The Effects of Epigallocatechin-3-Gallate Nutritional Supplementation in the Management of Multiple Sclerosis: A Systematic Review of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Data Collection and Selection Process
2.5. Data Items
2.6. Risk of Bias and Quality Assessment
2.7. Synthesis Methods
3. Results
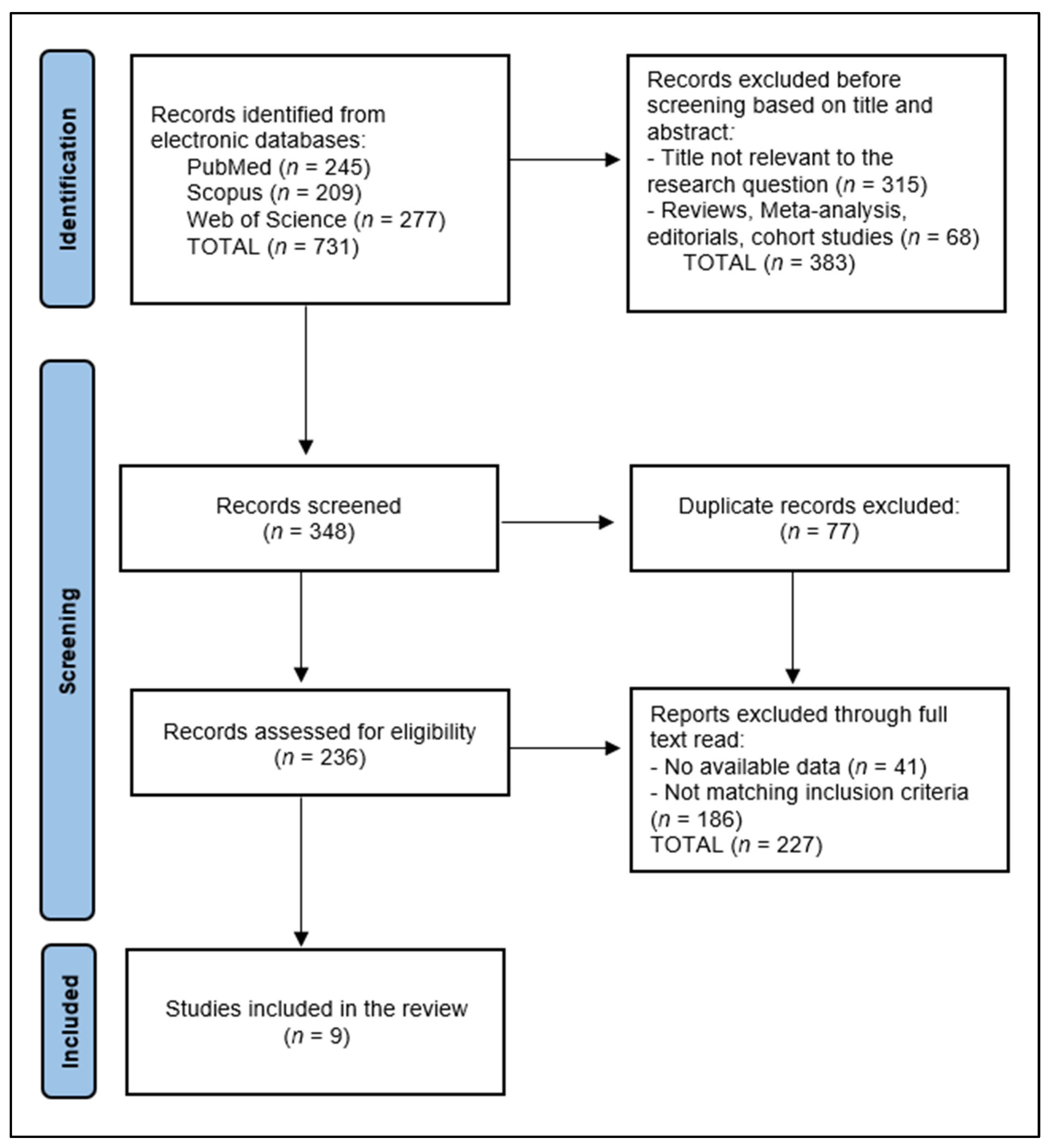
3.1. Study Selection and Study Characteristics
3.2. Results of Synthesis
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Talanki Manjunatha, R.; Habib, S.; Sangaraju, S.L.; Yepez, D.; Grandes, X.A. Multiple Sclerosis: Therapeutic Strategies on the Horizon. Cureus 2022, 14, e24895. [Google Scholar] [CrossRef] [PubMed]
- Ellen, O.; Ye, S.; Nheu, D.; Dass, M.; Pagnin, M.; Ozturk, E.; Theotokis, P.; Grigoriadis, N.; Petratos, S. The Heterogeneous Multiple Sclerosis Lesion: How Can We Assess and Modify a Degenerating Lesion? Int. J. Mol. Sci. 2023, 24, 11112. [Google Scholar] [CrossRef]
- Inojosa, H.; Proschmann, U.; Akgün, K.; Ziemssen, T. The need for a strategic therapeutic approach: Multiple sclerosis in check. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063032. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Samjoo, I.A.; Drudge, C.; Walsh, S.; Tiwari, S.; Brennan, R.; Boer, I.; Häring, D.A.; Klotz, L.; Adlard, N.; Banhazi, J. Comparative efficacy of therapies for relapsing multiple sclerosis: A systematic review and network meta-analysis. J. Comp. Eff. Res. 2023, 12, e230016. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Moreo, N. Disease-Modifying Therapies in Multiple Sclerosis: Overview and Treatment Considerations. Fed. Pract. 2016, 33, 28–34. [Google Scholar] [PubMed]
