | 322 ab | 340 a | 272 b | 14.5 | 0.04 |
| Phosphorus, mg/100 g | 186 bc | 203 ab | 219 a | 170 c | 4.87 | <0.01 |
| Sodium, mg/100 g | 63.4 ab | 64.5 a | 62.5 ab | 60.1 b | 0.86 | 0.03 |
| Magnesium, mg/100 g | 21.8 c | 22.8 b | 23.7 a | 20.3 d | 0.167 | <0.01 |
| Calcium, mg/100 g | 2.79 | 2.57 | 3.72 | 2.42 | 0.372 | 0.14 |
| Zinc, mg/100 g | 2.30 b | 2.18 b | 2.47 ab | 2.68 a | 0.076 | <0.01 |
| Iron, mg/100 g | 1.31 | 1.46 | 1.57 | 1.39 | 0.139 | 0.63 |
| Copper, mg/100 g | 0.10 b | 0.15 a | 0.14 ab | 0.12 ab | 0.010 | 0.03 |
| Manganese, mg/100 g | ≤0.02 | ≤0.02 | ≤0.02 | ≤0.02 | - | - |
| Selenium, µg/100 g | 20.0 | 22.3 | 22.2 | 22.4 | 1.29 | 0.38 |
| Item, Units | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Ribeye Steak | Rib Roast | SEM 4 | p-Value |
|---|---|---|---|---|---|---|---|
| Proximates | |||||||
| Protein, % | 30.7 ab | 30.9 ab | 33.1 a | 30.2 ab | 29.2 b | 0.68 | 0.03 |
| Lipid, % | 14.0 ab | 12.1 bc | 9.2 c | 16.6 a | 17.0 a | 0.89 | <0.01 |
| Moisture, % | 54.7 ab | 57.2 ab | 58.5 a | 53.2 b | 53.8 b | 0.87 | <0.01 |
| Ash, % | 1.0 b | 1.3 a | 1.3 a | 0.9 b | 1.0 b | 0.03 | <0.01 |
| Cholesterol, mg/100 g | 77.4 | 82.9 | 85.8 | 81.7 | 76.7 | 3.37 | 0.34 |
| Vitamins | |||||||
| Vitamin A (total), IU/100 g 5 | * | 78.8 | * | * | 30.6 | 22.3 | 0.44 |
| Vitamin D (total D2 and D3), IU/100 g 6 | * | * | * | * | * | - | - |
| Vitamin E, IU/kg 7 | * | * | * | * | * | - | - |
| Vitamin K1, µg/100 g | 0.17 b | 2.27 a | 0.17 b | 0.47 ab | 1.07 ab | 0.445 | 0.04 |
| Vitamin B1 (Thiamin), mg/100 g | 0.13 | 0.11 | 0.13 | 0.13 | 0.17 | 0.026 | 0.64 |
| Vitamin B2 (Riboflavin), mg/100 g | 0.14 | 0.08 | 0.15 | 0.12 | 0.14 | 0.039 | 0.78 |
| Vitamin B3 (Niacin), mg/100 g | 6.64 a | 3.08 b | 6.38 a | 4.43 ab | 3.56 ab | 0.682 | 0.01 |
| Vitamin B5 (Pantothenic Acid), mg/100 g | 0.56 | 0.37 | 0.46 | 0.41 | 0.56 | 0.067 | 0.23 |
| Vitamin B6 (Pyridoxine), µg/100 g | 0.24 | 0.13 | 0.25 | 0.18 | 0.19 | 0.046 | 0.40 |
| Vitamin B12 (Cyanocobalamin), µg/100 g | 1.79 | 1.18 | 1.50 | 1.57 | 1.81 | 0.331 | 0.67 |
| Minerals | |||||||
| Potassium, mg/100 g | 310 | 363 | 308 | 342 | 310 | 19.0 | 0.22 |
| Phosphorus, mg/100 g | 222 bc | 244 ab | 270 a | 213 bc | 207 c | 7.3 | <0.01 |
