Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity
Highlights
- Exposure Risks: Our study uncovers significant evidence linking phthalates and bisphenol exposure in foods to heightened risks of early puberty and childhood obesity, particularly in vulnerable populations.
- Critical Age Window: We identify a critical developmental window during childhood where these endocrine-disrupting chemicals have the most profound effect on early-onset obesity and precocious puberty.
- Dietary Sources: Ultraprocessed and packaged foods were found to contain higher levels of these chemicals, suggesting a need for regulatory reviews and public health interventions.
- Call for Policy Action: The research highlights the urgent need for stricter food safety regulations to reduce exposure to phthalates and bisphenols in consumer products.
Abstract
1. Introduction
2. Methods
3. Results
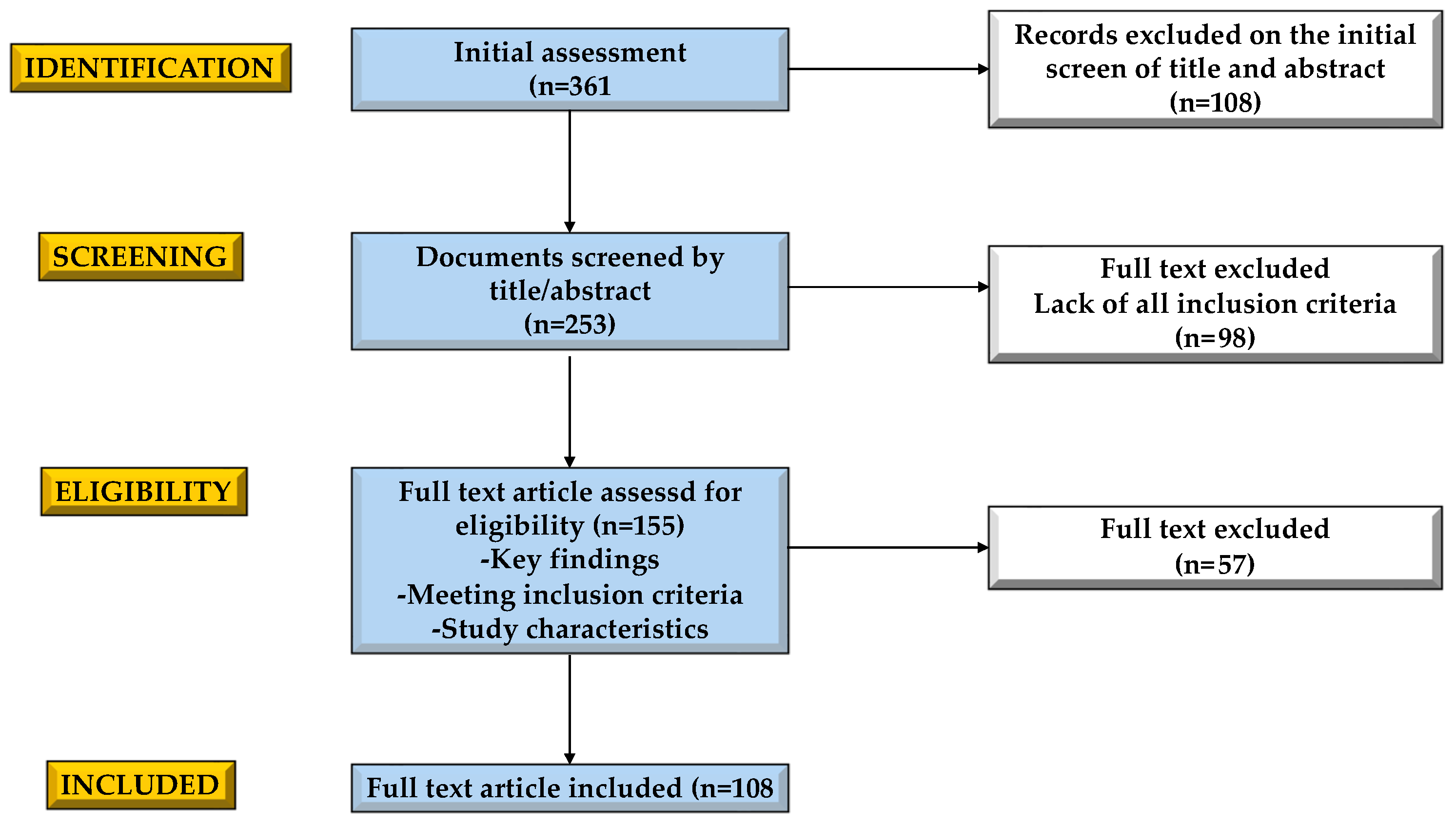
3.1. Selection Process
3.2. Define Phthalates and BPA Including Their Chemical Properties and Sources
3.2.1. Phthalates
| Reference | Matrix | Phthalate | Average Concentration |
|---|---|---|---|
| da Costa JM, et al., 2023 [29] | Baby food | DMP | ND–0.2 (ND) (μg/kg) |
| DEP | ND–1.6 (0.1) (μg/kg) | ||
| DiBP | 0.1–16.0 (2.7) (μg/kg) | ||
| DnBP | 0.1–32.0 (1.3) (μg/kg) | ||
| BBP | ND–16.0 (2.1) (μg/kg) | ||
| DEHP | ND–67.0 (22.0) (μg/kg) | ||
| DCHP | ND–1.8 (ND) (μg/kg) | ||
| DnOP | ND–3.0 (0.2) (μg/kg) | ||
| Cereals, mixed | DiBP | 2.41 (μg/kg) | |
| BBzP | <8.73 (μg/kg) | ||
| DEHP | 41.4 (μg/kg) | ||
| Popcorn, microwave | DiBP | 39.8 (μg/kg) | |
| DBP | 208 (μg/kg) | ||
| BBzP | 34.7 (μg/kg) | ||
| DEHP | 284 (μg/kg) | ||
| Chocolate bars | DiBP | <15.2 (μg/kg) | |
| DBP | <14.0 (μg/kg) | ||
| BBzP | 5.04 (μg/kg) | ||
| DEHP | 135 (μg/kg) | ||
| Wu CG, et al., 2014 [30] | Cola | DMP | 105 (µg L−1) |
| DEHP | 1123 (µg L−1) | ||
| Wu CG, et al., 2014 [30] | Fruit juices | DEHP | 22–126 (µg L−1) |
| Nanni N et al., 2011 [31] | Extra virgin olive | DEHP | 1134 (µg L−1) |
| DINP | 1722 (µg L−1) | ||
| DBP | 90 (µg L−1) | ||
| Fierens T et al., 2013 [32] | Milk, milked by hand | DEHP | <60 (µg L−1) |
| DIBP | 29 (µg L−1) | ||
| DBP | <15 (µg L−1) | ||
| BBP | <10 (µg L−1) | ||
| Fierens T et al., 2013 [32] | Milk, milked by machine | DEHP | 123.5(µg L−1) |
| DIBP | 15.1 (µg L−1) | ||
| DBP | ND | ||
| BBP | 14.3 (µg L−1) | ||
| Hou H et al., 2021 [25] | Apple pulp with label | Long chains PAEs | 0.08 (mg/kg) |
| Short chains PAEs | 0.16 (mg/kg) | ||
| Avocado with label | Short chains PAEs | 0.83 (mg/kg) | |
| Long chains PAEs | 1.04 (mg/kg) |
3.2.2. Biphenol A
3.3. The Role of Diet in Exposure to Phthalates and Bisphenol
3.4. The Influence of Nutrition on the Risk Factor for Precocious Puberty and Its Relation to Early-Onset of Obesity
3.5. Impact of Bisphenol and Phthalates in Foods
| Reference | Matrix | Bisphenol | Average Concentration |
|---|---|---|---|
| Lee J. et al., 2019 [88] | Baby food for 15-month-old children | BPA | 5.09 (ng/g) |
| Russo G. et al., 2019 [75] | Non-canned fruits, dried fruits, nuts, and seeds | BPA | Min–Max (μg kg−1) 0.105–2.130 |
| Russo G. et al., 2019 [75] | Canned tuna | BPA + BPs | Min–Max (μg kg−1) 6.3–187.0 |
| Schiano ME. et al., 2023 [76] | Canned legumes | BPA | 1.51–21.22 (ng/mL) |
| Schiano ME. et al., 2023 [76] | Sliced bread (plastic packaging) | BPA | ng/g (SD) 1.20 (0.3) |
| Schiano ME. et al., 2023 [76] | Salted snacks (plastic packaging) | BPA | ng/g (SD) 25.45 (23.54) |
| Robles-Aguilera V. et al., 2021 [89] | Ham (plastic packaging) | BPA | ng/g (SD) 6.6 (3.4) |
| Schiano ME. et al., 2023 [76] | Cake (not packaged) | BPs | ng/g (SD) 1.7 (0.7) |
| Robles-Aguilera V. et al., 2021 [89] | Rice (plastic packaging) | BPs | ng/g (SD) 3.3 (1.4) |
3.6. Studies Exploring the Impact of Phthalates and BPA in Precocious Puberty and Early-Onset Obesity
3.6.1. BPA and Precocious Puberty
3.6.2. Phthalates and Early Obesity
| BPA and Precocious Puberty | ||||
|---|---|---|---|---|
| Authors | Sample | Age | Type of Study | Main Results |
| Wolff et al., 2008 [94] | 192 multiethnic girls | 9 years old | Prospective cross-sectional study | No correlation between urinary BPA levels and puberty progression |
| Lee et al., 2009 [99] | 30 patients (29 girls and 1 boy) with idiopathic CPP + 30 healthy controls | 8.6 ± 0.9 vs. 7.8 ± 1.1 years old | Case-control study | Slightly higher urinary BPA levels in girls with peripheral precocious puberty |
| Wolff et al., 2010 [95] | 1151 American girls | 6–8 years old | Longitudinal study | No correlation between urinary BPA levels and puberty progression |