- Lee, C.Y.; Chan, K.H. Personalized Use of Disease-Modifying Therapies in Multiple Sclerosis. Pharmaceutics 2024, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Rafiee Zadeh, A.; Askari, M.; Azadani, N.N.; Ataei, A.; Ghadimi, K.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 95–104. [Google Scholar]
- Vivekanandan, G.; Abubacker, A.P.; Myneni, R.; Chawla, H.V.; Iqbal, A.; Grewal, A.; Ndakotsu, A.; Khan, S. Risk of Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis Patient Treated with Natalizumab: A Systematic Review. Cureus 2021, 13, e14764. [Google Scholar] [CrossRef]
- Moutinho, B.D.; de Barros, J.R.; Baima, J.P.; Saad-Hossne, R.; Sassaki, L.Y. Immunosuppression and Malignant Neoplasms: Risk-Benefit Assessment in Patients with Inflammatory Bowel Disease. Am. J. Case Rep. 2020, 21, e920949. [Google Scholar] [CrossRef] [PubMed]
- Tryfonos, C.; Mantzorou, M.; Fotiou, D.; Vrizas, M.; Vadikolias, K.; Pavlidou, E.; Giaginis, C. Dietary Supplements on Controlling Multiple Sclerosis Symptoms and Relapses: Current Clinical Evidence and Future Perspectives. Medicines 2019, 6, 95. [Google Scholar] [CrossRef]
- Ghareghani, M.; Zibara, K.; Rivest, S. Melatonin and vitamin D, two sides of the same coin, better to land on its edge to improve multiple sclerosis. Proc. Natl. Acad. Sci. USA 2023, 120, e2219334120. [Google Scholar] [CrossRef]
- Cai, F.; Liu, S.; Lei, Y.; Jin, S.; Guo, Z.; Zhu, D.; Guo, X.; Zhao, H.; Niu, X.; Xi, Y.; et al. Epigallocatechin-3 gallate regulates macrophage subtypes and immunometabolism to ameliorate experimental autoimmune encephalomyelitis. Cell. Immunol. 2021, 368, 104421. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 65064, Epigallocatechin Gallate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Epigallocatechin-Gallate (accessed on 8 June 2024).