| Sodium, mg/100 g | 66.2 | 72.4 | 69.9 | 71.4 | 68.3 | 2.78 | 0.56 |
| Magnesium, mg/100 g | 23.9 b | 26.1 a | 26.1 a | 22.2 bc | 21.3 c | 0.45 | <0.01 |
| Calcium, mg/100 g | 3.95 | 2.93 | 3.87 | 3.17 | 3.02 | 0.409 | 0.30 |
| Zinc, mg/100 g | 2.75 ab | 2.48 b | 2.88 ab | 2.99 a | 2.96 a | 0.087 | 0.01 |
| Iron, mg/100 g | 1.42 | 1.84 | 2.06 | 1.44 | 1.47 | 0.175 | 0.09 |
| Copper, mg/100 g | 0.14 | 0.16 | 0.28 | 0.14 | 0.12 | 0.081 | 0.67 |
| Manganese, mg/100 g | ≤0.02 | ≤0.02 | ≤0.02 | ≤0.02 | ≤0.02 | - | - |
| Selenium, µg/100 g | 29.4 | 31.6 | 33.2 | 29.3 | 29.7 | 2.12 | 0.64 |
| Item | Raw Beef Fat | Cooked Beef Fat | SEM | p-Value |
|---|---|---|---|---|
| Proximate Values | ||||
| Protein, % | 5.94 b | 8.62 a | 0.341 | 0.01 |
| Lipid, % | 70.9 | 70.3 | 0.531 | 0.48 |
| Moisture, % | 23.0 a | 18.5 b | 0.532 | <0.01 |
| Ash, % | 0.22 b | 0.48 a | 0.027 | <0.01 |
| Cholesterol, mg/g | 70.5 | 80.1 | 1.95 | 0.03 |
| Vitamins | ||||
| Vitamin A (total), IU/100 g 3 | * | 79.6 | 28.10 | - |
| Vitamin D (total D2 and D3), IU/100 g 4 | * | * | - | - |
| Vitamin E, IU/kg 5 | * | * | - | - |
| Vitamin K1, µg/mg | 0.40 b | 1.67 a | 0.655 | 0.02 |
| Vitamin B1 (Thiamin), mg/100 g | 0.12 | 0.11 | 0.029 | 0.82 |
| Vitamin B2 (Riboflavin), mg/100 g | 0.10 | 0.09 | 0.047 | 0.89 |
| Vitamin B3 (Niacin), mg/100 g | 4.93 a | 2.70 b | 0.518 | 0.04 |
| Vitamin B5 (Pantothenic Acid), mg/100 g | 0.47 | 0.49 | 0.161 | 0.91 |
| Vitamin B6 (Pyridoxine), µg/100 g | 0.22 | 0.15 | 0.075 | 0.55 |
| Vitamin B12 (Cyanocobalamin), µg/100 g | 1.40 | 1.21 | 0.332 | 0.71 |
| Minerals | ||||
| Potassium, mg/100 g | 132 b | 226 a | 18.1 | 0.02 |
| Phosphorus, mg/100 g | 54.9 b | 93.9 a | 1.50 | <0.01 |
| Sodium, mg/100 g | 35.2 b | 49.8 a | 2.96 | 0.03 |
| Magnesium, mg/100 g | 6.31 b | 12.1 a | 0.562 | <0.01 |
| Calcium, mg/100 g | 3.96 | 3.05 | 0.686 | 0.40 |
| Zinc, mg/100 g | 0.99 b | 1.39 a | 0.058 | <0.01 |
| Iron, mg/100 g | 0.69 b | 1.16 a | 0.042 | <0.01 |
| Copper, mg/100 g | 0.13 | 0.06 | 0.041 | 0.31 |
| Manganese, mg/100 g | ≤0.02 | ≤0.02 | - | - |
| Selenium, µg/100 g | 0.00 | 7.13 | 2.52 | 0.12 |
| Nutrient | Daily Value | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Ribeye Steak |
|---|---|---|---|---|---|