| Buttke et al., 2012 [97] | 461 American girls | 12–16 years old | Cross-sectional study | No significant link between urinary BPA levels and age at menarche |
| Frederiksen et al., 2013 [102] | 129 Danish children and adolescents | 6–21 years old | Cross-sectional study | No correlation between urinary BPA levels and puberty progression |
| Zhang et al., 2015 [12] | 430 | 6–14 years | Cross-sectional study | Subtle effects of phthalate metabolites associated with pubertal onset and progression. MnBP exposure may be associated with delayed pubic hair development in boys, while MnBP, MMP, MEP, and MEHP exposure may be associated with breast development onset, and MEHP metabolites may be associated with a speedup in breast development and an earlier menarche onset in girls |
| Mouritsen et al., 2013 [103] | 168 | 5–10 years | Longitudinal study | High exposure to DBP was associated with earlier age at pubarche in boys. In girls, no associations between phthalate exposure and age at pubertal milestones were observed |
| Lomenick et al., 2010 [104] | 56 | 7 years | Cross-sectional study | Phthalates may be associated with certain other toxicities in humans; our study suggests that their exposure is not associated with precocious puberty in female children |
| Yum et al., 2013 [98] | 150 Korean girls with ICPP + 90 healthy controls | 6–12 years old | Case-control study | No significant association between BPA levels and precocious puberty |
| Lee et al., 2014 [111] | 42 Korean girls with ICPP + 40 with IPPP + 32 healthy controls | 8.7 ± 1.0 vs. 8.4 ± 0.7 vs. 8.5 ± 0.9 | Case-control study | Non-statistically significant slightly higher BPA levels in ICPP and IPPP than controls |
| Ferguson et al., 2014 [113] | 250 boys | 8.10–14.4 years old | Prospective cohort | No association between prenatal BPA exposure and sex hormone levels in boys |
| Wolff et al., 2015 [96] | 1239 Black or Hispanic girls | 6–8 years old | Prospective longitudinal cohort study | No statistical link between urinary BPA levels and puberty progression |
| McGuinn et al., 2015 [107] | 987 American girls | 12–19 years old | Cross-sectional study | No significant association was found between urinary BPA levels and earlier menarche. Delayed menarche was found in girls with moderate urinary BPA levels |
| Supornsilchai et al., 2016 [110] | 29 Thai girls with ICPP + 12 with early puberty + 47 healthy girls | 7.44 ± 1.03 years old | Case-control cross-sectional study | Higher urinary BPA levels in obese girls with precocious puberty |
| Buluş et al., 2016 [101] | 42 Turkish girl with ICPP + 42 with IPPP + 50 healthy controls | 7.4 ± 0.6 years old | Case-control study | No significant differences in urinary BPA levels in girls with ICPP-IPPP |
| Wang et al., 2017 [114] | 671 Chinese boys | 9–18 years old | Cross-sectional study | Association between peripubertal BPA exposure and earlier pubertal onset, but delayed pubertal progression in boys |
| Durmaz et al., 2018 [93] | 28 Turkish girls with ICPP + 25 healthy girls | 4–8 years old | Case-control cross-sectional study | Higher urinary BPA levels in girls with CPP, no correlation with hormone levels |
| Chen et al., 2018 [63] | 285 Chinese girls with ICPP and 136 healthy controls | 6–9 years old | Case-control study | Higher urinary BPA concentrations linked to increased risk of ICPP |
| Jung et al., 2019 [105] | 47 Korean girls with ICPP + 47 healthy controls | 5–12 years old | Case-control study | No significant difference in urinary BPA levels between ICPP and controls |
| Bigambo et al., 2020 [106] | 4737 girls | - | Meta-analysis | Significant link between 2,5-dichlorophenol exposure and earlier puberty; no significant association between earlier puberty and bisphenol A, triclosan, and benzophenone 3 |
| Bigambo et al., 2023 [90] | 21 Chinese girls with ICPP + 149 healthy girls | 2–10 years old | Case-control study | BPA substitutes linked to higher risk of precocious puberty in girls |
| Huynh et al., 2024 [112] | 124 Vietnamese children with ICPP and 126 healthy controls | - | Case-control study | Higher urinary BPA levels in children with precocious puberty |
| Phthalates and Obesity | ||||
| Buser et al., 2014 [125] | 100 | 6–19 years | Cross-sectional study | Urinary concentrations of LMW phthalate metabolites are linked to higher obesity rates in male children and adolescents |
| Deierlein et al., 2016 [130] | 1239 | 6–8 years | Longitudinal study | The results showed that LMW PAEs (MEP, MBP, and MiBP) were positively associated with increased BMI and waist circumference scores in these girls |