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Rani, A.; Saini, V.; Patra, P.; Prashar, T.; Pandey, R.K.; Mishra, A.; Jha, H.C. Epigallocatechin Gallate: A Multifaceted Molecule for Neurological Disorders and Neurotropic Viral Infections. ACS Chem. Neurosci. 2023, 14, 2968–2980. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; Ortí, J.E.R. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Bellmann-Strobl, J.; Paul, F.; Wuerfel, J.; Dörr, J.; Infante-Duarte, C.; Heidrich, E.; Körtgen, B.; Brandt, A.; Pfüller, C.; Radbruch, H.; et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis: A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e981. [Google Scholar] [CrossRef]
- Mähler, A.; Steiniger, J.; Bock, M.; Klug, L.; Parreidt, N.; Lorenz, M.; Zimmermann, B.F.; Krannich, A.; Paul, F.; Boschmann, M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2015, 101, 487–495. [Google Scholar] [CrossRef]
- Benlloch, M.; Cuerda Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.Á.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Platero Armero, J.L.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Navarro-Illana, E.; Lajara-Romance, J.M.; Benlloch, M.; Ceron, J.J.; Tvarijonaviciute, A.; Proaño, B. Lipid Profile in Multiple Sclerosis: Functional Capacity and Therapeutic Potential of Its Regulation after Intervention with Epigallocatechin Gallate and Coconut Oil. Foods 2023, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.; Chien, C.; Scheel, M.; Brandt, A.U.; Dörr, J.; Wuerfel, J.; Klumbies, K.; Zimmermann, H.; Lorenz, M.; Wernecke, K.D.; et al. Epigallocatechin Gallate in Progressive MS: A Randomized, Placebo-Controlled Trial. Neurol. (R) Neuroimmunol. Neuroinflamm. 2021, 8, e964. [Google Scholar] [CrossRef]
- Cuerda-Ballester, M.; Proaño, B.; Alarcón-Jimenez, J.; de Bernardo, N.; Villaron-Casales, C.; Lajara Romance, J.M.; de la Rubia Ortí, J.E. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food Funct. 2023, 14, 1062–1071. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Sancho-Cantus, D.; Benlloch, M.; Ceron, J.J.; Peres Rubio, C.; García-Pardo, M.P.; López-Rodríguez, M.M.; de la Rubia Ortí, J.E. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life 2021, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Ortí, J.E.; Platero, J.L.; Benlloch, M.; Franco-Martinez, L.; Tvarijonaviciute, A.; Escribá-Alepuz, J.; Sancho-Castillo, S. Role of Haptoglobin as a Marker of Muscular Improvement in Patients with Multiple Sclerosis after Administration of Epigallocatechin Gallate and Increase of Beta-Hydroxybutyrate in the Blood: A Pilot Study. Biomolecules 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Oh, H.; Kang, B.G.; Kang, M.K.; Kim, D.Y.; Kim, Y.H.; Lee, J.Y.; Ji, J.G.; Lim, S.S.; Kang, Y.H. Lipid-Lowering Effects of Medium-Chain Triglyceride-Enriched Coconut Oil in Combination with Licorice Extracts in Experimental Hyperlipidemic Mice. J. Agric. Food Chem. 2018, 66, 10447–10457. [Google Scholar] [CrossRef]