| Protein | 50 g | 43.4 E | 43.6 E | 46.4 E | 43.8 E |
| Thiamin (B1) | 1.2 mg | 6.7 | 11.7 G | 10.0 G | 12.5 G |
| Riboflavin (B2) | 1.3 µg | 16.9 G | 13.8 G | 10.0 G | 8.5 |
| Niacin (B3) | 16 mg | 30.6 E | 36.6 E | 27.6 E | 27.3 E |
| Vitamin B6 | 1.7 mg | 5.9 | 15.3 G | 11.8 G | 18.8 G |
| Vitamin B12 | 2.4 µg | 61.7 E | 62.9 E | 73.3 E | 70.4 E |
| Selenium | 55 µg | 36.4 E | 42.4 E | 40.4 E | 40.7 E |
| Phosphorus | 1250 mg | 14.9 G | 16.2 G | 17.5 G | 13.6 G |
| Zinc | 11 mg | 20.9 E | 19.8 G | 22.5 E | 24.4 E |
| Iron | 18 mg | 7.2 | 8.1 | 8.7 | 7.7 |
| Fatty Acid, g/100 g | Cooked Beef Fat | Raw Beef Fat | SEM | p-Value 3 |
|---|---|---|---|---|
| Saturated Fatty Acids | 34.03 | 33.22 | 0.841 | 0.53 |
| 10:0 | 0.03 | 0.03 | 0.002 | 0.60 |
| 12:0 | 0.05 | 0.05 | 0.003 | 0.39 |
| 14:0 | 2.33 | 2.32 | 0.086 | 0.95 |
| 15:0 | 0.37 | 0.36 | 0.024 | 0.89 |
| 16:0 | 18.92 | 18.4 | 0.516 | 0.51 |
| 17:0 | 1.00 | 0.96 | 0.053 | 0.63 |
| 18:0 | 11.16 | 10.93 | 0.212 | 0.49 |
| 19:0 | 0.03 | 0.03 | <0.001 | 0.28 |
| 20:0 | 0.13 | 0.13 | 0.005 | 0.96 |
| 22:0 | 0.01 | 0.01 | 0.002 | 0.88 |
| 24:0 | 0.01 | 0.01 | <0.001 | 0.42 |
| Monounsaturated Fatty Acids | 32.21 | 31.29 | 0.726 | 0.42 |
| 14:1n5 | 0.65 | 0.63 | 0.027 | 0.73 |
| 16:1 trans | 0.05 | 0.06 | 0.009 | 0.54 |
| 16:1n7 | 2.04 | 1.95 | 0.047 | 0.25 |
| 17:1 | 0.01 | 0.01 | 0.001 | 0.59 |
| 18:1 trans | 3.29 | 3.19 | 0.192 | 0.71 |
| 18:1n9 | 25.35 | 24.59 | 0.483 | 0.33 |
| 18:1n7 | 0.21 | 0.22 | 0.006 | 0.38 |
| 19:1 | 0.01 | 0.01 | 0.001 | 0.46 |
| 20:1n5 | 0.00 | 0.04 | 0.029 | 0.37 |
| 20:1n8 | 0.08 | 0.08 | 0.002 | 0.69 |
| 20:1n11 | 0.47 | 0.47 | 0.02 | 1.00 |
| 24:1n9 | 0.02 | 0.02 | 0.001 | 0.62 |
| Polyunsaturated Fatty Acids | 2.03 | 1.93 | 0.139 | 0.62 |
| 18:2 trans | 0.08 | 0.08 | 0.039 | 0.97 |
| 18:3n6 | 0.18 | 0.18 | 0.004 | 0.93 |
| 18:2n6 | 1.56 | 1.46 | 0.101 | 0.51 |
| 20:2 | 0.03 | 0.03 | 0.001 | 0.24 |
| 20:3n6 | 0.06 | 0.06 | 0.002 | 0.62 |
| 20:3n3 | 0.01 | 0.00 | 0.001 | 0.41 |
| 20:4n6 | 0.04 | 0.03 | 0.003 | 0.21 |
| 22:4 | 0.02 | 0.02 | 0.001 | 0.62 |
| 22:5n3 | 0.04 | 0.05 | 0.006 | 0.68 |
| 22:6n3 | 0.02 | 0.02 | 0.007 | 0.66 |
| n-3 Fatty Acids | 0.07 | 0.08 | 0.008 | 0.60 |
| n-6 Fatty Acids | 1.83 | 1.72 | 0.105 | 0.50 |