| Bulus et al., 2016 [101] | 134 | 7–8 years | Case-control study | Higher phthalate levels in girls with CPP suggest that phthalates might impact the central nervous system and trigger puberty-related pathways |
| Srilanchakon et al., 2017 [131] | 136 | 7–9 years | Cross-sectional study | Girls with precocious puberty had an association with increased MEP concentration. This is the first report of the association between urinary phthalate levels and precocious puberty in Thai girls |
| Hashemipour et al., 2018 [132] | 150 | 7–9 years | Case-control study | Diethyl hexyl phthalate metabolites (MEHP, 5OH-MEHP, and 5oxo-MEHP) in girls with precocious puberty were significantly higher than those in the control group, indicating the possible role of these metabolites as endocrine-disrupting agents, in particular in the reproductive system |
| Amin et al., 2018 [133] 7/21/24 8:16:00 a.m. | 242 | 6–18 years | Cross-sectional study | Urinary PAE levels of MBzP, MBP, MMP, MEHP, and MEHHP were significantly associated with childhood obesity. Additionally, MBzP and MEHP were related to triglyceride levels and obesity |
| Xia et al., 2018 [134] | 149 | 10–15 years | Case-control study | Phthalate exposure might contribute to the development of high weight and obesity in school-age children |
| Wang et al., 2022 [128] | 798 | 7–10 years | Cross-sectional study | The level of PAE metabolite exposure was linked to the risk of abdominal obesity in Chinese students aged 7–10 years |
| Dong et al., 2022 [126] | 2298 | 7–13 years | Case-control study | The results suggested that children in Xiamen City, China, were widely exposed to environmental PAE pollutants. Furthermore, this high exposure could increase the risk of high weight and obesity, particularly in girls |
| Su et al., 2023 [135] | 220 | 2–14 years | Cohort study | Phthalate exposure at certain times may affect children’s reproductive development during puberty |
| Li et al., 2023 [127] | 480 | 6–8 years | Case-control study | Diet and physical activity, but not phthalate metabolites, were associated with childhood obesity |
| Huynh et al., 2024 [112] | 250 | 6–8 years | Case-control study | This study found BPA-glucuronide in 11.3% of the PP group but not in the control group, suggesting a potential link. The PP group also had a higher prevalence of MBP (8.1%) compared to the control group (2.4%) |
4. Limits
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Gibert, Y.; Sargis, R.M.; Nadal, A. Editorial: Endocrine Disrupters and Metabolism. Front. Endocrinol. 2019, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B. Adipose Tissue and Endocrine-Disrupting Chemicals: Does Sex Matter? Int. J. Environ. Res. Public Health 2020, 17, 9403. [Google Scholar] [CrossRef]
- Vacca, M.; Calabrese, F.M.; Loperfido, F.; Maccarini, B.; Cerbo, R.M.; Sommella, E.; Salviati, E.; Voto, L.; De Angelis, M.; Ceccarelli, G.; et al. Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines 2024, 12, 234. [Google Scholar] [CrossRef]
- Ghassabian, A.; Vandenberg, L.; Kannan, K.; Trasande, L. Endocrine-Disrupting Chemicals and Child Health. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 573–594. [Google Scholar] [CrossRef]
- Wu, W.; Li, M.; Liu, A.; Wu, C.; Li, D.; Deng, Q.; Zhang, B.; Du, J.; Gao, X.; Hong, Y. Bisphenol A and the Risk of Obesity a Systematic Review with Meta-Analysis of the Epidemiological Evidence. Dose-Response 2020, 18, 155932582091694. [Google Scholar] [CrossRef]
- Pérez-Bermejo, M.; Mas-Pérez, I.; Murillo-Llorente, M.T. The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines 2021, 9, 666. [Google Scholar] [CrossRef]
- Maniradhan, M.; Calivarathan, L. Bisphenol A-Induced Endocrine Dysfunction and Its Associated Metabolic Disorders. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 515–529. [Google Scholar] [CrossRef]
- Zhou, M.; Ford, B.; Lee, D.; Tindula, G.; Huen, K.; Tran, V.; Bradman, A.; Gunier, R.; Eskenazi, B.; Nomura, D.K.; et al. Metabolomic Markers of Phthalate Exposure in Plasma and Urine of Pregnant Women. Front. Public Health 2018, 6, 298. [Google Scholar] [CrossRef]
- Ouidir, M.; Cissé, A.H.; Botton, J.; Lyon-Caen, S.; Thomsen, C.; Sakhi, A.K.; Sabaredzovic, A.; Bayat, S.; Slama, R.; Heude, B.; et al. Fetal and Infancy Exposure to Phenols, Parabens, and Phthalates and Anthropometric Measurements up to 36 Months, in the Longitudinal SEPAGES Cohort. Environ. Health Perspect. 2024, 132, 057002. [Google Scholar] [CrossRef]