- Duarte, A.C.; Spiazzi, B.F.; Zingano, C.P.; Merello, E.N.; Wayerbacher, L.F.; Teixeira, P.P.; Farenzena, L.P.; de Araujo, C.; Amazarray, C.R.; Colpani, V.; et al. The effects of coconut oil on the cardiometabolic profile: A systematic review and meta-analysis of randomized clinical trials. Lipids Health Dis. 2022, 21, 83. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed]
- Herges, K.; Millward, J.M.; Hentschel, N.; Infante-Duarte, C.; Aktas, O.; Zipp, F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE 2011, 6, e25456. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wen, M.; Liu, M.; Wang, Q.; Liu, Q.; Li, L.; Siebert, H.C.; Loers, G.; Zhang, R.; Zhang, N. Effect of β-hydroxybutyrate on behavioral alterations, molecular and morphological changes in CNS of multiple sclerosis mouse model. Front. Aging Neurosci. 2022, 14, 1075161. [Google Scholar] [CrossRef] [PubMed]
- Semnani, M.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017, 55, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Cascella, M.; Schiavone, V.; Mehrabi-Kermani, F.; Cuomo, A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: An update on preclinical in vivo studies and future perspectives. Drug Des. Devel Ther. 2017, 11, 2737–2742. [Google Scholar] [CrossRef]
- Klumbies, K.; Rust, R.; Dörr, J.; Konietschke, F.; Paul, F.; Bellmann-Strobl, J.; Brandt, A.U.; Zimmermann, H.G. Retinal Thickness Analysis in Progressive Multiple Sclerosis Patients Treated with Epigallocatechin Gallate: Optical Coherence Tomography Results From the SUPREMES Study. Front. Neurol. 2021, 12, 615790. [Google Scholar] [CrossRef] [PubMed]
- Afshar, B.; Ganjalikhani-Hakemi, M.; Khalifezadeh Esfahani, Z.; Eskandari, N.; Shaygannajad, V.; Hosseininasab, F.; Alsahebfosoul, F. Evaluating the Effects of Epigallocatechin-3-Gallate on HIF-1α Protein and RORC Gene Expression in Peripheral Blood Mononuclear Cells in Patients With Multiple Sclerosis. Basic Clin. Neurosci. 2021, 12, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mische, L.J.; Mowry, E.M. The Evidence for Dietary Interventions and Nutritional Supplements as Treatment Options in Multiple Sclerosis: A Review. Curr. Treat. Options Neurol. 2018, 20, 8. [Google Scholar] [CrossRef]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Evans, E.; Piccio, L.; Cross, A.H. Use of Vitamins and Dietary Supplements by Patients with Multiple Sclerosis: A Review. JAMA Neurol. 2018, 75, 1013–1021. [Google Scholar] [CrossRef]
- Mandato, C.; Colucci, A.; Lanzillo, R.; Staiano, A.; Scarpato, E.; Schiavo, L.; Operto, F.F.; Serra, M.R.; Di Monaco, C.; Napoli, J.S.; et al. Multiple Sclerosis-Related Dietary and Nutritional Issues: An Updated Scoping Review with a Focus on Pediatrics. Children 2023, 10, 1022. [Google Scholar] [CrossRef] [PubMed]