| n-6:n-3 | 26 | 22 | -- | -- |
| Fatty Acid, g/100 g | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Rib Roast 4 | SEM | p-Value |
|---|---|---|---|---|---|---|
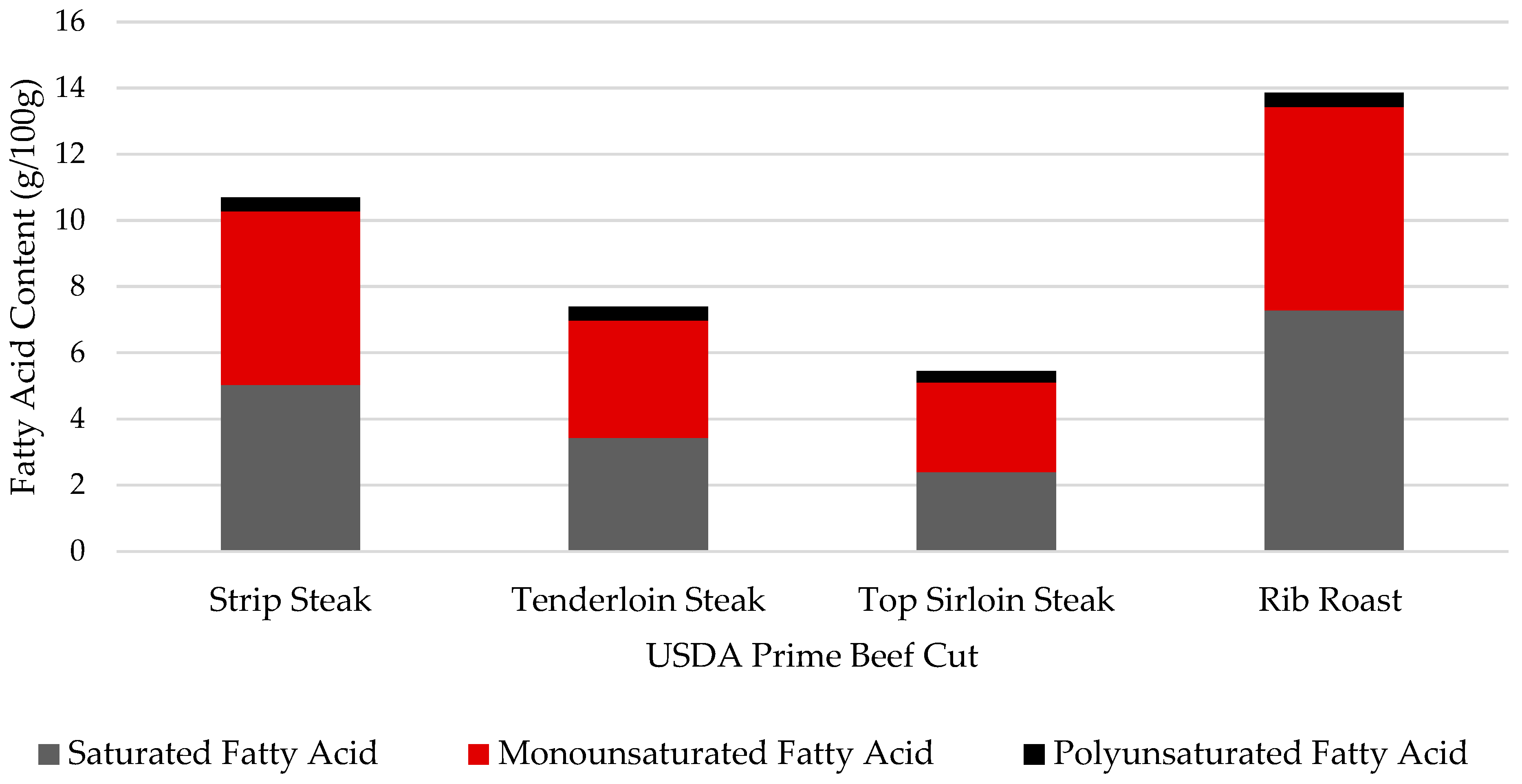
| Saturated Fatty Acids | 5.03 ab | 3.43 ab | 2.39 b | 7.28 a | 0.900 | 0.02 |
| 10:0 | 0.01 a | 0.00 b | 0.00 c | 0.01 a | <0.001 | <0.01 |
| 12:0 | 0.01 a | 0.01 b | 0.00 c | 0.01 a | <0.001 | <0.01 |
| 14:0 | 0.36 ab | 0.23 bc | 0.15 c | 0.45 a | 0.036 | <0.01 |
| 15:0 | 0.07 | 0.05 | 0.04 | 0.08 | 0.011 | 0.14 |
| 16:0 | 3.07 ab | 1.97 ab | 1.40 b | 4.37 a | 0.536 | 0.02 |
| 17:0 | 0.13 | 0.09 | 0.06 | 0.21 | 0.033 | 0.07 |
| 18:0 | 1.38 ab | 1.05 ab | 0.72 b | 2.13 a | 0.288 | 0.04 |
| 20:0 | 0.01 bc | 0.02 ab | 0.01 c | 0.02 a | 0.001 | <0.01 |
| Monounsaturated Fatty Acids | 5.25 a | 3.54 b | 2.71 b | 6.14 a | 0.342 | <0.01 |
| 14:1n5 | 0.11 a | 0.06 b | 0.04 c | 0.10 a | 0.003 | <0.01 |
| 16:1 trans | 0.01 | 0.00 | 0.00 | 0.01 | 0.001 | 0.10 |
| 16:1n7 | 0.40 a | 0.21 b | 0.17 b | 0.38 a | 0.012 | <0.01 |
| 17:1 | 0.04 | 0.07 | 0.05 | 0.04 | 0.023 | 0.75 |
| 18:1 trans | 0.35 ab | 0.29 bc | 0.21 c | 0.46 a | 0.030 | <0.01 |
| 18:1n9 | 4.19 ab | 2.74 bc | 2.10 c | 5.00 a | 0.337 | <0.01 |
| 18:1n7 | 0.08 | 0.11 | 0.09 | 0.08 | 0.033 | 0.86 |
| 20:1n8 | 0.01 | 0.00 | 0.00 | 0.01 | 0.002 | 0.10 |
| 20:1n11 | 0.05 a | 0.05 a | 0.04 b | 0.07 a | 0.004 | <0.01 |
| Polyunsaturated Fatty Acids | 0.42 | 0.43 | 0.35 | 0.43 | 0.023 | 0.09 |
| 18:2 trans | 0.02 | 0.01 | 0.01 | 0.01 | 0.004 | 0.20 |
| 18:3n6 | 0.02 ab | 0.02 bc | 0.01 c | 0.03 a | 0.002 | <0.01 |
| 18:2n6 | 0.30 ab | 0.31 a | 0.24 b | 0.30 ab | 0.015 | 0.04 |
| 20:2 | 0.00 ab | 0.00 ab | 0.00 b | 0.01 a | <0.001 | 0.01 |
| 20:3n6 | 0.01 | 0.01 | 0.01 | 0.02 | 0.005 | 0.51 |
| 20:4n6 | 0.05 ab | 0.05 a | 0.05 ab | 0.04 b | 0.003 | 0.03 |
| 22:4 | 0.01 | 0.00 | 0.00 | 0.01 | 0.001 | 0.10 |
| 22:5n3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.49 |
| n-3 Fatty Acids | 0.01 | 0.02 | 0.02 | 0.02 | 0.001 | 0.18 |
| n-6 Fatty Acids | 0.38 | 0.39 | 0.31 | 0.38 | 0.019 | 0.07 |
| n-6:n-3 | 38 | 20 | 16 | 19 | -- | -- |