- Gaston, S.A.; Tulve, N.S. Urinary Phthalate Metabolites and Metabolic Syndrome in U.S. Adolescents: Cross-Sectional Results from the National Health and Nutrition Examination Survey (2003–2014) Data. Int. J. Hyg. Environ. Health 2019, 222, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Shi, H.; Jiang, X.; Zhao, Y.; Fang, X.; Xie, C. Could Exposure to Phthalates Speed up or Delay Pubertal Onset and Development? A 1.5-Year Follow-up of a School-Based Population. Environ. Int. 2015, 83, 41–49. [Google Scholar] [CrossRef]
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X.; et al. Perinatal Exposure to Bisphenol A at Reference Dose Predisposes Offspring to Metabolic Syndrome in Adult Rats on a High-Fat Diet. Endocrinology 2011, 152, 3049–3061. [Google Scholar] [CrossRef]
- Li, D.-K.; Miao, M.; Zhou, Z.; Wu, C.; Shi, H.; Liu, X.; Wang, S.; Yuan, W. Urine Bisphenol-A Level in Relation to Obesity and Overweight in School-Age Children. PLoS ONE 2013, 8, e65399. [Google Scholar] [CrossRef]
- Menale, C.; Piccolo, M.T.; Cirillo, G.; Calogero, R.A.; Papparella, A.; Mita, L.; Del Giudice, E.M.; Diano, N.; Crispi, S.; Mita, D.G. Bisphenol A Effects on Gene Expression in Adipocytes from Children: Association with Metabolic Disorders. J. Mol. Endocrinol. 2015, 54, 289–303. [Google Scholar] [CrossRef]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet Quality and Exposure to Endocrine-Disrupting Chemicals among US Adults. Environ. Res. 2022, 211, 113049. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Kiess, W.; Häussler, G.; Vogel, M. Endocrine-Disrupting Chemicals and Child Health. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101516. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Assessment on the State of the Science of Endocrine Disruptors. Available online: https://www.who.int/publications/i/item/WHO-PSC-EDC-02.2 (accessed on 1 July 2024).
- Hunt, K.J.; Ferguson, P.L.; Bloom, M.S.; Neelon, B.; Pearce, J.; Commodore, S.; Newman, R.B.; Roberts, J.R.; Bain, L.; Baldwin, W.; et al. Phthalate and Phthalate Replacement Concentrations in Relationship to Adiposity in a Multi-Racial Cohort of Children. Int. J. Obes. 2024. [Google Scholar] [CrossRef]
- Barnes, S.J. Understanding Plastics Pollution: The Role of Economic Development and Technological Research. Environ. Pollut. 2019, 249, 812–821. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.-M. Metabolism of Phthalates in Humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Min, Y.; Liu, X.; Wang, P.; Zhou, Z.; Liu, D. Occurrence and Migration of Phthalates in Adhesive Materials to Fruits and Vegetables. J. Hazard. Mater. 2021, 418, 126277. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Guo, J.-L.; Xue, J.; Bai, C.-L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- da Costa, J.M.; Kato, L.S.; Galvan, D.; Lelis, C.A.; Saraiva, T.; Conte-Junior, C.A. Occurrence of Phthalates in Different Food Matrices: A Systematic Review of the Main Sources of Contamination and Potential Risks. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2043–2080. [Google Scholar] [CrossRef]
- Wu, C.-F.; Chang-Chien, G.-P.; Su, S.-W.; Chen, B.-H.; Wu, M.-T. Findings of 2731 Suspected Phthalate-Tainted Foodstuffs during the 2011 Phthalates Incident in Taiwan. J. Formos. Med. Assoc. Taiwan Yi Zhi 2014, 113, 600–605. [Google Scholar] [CrossRef]
- Nanni, N.; Fiselier, K.; Grob, K.; Di Pasquale, M.; Fabrizi, L.; Aureli, P.; Coni, E. Contamination of Vegetable Oils Marketed in Italy by Phthalic Acid Esters. Food Control 2011, 22, 209–214. [Google Scholar] [CrossRef]
- Fierens, T.; Van Holderbeke, M.; Willems, H.; De Henauw, S.; Sioen, I. Transfer of Eight Phthalates through the Milk Chain—A Case Study. Environ. Int. 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Plastics Europe Plastics—The Facts 2022. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 10 August 2024).
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The Gut Microbiota: A Major Player in the Toxicity of Environmental Pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A–Sources, Toxicity and Biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Neri, I.; Russo, G.; Grumetto, L. Bisphenol A and Its Analogues: From Their Occurrence in Foodstuffs Marketed in Europe to Improved Monitoring Strategies—A Review of Published Literature from 2018 to 2023. Arch. Toxicol. 2024, 98, 2441–2461. [Google Scholar] [CrossRef]
- Chen, M.; Lv, C.; Zhang, S.; Tse, L.A.; Hong, X.; Liu, X.; Ding, Y.; Xiao, P.; Tian, Y.; Gao, Y. Bisphenol A Substitutes and Childhood Obesity at 7 Years: A Cross-Sectional Study in Shandong, China. Environ. Sci. Pollut. Res. 2023, 30, 73174–73184. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Bisphenol A. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 24 June 2024).