| Study and Author | Country | Study Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 [21] Platero et al. | Spain | 2020 | Randomized trial | Medium |
| 2 [22] Bellmann-Strobl et al. | Germany | 2021 | Randomized trial | High |
| 3 [23] Mähler et al. | Germany | 2015 | Randomized trial | High |
| 4 [24] Benlloch et al. | Spain | 2020 | Randomized trial | High |
| 5 [25] de la Rubia Ortí et al. | Spain | 2023 | Randomized trial | High |
| 6 [26] Rust et al. | Germany | 2021 | Randomized trial | High |
| 7 [27] Cuerda-Ballester et al. | Spain | 2023 | Randomized trial | Medium |
| 8 [28] Platero et al. | Spain | 2021 | Randomized trial | High |
| 9 [29] de la Rubia Ortí et al. | Spain | 2021 | Randomized trial | High |
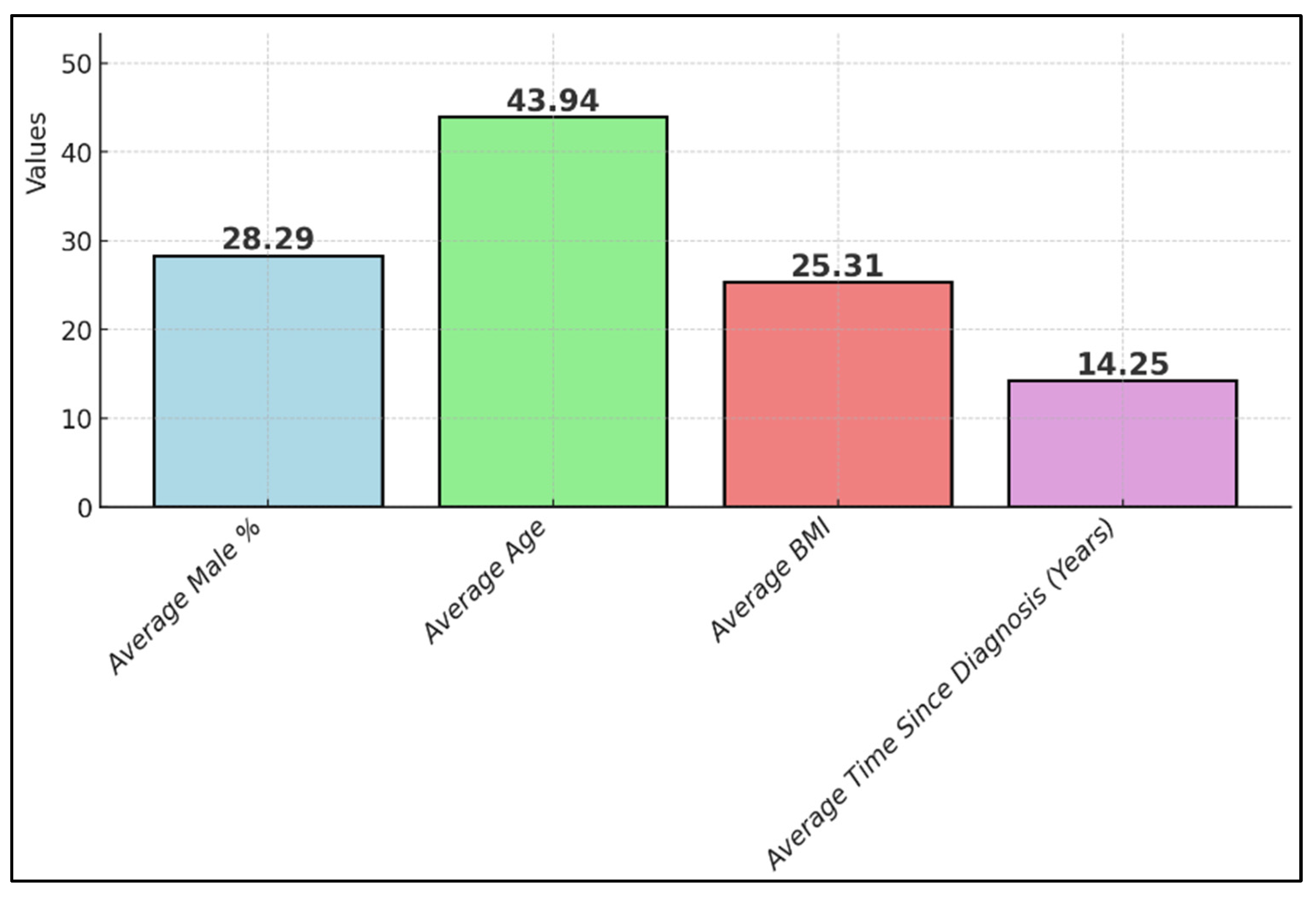
| Study Number | Sample Size (Intervention Group) | Mean Age/Age Range | Gender Distribution | Weight/BMI | Time Since Diagnosis |
|---|---|---|---|---|---|
| 1 [21] Platero et al. | 27 | 45 years | 18.5% male, 81.5% female | Pre-test BMI 23.43 kg/m2, post-test BMI 23.49 kg/m2 | Range 9–35 years |
| 2 [22] Bellmann-Strobl et al. | 62 | 39 years | 67% female, 33% male | NR | Median 6.1 years |
| 3 [23] Mähler et al. | 18 (8 men and 10 women) | 40 years for men, 45 years for women | 44.4% male, 55.6% female | Men 24.7 kg/m2, women 25.9 kg/m2 post-EGCG; men 24.0 kg/m2, women 25.8 kg/m2 post-placebo | 89 months (7–192) for men, 60 months (25–208) for women |
| 4 [24] Benlloch et al. | 51 | 44.5 years | 58.3% female, 41.7% male | Mean BMI: 25.92 kg/m2 | At least 6 months |
| 5 [25] de la Rubia Ortí et al. | 25 | 44 years | 20% male, 80% female | 25.9 kg/m2 | Mean 11.7 years |
| 6 [26] Rust et al. | 30 | 18–65 years | NR | NR | NR |
| 7 [27] Cuerda-Ballester et al. | 51 | 50 years | 31.7% male, 58.3% female | 18.9% fat mass | Median 14.5 years |
| 8 [28] Platero et al. | 27 | 44.5 years | 18.5% male, 81.5% female | 19.4% fat mass | Mean 11.8 years |
| 9 [29] de la Rubia Ortí et al. | 27 | 44.5 years | 18.5% male, 81.5% female | 25.97 kg/m2 | Mean 12 years |
| Study Number | Measurement/Dose/Administration | Follow-Up | Multiple Sclerosis Disease Features (Activity/Disability) | Therapeutic Effects | Interpretation |
|---|---|---|---|---|---|
| 1 [21] Platero et al. | 800 mg of EGCG and 60 mL of coconut oil | 4 months | EDSS pre-test: 3.00 EDSS Post-test: 3.00 (indicating no significant change in disability status) | IL-6: pre-test (pg/mL): 2.18, post-test (pg/mL): 0.84 (significant reduction) State anxiety (STAI): pre-test: 23.00, post-test: 17.00 (significant reduction) | Both EGCG and coconut oil contribute to reducing state anxiety and IL-6 levels, with a slight improvement in functional capacity |