| Fatty Acid, g/100 g | Strip Loin Steak | Tender-Loin Steak | Top Sirloin Steak | Ribeye | Rib Roast | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Saturated Fatty Acids | 6.04 a | 5.74 ab | 3.55 b | 7.94 a | 7.34 a | 0.495 | <0.01 |
| 10:0 | 0.01 ab | 0.01 ab | 0.00 b | 0.01 a | 0.01 a | 0.001 | <0.01 |
| 12:0 | 0.01 ab | 0.02 a | 0.01 ab | 0.01 ab | 0.01 b | 0.001 | 0.04 |
| 14:0 | 0.43 ab | 0.36 bc | 0.21 c | 0.53 a | 0.52 ab | 0.035 | <0.01 |
| 15:0 | 0.07 a | 0.08 a | 0.05 b | 0.09 a | 0.08 a | 0.004 | <0.01 |
| 16:0 | 3.66 ab | 3.26 bc | 2.00 c | 4.69 a | 4.38 ab | 0.286 | <0.01 |
| 17:0 | 0.16 ab | 0.16 ab | 0.10 b | 0.22 a | 0.19 a | 0.017 | <0.01 |
| 18:0 | 1.67 ab | 1.82 ab | 1.14 b | 2.35 a | 2.11 a | 0.166 | <0.01 |
| 19:0 | 0.01 a | 0.00 b | 0.00 b | 0.01 a | 0.01 a | <0.001 | <0.01 |
| 20:0 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.003 | 0.08 |
| Monounsaturated Fatty Acids | 6.42 ab | 5.10 bc | 4.04 c | 7.52 a | 7.64 a | 0.424 | <0.01 |
| 13:1 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.004 | 0.57 |
| 14:1n5 | 0.12 a | 0.08 b | 0.05 b | 0.14 a | 0.14 a | 0.008 | <0.01 |
| 16:1 trans | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.002 | 0.59 |
| 16:1n7 | 0.49 a | 0.30 b | 0.23 b | 0.51 a | 0.53 a | 0.032 | <0.01 |
| 17:1 | 0.08 | 0.1 | 0.08 | 0.11 | 0.09 | 0.033 | 0.95 |
| 18:1 trans | 0.46 | 0.45 | 0.34 | 0.58 | 0.59 | 0.063 | 0.09 |
| 18:1n9 | 5.02 ab | 3.92 bc | 3.14 c | 5.90 a | 5.99 a | 0.344 | <0.01 |
| 18:1n7 | 0.14 | 0.16 | 0.14 | 0.17 | 0.17 | 0.046 | 0.97 |
| 20:1n8 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.003 | 0.44 |
| 20:1n11 | 0.07 bc | 0.07 bc | 0.05 c | 0.09 ab | 0.10 a | 0.006 | <0.01 |
| Polyunsaturated Fatty Acids | 0.54 | 0.64 | 0.54 | 0.61 | 0.62 | 0.050 | 0.48 |
| 18:2 trans | 0.02 | 0.02 | 0.01 | 0.02 | 0.03 | 0.004 | 0.20 |
| 18:3n6 | 0.03 ab | 0.02 ab | 0.02 b | 0.03 a | 0.03 a | 0.003 | 0.01 |
| 18:2n6 | 0.38 | 0.45 | 0.37 | 0.44 | 0.44 | 0.037 | 0.39 |
| 20:2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.13 |
| 20:3n6 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.002 | 0.76 |
| 20:4n6 | 0.06 a | 0.08 a | 0.08 a | 0.05 a | 0.05 a | 0.005 | 0.03 |
| 22:4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.30 |
| 22:5n3 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.002 | 0.60 |