- Rashid, H.; Alqahtani, S.S.; Alshahrani, S. Diet: A Source of Endocrine Disruptors. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 633–645. [Google Scholar] [CrossRef]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef]
- Cunha, S.C.; Menezes-Sousa, D.; Mello, F.V.; Miranda, J.A.T.; Fogaca, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Survey on Endocrine-Disrupting Chemicals in Seafood: Occurrence and Distribution. Environ. Res. 2022, 210, 112886. [Google Scholar] [CrossRef]
- Serrano, S.; Karr, C.; Seixas, N.; Nguyen, R.; Barrett, E.; Janssen, S.; Redmon, B.; Swan, S.; Sathyanarayana, S. Dietary Phthalate Exposure in Pregnant Women and the Impact of Consumer Practices. Int. J. Environ. Res. Public Health 2014, 11, 6193–6215. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Dietrich, J.P.; Martinelli, E.; Stenstad, A.; Pradhan, P.; Gabrysch, S.; Mishra, A.; Weindl, I.; Le Mouël, C.; Rolinski, S.; et al. The Ongoing Nutrition Transition Thwarts Long-Term Targets for Food Security, Public Health and Environmental Protection. Sci. Rep. 2020, 10, 19778. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The Nutrition Transition to a Stage of High Obesity and Noncommunicable Disease Prevalence Dominated by Ultra-processed Foods Is Not Inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Sauer, P. Obesity in Early Life: Its Causes, Prevention and Risks in Later Life. Nutrients 2023, 15, 2999. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Herrmann, S.; Lucas, M. The Role of Endocrine-Disrupting Phthalates and Bisphenols in Cardiometabolic Disease: The Evidence Is Mounting. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-Processed Food Consumption and Exposure to Phthalates and Bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef]
- Van Woerden, I.; Payne-Sturges, D.C.; Whisner, C.M.; Bruening, M. Dietary Quality and Bisphenols: Trends in Bisphenol A, F, and S Exposure in Relation to the Healthy Eating Index Using Representative Data from the NHANES 2007–2016. Am. J. Clin. Nutr. 2021, 114, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, I.; Tagliaferri, S.; Sommella, E.; Salviati, E.; Porri, D.; Raspini, B.; Cena, H.; Campiglia, P.; La Rocca, C.; Cerbo, R.M.; et al. Lifestyle Habits and Exposure to BPA and Phthalates in Women of Childbearing Age from Northern Italy: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 9710. [Google Scholar] [CrossRef]
- Magalhães, V.; Severo, M.; Costa, S.A.; Correia, D.; Carvalho, C.; Torres, D.; Casal, S.; Cunha, S.; Lopes, C. Bisphenol A and Cardiometabolic Risk in Adolescents: Data from the Generation XXI Cohort. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1088–1096. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Annunziata, G.; Camajani, E.; Caprio, M.; Sojat, A.S.; Marina, L.V.; Guarnotta, V.; Colao, A.; Muscogiuri, G. Role of Mediterranean Diet in Endocrine Diseases: A Joint Overview by the Endocrinologist and the Nutritionist. J. Endocrinol. Investig. 2023, 47, 17–33. [Google Scholar] [CrossRef]
- Abreu, A.P.; Kaiser, U.B. Pubertal Development and Regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Magenes, V.C.; Pascuzzi, M.C.; Rossi, V.; Sangiorgio, A.; Bosetti, A.; Zuccotti, G.; Mameli, C. The Role of Pediatric Nutrition as a Modifiable Risk Factor for Precocious Puberty. Life 2021, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Chan, Y.-M. Normal Puberty. Available online: https://www.uptodate.com/contents/normal-puberty?search=normal%20puberty&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 10 August 2024).
- Calcaterra, V.; Cena, H.; Regalbuto, C.; Vinci, F.; Porri, D.; Verduci, E.; Mameli, C.; Zuccotti, G.V. The Role of Fetal, Infant, and Childhood Nutrition in the Timing of Sexual Maturation. Nutrients 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, Diagnosis, and Treatment of Central Precocious Puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.H.; Lawrence, N.; Steele, C.; Mohamed, Z. Precocious Puberty. BMJ 2020, 368, l6597. [Google Scholar] [CrossRef] [PubMed]
- Eckert-Lind, C.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.V.; Juul, A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.F. Physiology of Puberty in Boys and Girls and Pathological Disorders Affecting Its Onset. J. Adolesc. 2019, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dai, L.; Dong, Y.; Wang, N.; Zhang, J.; Liu, C.; Li, Z.; Chu, L.; Chen, S. Analysis of Risk Factors of Precocious Puberty in Children. BMC Pediatr. 2023, 23, 456. [Google Scholar] [CrossRef]
- Aghaee, S.; Deardorff, J.; Greenspan, L.C.; Quesenberry, C.P.; Kushi, L.H.; Kubo, A. Correction to: Breastfeeding and Timing of Pubertal Onset in Girls: A Multiethnic Population-Based Prospective Cohort Study. BMC Pediatr. 2019, 19, 317. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Zhang, Y.; Sun, W.; Jiang, Y.; Song, Y.; Zhu, Q.; Mei, H.; Wang, X.; Liu, S.; et al. Association between Dietary Patterns and Precocious Puberty in Children: A Population-Based Study. Int. J. Endocrinol. 2018, 2018, 4528704. [Google Scholar] [CrossRef]
- Günther, A.L.B.; Karaolis-Danckert, N.; Kroke, A.; Remer, T.; Buyken, A.E. Dietary Protein Intake throughout Childhood Is Associated with the Timing of Puberty. J. Nutr. 2010, 140, 565–571. [Google Scholar] [CrossRef]