| 2 [22] Bellmann-Strobl et al. | 800 mg of EGCG daily | 18 months | EDSS score at baseline: median 2.0, range 0–6.0 EDSS score at 18 months: median 2.2, change from baseline 0.14 | Annualized relapse rate: EGCG group: 0.47, placebo group: 0.50 MRI lesion activity: proportion of patients without new T2w lesions at 18 months: EGCG group: 29% (18 of 62 patients), placebo group: 25% (15 of 60 patients) (no significant difference) Number of new T2w lesions: EGCG group: mean 3.1, placebo group: mean 1.9 (no significant difference) | EGCG, added to glatiramer acetate, did not demonstrate superiority over placebo in reducing MRI and clinical disease activity over 18 months. It was safe to use at the tested dosage |
| 3 [23] Mähler et al. | 600 mg of EGCG daily | 12 months | EDSS score: ≤4.5 for all participants | Therapeutic effects: fat oxidation (FAOx) at rest (postprandial, g/4 h): placebo: men 9.2 ± 4.9, women 7.8 ± 4.2; EGCG: men 12.9 ± 5.7, women 6.2 ± 3.6 Energy expenditure efficiency during exercise (%): placebo: men 21 ± 3, women 20 ± 3; EGCG: men 27 ± 6, women 25 ± 7 | EGCG administration led to a sex-specific response in energy metabolism at rest and during exercise. In men, EGCG increased fat oxidation and exercise efficiency more than in women. The therapeutic effects suggest that EGCG may improve metabolic function in MS patients, particularly in men |
| 4 [24] Benlloch et al. | 800 mg of EGCG and 60 mL of coconut oil daily | 4 months | EDSS pre-test: 3.80 ± 2.00, EDSS post-test: 3.37 ± 2.03 | WHR decreased from 0.95 to 0.87, WHtR decreased from 0.60 to 0.55, fat mass decreased from 19.01% to 17.74%, muscle mass increased from 38.01% to 41.10%, albumin increased from 4.55 g/dL to 4.83 g/dL, BHB increased from 0.04 mMol/L to 0.10 mMol/L, PON1 increased from 2.49 UI/L to 2.97 UI/L | EGCG combined with coconut oil significantly altered cardiac risk markers, improving metabolic health in MS patients, potentially lowering cardiovascular risk |
| 5 [25] de la Rubia Ortí et al. | 800 mg of EGCG and 60 mL of coconut oil | 4 months | NR | Triglycerides: control: pre: 112.8, post: 142 mg/dL; intervention: pre: 103.6, post: 88.7 mg/dL Total cholesterol (TC): control: pre: 210.8, post: 236.4 mg/dL; intervention: pre: 220.9, post: 232.5 mg/dL HDL cholesterol: control: pre: 76.56, post: 86 mg/dL; intervention: 84.81 mg/dL | EGCG and coconut oil can positively influence lipid metabolism, particularly by reducing triglyceride levels, which correlates with improvements in functional disability in MS patients |
| 6 [26] Rust et al. | 1200 mg of EGCG daily | 36 months with an optional 12-month extension | EDSS at screening was 3–8.37% in the EGCG group and 39% in the placebo group (with primary progressive disease) | Study did not meet its primary endpoint as the rate of decrease in brain parenchymal fraction over 36 months was 0.0092 in the EGCG group and −0.0078 in the placebo group. Annualized atrophy rates (AARs) were 0.31% for the EGCG group and 0.26% for the placebo group | Primary endpoint of reducing brain atrophy not met; no significant changes in MRI and clinical end points |