| n-3 Fatty Acids | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.003 | 0.36 |
| n-6 Fatty Acids | 0.49 | 0.58 | 0.48 | 0.55 | 0.55 | 0.044 | 0.50 |
| n-6:n-3 | 25 | 19 | 16 | 28 | 28 | -- | -- |
| Amino Acid, g/100 g | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Rib Roast 3 |
|---|---|---|---|---|
| Essential | ||||
| Histidine | 0.74 | 0.69 | 0.78 | 0.69 |
| Isoleucine | 1.00 | 1.03 | 1.00 | 0.97 |
| Leucine | 1.71 | 1.78 | 1.74 | 1.67 |
| Lysine | 1.83 | 1.89 | 1.85 | 1.79 |
| Methionine | 0.60 | 0.62 | 0.60 | 0.57 |
| Phenylalanine | 0.86 | 0.87 | 0.87 | 0.83 |
| Threonine | 0.95 | 0.98 | 0.96 | 0.92 |
| Tryptophan | 0.25 | 0.25 | 0.25 | 0.25 |
| Valine | 1.05 | 1.07 | 1.07 | 1.02 |
| Non-Essential | ||||
| Alanine | 1.36 | 1.25 | 1.27 | 1.27 |
| Arginine | 1.42 | 1.39 | 1.38 | 1.34 |
| Aspartic Acid | 1.95 | 1.97 | 1.95 | 1.88 |
| Cystine | 0.24 | 0.25 | 0.25 | 0.24 |
| Glutamic Acid | 3.17 | 3.21 | 3.16 | 3.05 |
| Glycine | 1.18 | 0.86 | 0.92 | 0.95 |
| Proline | 1.02 | 0.85 | 0.87 | 0.90 |
| Serine | 0.83 | 0.83 | 0.82 | 0.80 |
| Tyrosine | 0.72 | 0.75 | 0.75 | 0.71 |
| Amino Acid, g/100 g | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Ribeye Steak | Rib Roast |
|---|---|---|---|---|---|
| Essential | |||||
| Histidine | 0.98 | 0.93 | 1.05 | 0.91 | 0.87 |
| Isoleucine | 1.46 | 1.47 | 1.48 | 1.40 | 1.32 |
| Leucine | 2.46 | 2.56 | 2.58 | 2.41 | 2.25 |
| Lysine | 2.67 | 2.72 | 2.76 | 2.61 | 2.44 |
| Methionine | 0.84 | 0.88 | 0.88 | 0.82 | 0.77 |
| Phenylalanine | 1.21 | 1.25 | 1.29 | 1.20 | 1.13 |
| Threonine | 1.38 | 1.40 | 1.43 | 1.33 | 1.25 |
| Tryptophan | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Valine | 1.51 | 1.53 | 1.56 | 1.45 | 1.37 |
| Non-Essential | |||||
| Alanine | 1.82 | 1.81 | 1.88 | 1.79 | 1.76 |
| Arginine | 1.96 | 1.95 | 2.02 | 1.96 | 1.80 |
| Aspartic Acid | 2.79 | 2.86 | 2.92 | 2.76 | 2.59 |
| Cystine | 0.34 | 0.34 | 0.34 | 0.33 | 0.30 |
| Glutamic Acid | 4.45 | 4.62 | 4.62 | 4.38 | 4.18 |
| Glycine | 1.37 | 1.26 | 1.45 | 1.40 | 1.52 |
| Proline | 1.28 | 1.24 | 1.34 | 1.30 | 1.34 |
| Serine | 1.17 | 1.18 | 1.22 | 1.14 | 1.10 |
| Tyrosine | 1.08 | 1.09 | 1.11 | 1.05 | 0.97 |
| Item, Unit | USDA Prime Nutrient Data | USDA Choice Nutrient Data |
|---|---|---|