- Jansen, E.C.; Zhou, L.; Perng, W.; Song, P.X.; Rojo, M.M.T.; Mercado, A.; Peterson, K.E.; Cantoral, A. Vegetables and Lean Proteins–Based and Processed Meats and Refined Grains –Based Dietary Patterns in Early Childhood Are Associated with Pubertal Timing in a Sex-Specific Manner: A Prospective Study of Children from Mexico City. Nutr. Res. 2018, 56, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wiley, A.S. Milk Intake and Total Dairy Consumption: Associations with Early Menarche in NHANES 1999–2004. PLoS ONE 2011, 6, e14685. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Willett, W.C.; Wang, M.; Rich-Edwards, J.; Frazier, A.L.; Michels, K.B. Milk Consumption after Age 9 Years Does Not Predict Age at Menarche. J. Nutr. 2015, 145, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wu, Y.; Feng, Z.; Chai, Y.; Hou, S.; Yu, Z.; Shen, X. Dietary Pattern and Precocious Puberty Risk in Chinese Girls: A Case-Control Study. Nutr. J. 2024, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, S.; Chen, C.; Yuan, Y.; Dai, Z.; Chen, A.; Zhang, B.; Liu, S.; Lin, C. Association of Traditional Dietary Pattern with Early and Precocious Puberty: A Population-Based Cross-Sectional Study. Pediatr. Res. 2024, 96, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kong, Y.; Xie, X.; Wang, Y.; Wang, N. Association between Precocious Puberty and Obesity Risk in Children: A Systematic Review and Meta-Analysis. Front. Pediatr. 2023, 11, 1226933. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Hruby, C.; Siccardo, F.; Mari, A.; Cordaro, E.; Fabiano, V.; Zuccotti, G. Links between Childhood Obesity, High-Fat Diet, and Central Precocious Puberty. Children 2023, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, Y.; Gaskins, A.J.; Li, L.-J.; Huang, Z.; Eriksson, J.G.; Hu, F.B.; Chong, Y.S.; Zhang, C. Mediterranean Diet and Female Reproductive Health over Lifespan: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2023, 229, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Asghari, G.; Mirmiran, P.; Azizi, F. Longitudinal Association of Dietary Sources of Animal and Plant Protein throughout Childhood with Menarche. BMC Pediatr. 2021, 21, 206. [Google Scholar] [CrossRef]
- Tang, J.; Xue, P.; Huang, X.; Lin, C.; Liu, S. Diet and Nutrients Intakes during Infancy and Childhood in Relation to Early Puberty: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 5004. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Mita, D.G.; Grumetto, L. Occurrence of Bisphenol A and Its Analogues in Some Foodstuff Marketed in Europe. Food Chem. Toxicol. 2019, 131, 110575. [Google Scholar] [CrossRef]
- Schiano, M.E.; Sodano, F.; Magli, E.; Corvino, A.; Fiorino, F.; Rimoli, M.G.; Seccia, S.; Albrizio, S. Quantitative Determination of BPA, BPB, BPF and BPS Levels in Canned Legumes from Italian Market. Food Chem. 2023, 416, 135642. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Cofini, M.; Rigante, D.; Lucchetti, L.; Cipolla, C.; Penta, L.; Esposito, S. The Effect of Bisphenol A on Puberty: A Critical Review of the Medical Literature. Int. J. Environ. Res. Public Health 2017, 14, 1044. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An Estrogenic Substance Is Released from Polycarbonate Flasks during Autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Laribi, O.; Ropero, A.B.; Fuentes, E.; Ripoll, C.; Soria, B.; Nadal, A. Low Doses of Bisphenol A and Diethylstilbestrol Impair Ca2+ Signals in Pancreatic α-Cells through a Nonclassical Membrane Estrogen Receptor within Intact Islets of Langerhans. Environ. Health Perspect. 2005, 113, 969–977. [Google Scholar] [CrossRef]
- Matsushima, A.; Kakuta, Y.; Teramoto, T.; Koshiba, T.; Liu, X.; Okada, H.; Tokunaga, T.; Kawabata, S.; Kimura, M.; Shimohigashi, Y. Structural Evidence for Endocrine Disruptor Bisphenol A Binding to Human Nuclear Receptor ERRγ. J. Biochem. (Tokyo) 2007, 142, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Chattopadhyay, S.; Gong, E.-Y.; Ahn, R.S.; Lee, K. Antiandrogenic Effects of Bisphenol A and Nonylphenol on the Function of Androgen Receptor. Toxicol. Sci. 2003, 75, 40–46. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an Environmental Contaminant That Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Caserta, D.; Di Segni, N.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol a and the Female Reproductive Tract: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Reprod. Biol. Endocrinol. 2014, 12, 37. [Google Scholar] [CrossRef]
- Costa, E.M.F.; Spritzer, P.M.; Hohl, A.; Bachega, T.A.S.S. Effects of Endocrine Disruptors in the Development of the Female Reproductive Tract. Arq. Bras. Endocrinol. Metabol. 2014, 58, 153–161. [Google Scholar] [CrossRef]
- Braun, J.M.; Yolton, K.; Dietrich, K.N.; Hornung, R.; Ye, X.; Calafat, A.M.; Lanphear, B.P. Prenatal Bisphenol A Exposure and Early Childhood Behavior. Environ. Health Perspect. 2009, 117, 1945–1952. [Google Scholar] [CrossRef]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The Effect of Maternal Exposure to Endocrine Disrupting Chemicals on Fetal and Neonatal Development: A Review on the Major Concerns. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 224–242. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, Y.-A.; Choi, K.; Park, J.; Moon, H.-B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.-J.; Eun, S.-H.; et al. Bisphenol A in Infant Urine and Baby-Food Samples among 9- to 15-Month-Olds. Sci. Total Environ. 2019, 697, 133861. [Google Scholar] [CrossRef]
- Robles-Aguilera, V.; Gálvez-Ontiveros, Y.; Rodrigo, L.; Salcedo-Bellido, I.; Aguilera, M.; Zafra-Gómez, A.; Monteagudo, C.; Rivas, A. Factors Associated with Exposure to Dietary Bisphenols in Adolescents. Nutrients 2021, 13, 1553. [Google Scholar] [CrossRef]