| 7 [27] Cuerda-Ballester et al. | 800 mg of EGCG and 60 mL of coconut oil daily | 4 months | EDSS significant improvement from 3.7 | Berg balance scale score increasing from an initial average of 49 ± 9.6 to 52 ± 6.9 after treatment; significant improvement in the intervention group, where the 10 m walk test (10 MWT) times improved from 1.56 ± 0.58 m/s to 1.73 ± 0.61 m/s; 2 WMT distance increased from 113 ± 38 m to 136 ± 41 m | EGCG and coconut oil improve gait speed and balance, contributing to enhanced functionality in MS patients |
| 8 [28] Platero et al. | 800 mg of EGCG and 60 mL of coconut oil daily | 4 months | NR | Depression (BDI-II scale) decreased from a median of 12.0 to 8.0 | Significant reduction in depression levels and abdominal fat; increase in albumin levels in the intervention group |
| 9 [29] de la Rubia Ortí et al. | 800 mg of EGCG and 60 mL of coconut oil daily | 4 months | NR | BHB levels increased from 0.05 to 0.10 mMol/L, muscle mass increased significantly, IL-6 levels decreased | Intervention showed improvements in muscle mass and reductions in inflammation markers, indicating positive therapeutic effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuldesz, A.C.; Tudor, R.; Nandarge, P.S.; Elagez, A.; Cornea, A.; Ion, R.; Bratosin, F.; Prodan, M.; Simu, M. The Effects of Epigallocatechin-3-Gallate Nutritional Supplementation in the Management of Multiple Sclerosis: A Systematic Review of Clinical Trials. Nutrients 2024, 16, 2723. https://doi.org/10.3390/nu16162723
Schuldesz AC, Tudor R, Nandarge PS, Elagez A, Cornea A, Ion R, Bratosin F, Prodan M, Simu M. The Effects of Epigallocatechin-3-Gallate Nutritional Supplementation in the Management of Multiple Sclerosis: A Systematic Review of Clinical Trials. Nutrients. 2024; 16(16):2723. https://doi.org/10.3390/nu16162723
Chicago/Turabian StyleSchuldesz, Amanda Claudia, Raluca Tudor, Prashant Sunil Nandarge, Ahmed Elagez, Amalia Cornea, Radu Ion, Felix Bratosin, Mihaela Prodan, and Mihaela Simu. 2024. "The Effects of Epigallocatechin-3-Gallate Nutritional Supplementation in the Management of Multiple Sclerosis: A Systematic Review of Clinical Trials" Nutrients 16, no. 16: 2723. https://doi.org/10.3390/nu16162723
APA StyleSchuldesz, A. C., Tudor, R., Nandarge, P. S., Elagez, A., Cornea, A., Ion, R., Bratosin, F., Prodan, M., & Simu, M. (2024). The Effects of Epigallocatechin-3-Gallate Nutritional Supplementation in the Management of Multiple Sclerosis: A Systematic Review of Clinical Trials. Nutrients, 16(16), 2723. https://doi.org/10.3390/nu16162723