| Strip Steak 3 | ||
| Protein, % | 21.7 | 22.9 |
| Total Lipid, % | 11.6 | 6.34 |
| Zinc, mg/100 g | 2.3 | 3.8 |
| Iron, mg/100 g | 1.3 | 1.9 |
| Niacin, mg/100 g | 4.9 | 7.0 |
| Vitamin B12, µg/100 g | 1.5 | 1.7 |
| SFA, g/100 g | 5.03 | 2.52 |
| MUFA, g/100 g | 5.25 | 2.95 |
| Oleic Acid, C18:1n9 | 4.19 | 2.36 |
| PUFA, g/100 g | 0.42 | 0.39 |
| Cholesterol, mg/100 g | 55 | 58 |
| Tenderloin Steak 4 | ||
| Protein, % | 21.8 | 21.8 |
| Total Lipid, % | 9.2 | 6.2 |
| Zinc, mg/100 g | 2.4 | 3.3 |
| Iron, mg/100 g | 1.4 | 2.6 |
| Niacin, mg/100 g | 5.9 | 4.5 |
| Vitamin B12, µg/100 g | 1.5 | 3.4 |
| SFA, g/100 g | 3.43 | 2.12 |
| Oleic Acid, C18:1n9 | 2.74 | 1.85 |
| MUFA, g/100 g | 3.54 | 2.34 |
| PUFA, g/100 g | 0.43 | 0.45 |
| Cholesterol, mg/100 g | 57 | 60 |
| Ribeye Steak 5 | ||
| Protein, % | 21.9 | 21.4 |
| Total Lipid, % | 12.7 | 8.5 |
| Zinc, mg/100 g | 2.7 | 5.6 |
| Iron, mg/100 g | 1.6 | 2.0 |
| Niacin, mg/100 g | 4.4 | 5.5 |
| Vitamin B12, µg/100 g | 1.7 | 2.0 |
| SFA, g/100 g | 7.28 | 3.06 |
| MUFA, g/100 g | 6.14 | 3.58 |
| Oleic Acid, C18:1n9 | 5.00 | 2.86 |
| PUFA, g/100 g | 0.43 | 0.47 |
| Cholesterol, mg/100 g | 58 | 63 |
| Top Sirloin Steak 6 | ||
| Protein, % | 23.2 | 21.9 |
| Total Lipid, % | 6.5 | 4.6 |
| Zinc, mg/100 g | 2.5 | 4.1 |
| Iron, mg/100 g | 1.5 | 1.6 |
| Niacin, mg/100 g | 4.4 | 7.4 |
| Vitamin B12, µg/100 g | 1.8 | 1.2 |
| SFA, g/100 g | 2.39 | 1.71 |
| Oleic Acid, C18:1n9 | 2.10 | 1.72 |
| MUFA, g/100 g | 2.71 | 1.86 |
| PUFA, g/100 g | 0.35 | 0.21 |
| Cholesterol, mg/100 g | 59 | 61 |
| Item | Total Caloric Content |
|---|---|
| Raw (kcal/100 g sample) | |
| Strip Loin Steak | 191.2 |
| Tenderloin Steak | 170.0 |
| Top Sirloin Steak | 151.3 |
| Rib Roast | 201.9 |
| Cooked (kcal/85 g sample) | |
| Strip Loin Steak | 211.8 |
| Tenderloin Steak | 197.6 |
| Top Sirloin Steak | 182.9 |
| Ribeye Steak | 229.7 |
| Rib Roast | 229.3 |
| Item | Strip Loin Steak | Tenderloin Steak | Top Sirloin Steak | Ribeye Steak | Rib Roast |
|---|---|---|---|---|---|
| Percent Cook Loss ± SEM | 23.6± 0.93 | 24.8 ± 0.91 | 25.3 ± 0.91 | 23.9 ± 0.93 | 25.4 ± 0.91 |
| Proximates | |||||
| Protein, % | 1.8 | 1.4 | 1.5 | 1.1 | −0.1 |
| Lipid, % | −0.9 | −0.1 | 0.4 | −0.1 | 0.0 |