- Bigambo, F.M.; Wang, D.; Sun, J.; Ding, X.; Li, X.; Gao, B.; Wu, D.; Gu, W.; Zhang, M.; Wang, X. Association between Urinary BPA Substitutes and Precocious Puberty among Girls: A Single-Exposure and Mixed Exposure Approach from a Chinese Case—Control Study. Toxics 2023, 11, 905. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 097004. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, J.; Fang, Y.; Liang, L.; Chen, W.; Chen, X. Serum Bisphenol A Concentration and Premature Thelarche in Female Infants Aged 4-Month to 2-Year. Indian J. Pediatr. 2015, 82, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Asci, A.; Erkekoglu, P.; Balcı, A.; Bircan, I.; Koçer-Gumusel, B. Urinary Bisphenol A Levels in Turkish Girls with Premature Thelarche. Hum. Exp. Toxicol. 2018, 37, 1007–1016. [Google Scholar] [CrossRef]
- Wolff, M.S.; Britton, J.A.; Boguski, L.; Hochman, S.; Maloney, N.; Serra, N.; Liu, Z.; Berkowitz, G.; Larson, S.; Forman, J. Environmental Exposures and Puberty in Inner-City Girls. Environ. Res. 2008, 107, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.; Liao, L.; Biro, F.; Kushi, L.H.; Erdmann, C.; Hiatt, R.A.; Rybak, M.E.; et al. Investigation of Relationships between Urinary Biomarkers of Phytoestrogens, Phthalates, and Phenols and Pubertal Stages in Girls. Environ. Health Perspect. 2010, 118, 1039–1046. [Google Scholar] [CrossRef]
- Wolff, M.S.; Teitelbaum, S.L.; McGovern, K.; Pinney, S.M.; Windham, G.C.; Galvez, M.; Pajak, A.; Rybak, M.; Calafat, A.M.; Kushi, L.H.; et al. Environmental Phenols and Pubertal Development in Girls. Environ. Int. 2015, 84, 174–180. [Google Scholar] [CrossRef]
- Buttke, D.E.; Sircar, K.; Martin, C. Exposures to Endocrine-Disrupting Chemicals and Age of Menarche in Adolescent Girls in NHANES (2003–2008). Environ. Health Perspect. 2012, 120, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Yum, T.; Lee, S.; Kim, Y. Association between Precocious Puberty and Some Endocrine Disruptors in Human Plasma. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2013, 48, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, W.; Chae, H.; Lee, E.; Kim, J.H.; Kim, D.-H.; Kim, H.-S. Determination of Serum Di-(2-Ethylhexyl) Phthalate and Bisphenol A Level in Children with Idiopathic Central Precocious Puberty. J. Korean Soc. Pediatr. Endocrinol. 2009, 14, 154–162. [Google Scholar]
- Han, E.; Yim, O.; Chung, J.; Baek, S.; Kim, Y. The Study of Relationship between the Concentrations of Bisphenol A and DEHP in Human Plasma and Precocious Puberty. Anal. Sci. Technol. 2008, 21, 375–382. [Google Scholar]
- Buluş, A.D.; Aşci, A.; Erkekoglu, P.; Balci, A.; Andiran, N.; Koçer-Gümüşel, B. The Evaluation of Possible Role of Endocrine Disruptors in Central and Peripheral Precocious Puberty. Toxicol. Mech. Methods 2016, 26, 493–500. [Google Scholar] [CrossRef]
- Frederiksen, H.; Aksglaede, L.; Sorensen, K.; Nielsen, O.; Main, K.M.; Skakkebaek, N.E.; Juul, A.; Andersson, A.-M. Bisphenol A and Other Phenols in Urine from Danish Children and Adolescents Analyzed by Isotope Diluted TurboFlow-LC-MS/MS. Int. J. Hyg. Environ. Health 2013, 216, 710–720. [Google Scholar] [CrossRef]
- Mouritsen, A.; Frederiksen, H.; Sørensen, K.; Aksglaede, L.; Hagen, C.; Skakkebaek, N.E.; Main, K.M.; Andersson, A.M.; Juul, A. Urinary Phthalates from 168 Girls and Boys Measured Twice a Year during a 5-Year Period: Associations with Adrenal Androgen Levels and Puberty. J. Clin. Endocrinol. Metab. 2013, 98, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Lomenick, J.P.; Calafat, A.M.; Melguizo Castro, M.S.; Mier, R.; Stenger, P.; Foster, M.B.; Wintergerst, K.A. Phthalate Exposure and Precocious Puberty in Females. J. Pediatr. 2010, 156, 221–225. [Google Scholar] [CrossRef]
- Jung, M.K.; Choi, H.S.; Suh, J.; Kwon, A.; Chae, H.W.; Lee, W.J.; Yoo, E.-G.; Kim, H.-S. The Analysis of Endocrine Disruptors in Patients with Central Precocious Puberty. BMC Pediatr. 2019, 19, 323. [Google Scholar] [CrossRef] [PubMed]
- Bigambo, F.M.; Sun, H.; Yan, W.; Wu, D.; Xia, Y.; Wang, X.; Wang, X. Association between Phenols Exposure and Earlier Puberty in Children: A Systematic Review and Meta-Analysis. Environ. Res. 2020, 190, 110056. [Google Scholar] [CrossRef] [PubMed]
- McGuinn, L.A.; Ghazarian, A.A.; Joseph Su, L.; Ellison, G.L. Urinary Bisphenol A and Age at Menarche among Adolescent Girls: Evidence from NHANES 2003–2010. Environ. Res. 2015, 136, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Khoury, P.; Morrison, J.A. Influence of Obesity on Timing of Puberty. Int. J. Androl. 2006, 29, 272–277; discussion 286–290. [Google Scholar] [CrossRef]
- Durmaz, E.; Aşçı, A.; Erkekoğlu, P.; Akçurin, S.; Gümüşel, B.K.; Bircan, I. Urinary Bisphenol a Levels in Girls with Idiopathic Central Precocious Puberty. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Supornsilchai, V.; Jantarat, C.; Nosoognoen, W.; Pornkunwilai, S.; Wacharasindhu, S.; Soder, O. Increased Levels of Bisphenol A (BPA) in Thai Girls with Precocious Puberty. J. Pediatr. Endocrinol. Metab. JPEM 2016, 29, 1233–1239. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, S.M.; Choi, M.H.; Lee, J.; Park, M.J.; Kim, S.H.; Lee, W.-Y.; Hong, J.; Chung, B.C. Changes in Steroid Metabolism among Girls with Precocious Puberty May Not Be Associated with Urinary Levels of Bisphenol A. Reprod. Toxicol. 2014, 44, 1–6. [Google Scholar] [CrossRef]
- Vu Huynh, Q.T.; Ban, H.T.; Vuong, N.L.; Khanh, N.P. The Relationship between Bisphenol A and Phthalates with Precocious Puberty in Vietnamese Children. J. Pediatr. Endocrinol. Metab. 2024, 37, 644–651. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Peterson, K.E.; Lee, J.M.; Mercado-García, A.; Blank-Goldenberg, C.; Téllez-Rojo, M.M.; Meeker, J.D. Prenatal and Peripubertal Phthalates and Bisphenol A in Relation to Sex Hormones and Puberty in Boys. Reprod. Toxicol. 2014, 47, 70–76. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Miao, M.; Liang, H.; Chen, J.; Zhou, Z.; Wu, C.; Yuan, W. Urine Bisphenol A and Pubertal Development in Boys. Int. J. Hyg. Environ. Health 2017, 220, 43–50. [Google Scholar] [CrossRef]
- Bordini, B.; Rosenfield, R.L. Normal Pubertal Development: Part II: Clinical Aspects of Puberty. Pediatr. Rev. 2011, 32, 281–292. [Google Scholar] [CrossRef]
- Kaplowitz, P.B. Link between Body Fat and the Timing of Puberty. Pediatrics 2008, 121 (Suppl. 3), S208–S217. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; Van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of Urinary Phthalate Metabolites Are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef]
- Hatch, E.E.; Nelson, J.W.; Stahlhut, R.W.; Webster, T.F. Association of Endocrine Disruptors and Obesity: Perspectives from Epidemiological Studies. Int. J. Androl. 2010, 33, 324–332. [Google Scholar] [CrossRef]
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of Exposure to Phthalates with Cardiometabolic Risk Factors in Children and Adolescents: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2019, 26, 35670–35686. [Google Scholar] [CrossRef]
- Zarean, M.; Poursafa, P.; Amin, M.M.; Kelishadi, R. Association of Endocrine Disrupting Chemicals, Bisphenol A and Phthalates, with Childhood Obesity: A Systematic Review. J. Pediatr. Rev. 2017, 6, e11894. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Beserra, B.T.S.; Silva, N.G.; Lima, C.L.; Rocha, P.R.S.; Coelho, M.S.; Neves, F.D.A.R.; Amato, A.A. Exposure to Endocrine-Disrupting Chemicals and Anthropometric Measures of Obesity: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e033509. [Google Scholar] [CrossRef]
- Wu, Q.; Li, G.; Zhao, C.-Y.; Na, X.-L.; Zhang, Y.-B. Association between Phthalate Exposure and Obesity Risk: A Meta-Analysis of Observational Studies. Environ. Toxicol. Pharmacol. 2023, 102, 104240. [Google Scholar] [CrossRef] [PubMed]
- Buser, M.C.; Murray, H.E.; Scinicariello, F. Age and Sex Differences in Childhood and Adulthood Obesity Association with Phthalates: Analyses of NHANES 2007–2010. Int. J. Hyg. Environ. Health 2014, 217, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, D.; Li, Y.; Yang, Z.; Wang, X.; Chen, M.; Wang, Z.; Song, Y.; Zou, Z.; Ma, J. Effect of Childhood Phthalates Exposure on the Risk of Overweight and Obesity: A Nested Case-Control Study in China. Environ. Int. 2022, 158, 106886. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yao, Y.; Chen, D.; Wu, Y.; Liao, Y.; Zhou, L. Phthalates, Physical Activity, and Diet, Which Are the Most Strongly Associated with Obesity? A Case-Control Study of Chinese Children. Endocrine 2023, 82, 69–77. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, D.; Zou, Z. The Association of Phthalate Metabolites with Childhood Waist Circumference and Abdominal Obesity. Eur. J. Pediatr. 2022, 182, 803–812. [Google Scholar] [CrossRef]
- Wassenaar, P.N.H.; Legler, J. Systematic Review and Meta-Analysis of Early Life Exposure to Di(2-Ethylhexyl) Phthalate and Obesity Related Outcomes in Rodents. Chemosphere 2017, 188, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Deierlein, A.L.; Wolff, M.S.; Pajak, A.; Pinney, S.M.; Windham, G.C.; Galvez, M.P.; Silva, M.J.; Calafat, A.M.; Kushi, L.H.; Biro, F.M.; et al. Longitudinal Associations of Phthalate Exposures during Childhood and Body Size Measurements in Young Girls. Epidemiology 2016, 27, 492–499. [Google Scholar] [CrossRef]
- Srilanchakon, K.; Thadsri, T.; Jantarat, C.; Thengyai, S.; Nosoognoen, W.; Supornsilchai, V. Higher Phthalate Concentrations Are Associated with Precocious Puberty in Normal Weight Thai Girls. J. Pediatr. Endocrinol. Metab. 2017, 30, 1293–1298. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kelishadi, R.; Amin, M.M.; Ebrahim, K. Is There Any Association between Phthalate Exposure and Precocious Puberty in Girls? Environ. Sci. Pollut. Res. 2018, 25, 13589–13596. [Google Scholar] [CrossRef]
- Amin, M.M.; Ebrahimpour, K.; Parastar, S.; Shoshtari-Yeganeh, B.; Hashemi, M.; Mansourian, M.; Poursafa, P.; Fallah, Z.; Rafiei, N.; Kelishadi, R. Association of Urinary Concentrations of Phthalate Metabolites with Cardiometabolic Risk Factors and Obesity in Children and Adolescents. Chemosphere 2018, 211, 547–556. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate Exposure and Childhood Overweight and Obesity: Urinary Metabolomic Evidence. Environ. Int. 2018, 121, 159–168. [Google Scholar] [CrossRef]
- Su, P.-H.; Huang, J.-Y.; Wang, S.-L.J.; Chang, H.-P. Phthalates Exposure and Pubertal Development in a 15-Year Follow-up Birth Cohort Study in Taiwan. Front. Endocrinol. 2023, 14, 1065918. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Labra, M.; NBFC Collaborator Group. Biodiversity and planetary health: A call for integrated action. Lancet 2024, 403, 1985–1986. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Cena, H.; Loperfido, F.; Rossi, V.; Grazi, R.; Quatrale, A.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G. Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity. Nutrients 2024, 16, 2732. https://doi.org/10.3390/nu16162732
Calcaterra V, Cena H, Loperfido F, Rossi V, Grazi R, Quatrale A, De Giuseppe R, Manuelli M, Zuccotti G. Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity. Nutrients. 2024; 16(16):2732. https://doi.org/10.3390/nu16162732
Chicago/Turabian StyleCalcaterra, Valeria, Hellas Cena, Federica Loperfido, Virginia Rossi, Roberta Grazi, Antonia Quatrale, Rachele De Giuseppe, Matteo Manuelli, and Gianvincenzo Zuccotti. 2024. "Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity" Nutrients 16, no. 16: 2732. https://doi.org/10.3390/nu16162732
APA StyleCalcaterra, V., Cena, H., Loperfido, F., Rossi, V., Grazi, R., Quatrale, A., De Giuseppe, R., Manuelli, M., & Zuccotti, G. (2024). Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity. Nutrients, 16(16), 2732. https://doi.org/10.3390/nu16162732