| Moisture, % | −24.1 | −26.2 | −26.4 | −24.1 | −24.5 |
| Ash, % | −0.1 | 0.0 | −0.1 | −0.1 | −0.1 |
| Cholesterol, mg/100 g | 3.7 | 5.1 | 5.2 | 4.1 | −0.9 |
| Vitamins | |||||
| Vitamin A (total), IU/100 g | −0.3 | 59.3 | 0.0 | 0.0 | 22.8 |
| Vitamin D (total D2 and D3), IU/100 g | -- | -- | -- | -- | -- |
| Vitamin E, IU/kg | -- | -- | -- | -- | -- |
| Vitamin K1, µg/100 g | −0.8 | 1.2 | -- | −0.5 | -- |
| Vitamin B1 (Thiamin), mg/100 g | -- | −0.1 | -- | −0.1 | -- |
| Vitamin B2 (Riboflavin), mg/100 g | −0.1 | −0.1 | -- | -- | -- |
| Vitamin B3 (Niacin), mg/100 g | 0.2 | −3.5 | 0.3 | −1.0 | −1.7 |
| Vitamin B5 (Pantothenic Acid), mg/100 g | -- | −0.3 | −0.2 | −0.2 | −0.1 |
| Vitamin B6 (Pyridoxine), mg/100 g | 0.1 | −0.2 | -- | −0.2 | −0.2 |
| Vitamin B12 (Cyanocobalamin), µg/100 g | −0.1 | −0.6 | −0.6 | −0.5 | −0.3 |
| Minerals | |||||
| Potassium, mg/100 g | −52.2 | −49.0 | −109.9 | −11.7 | −40.7 |
| Phosphorus, mg/100 g | −16.4 | −19.5 | −17.3 | −7.9 | −15.6 |
| Sodium, mg/100 g | −12.8 | −10.1 | −10.3 | −5.8 | −9.1 |
| Magnesium, mg/100 g | −3.5 | −3.2 | −4.2 | −3.4 | −4.4 |
| Calcium, mg/100 g | 0.2 | −0.4 | −0.8 | -- | −0.2 |
| Zinc, mg/100 g | −0.2 | −0.3 | −0.3 | −0.4 | −0.5 |
| Iron, mg/100 g | −0.2 | −0.1 | -- | −0.3 | −0.3 |
| Copper, mg/100 g | -- | -- | 0.1 | -- | -- |
| Selenium, µg/100 g | 2.5 | 0.5 | 2.6 | −0.1 | −0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortensen, E.G.; Fuerniss, H.F.; Legako, J.F.; Thompson, L.D.; Woerner, D.R. Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts. Nutrients 2024, 16, 2912. https://doi.org/10.3390/nu16172912
Mortensen EG, Fuerniss HF, Legako JF, Thompson LD, Woerner DR. Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts. Nutrients. 2024; 16(17):2912. https://doi.org/10.3390/nu16172912
Chicago/Turabian StyleMortensen, Emma G., Hannah F. Fuerniss, Jerrad F. Legako, Leslie D. Thompson, and Dale R. Woerner. 2024. "Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts" Nutrients 16, no. 17: 2912. https://doi.org/10.3390/nu16172912
APA StyleMortensen, E. G., Fuerniss, H. F., Legako, J. F., Thompson, L. D., & Woerner, D. R. (2024). Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts. Nutrients, 16(17), 2912. https://doi.org/10.3390/nu16172912






