Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria
Abstract
1. Introduction
2. Methods
2.1. The SN2 Study Cohort
2.2. The NAC Cohort
2.3. Assessment at Every Visit
2.4. Nitisinone Therapy
2.5. Dietetic Assessments and Management in the NAC
2.6. Statistical Analysis
3. Results
3.1. Baseline Comparisons
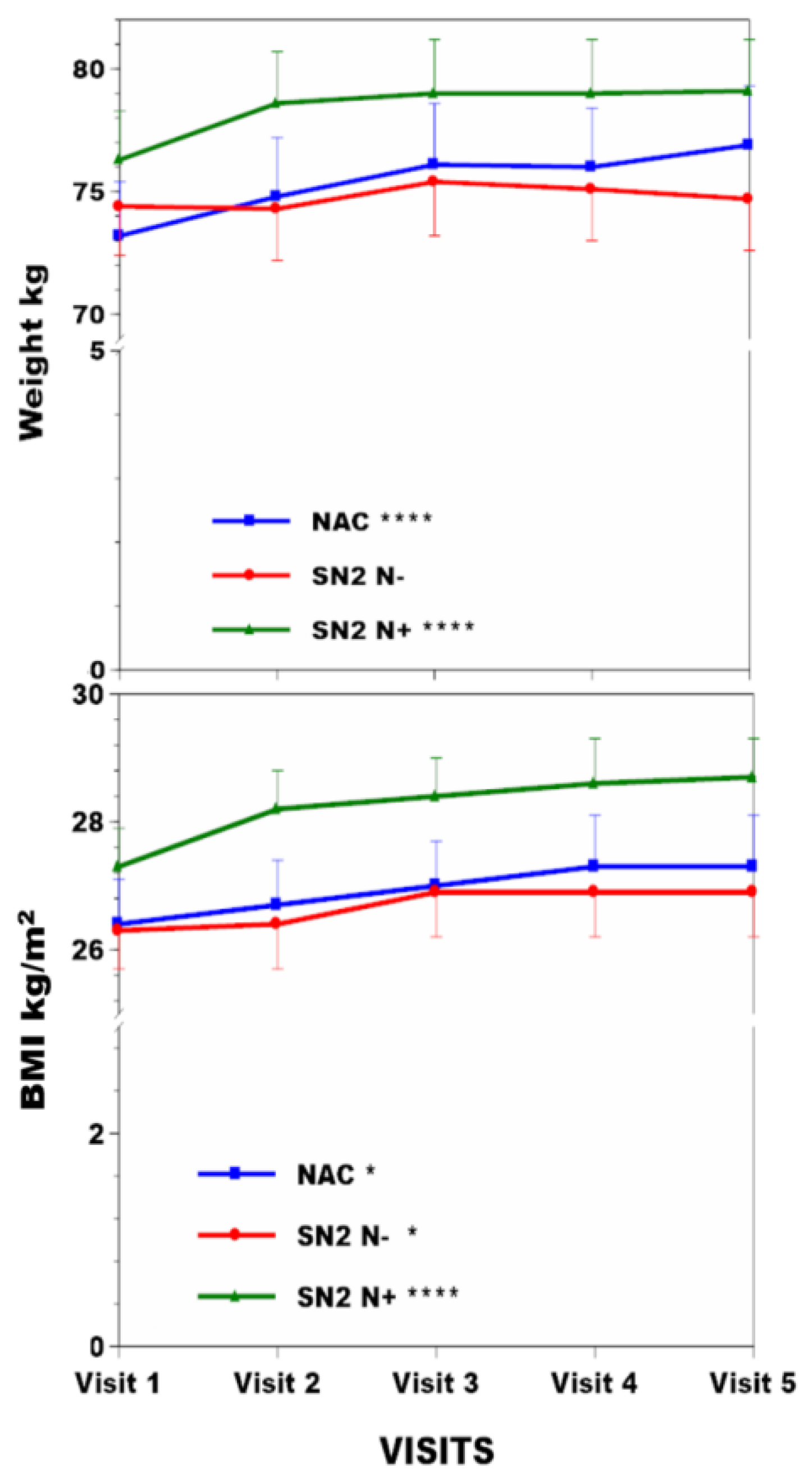
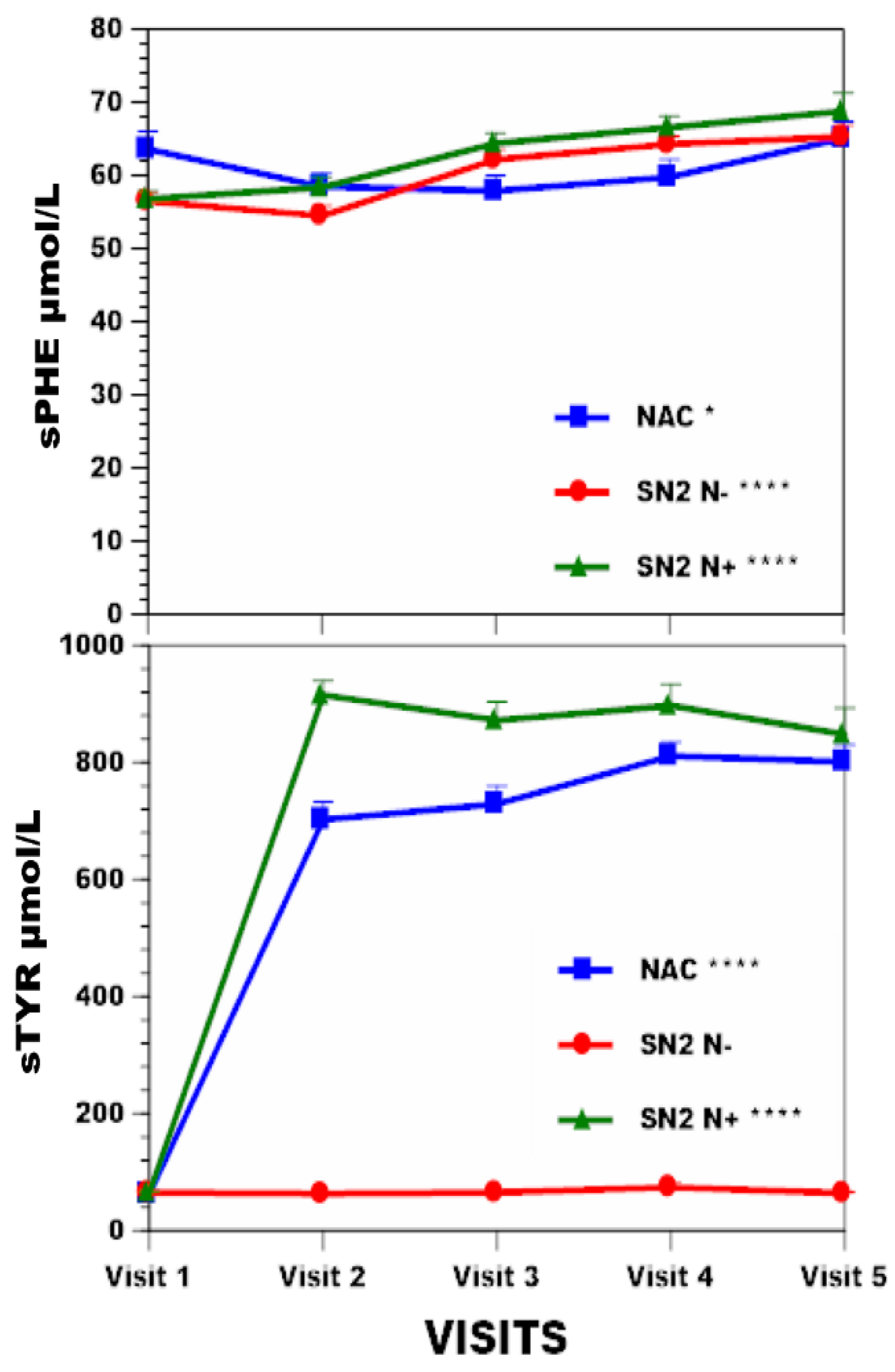
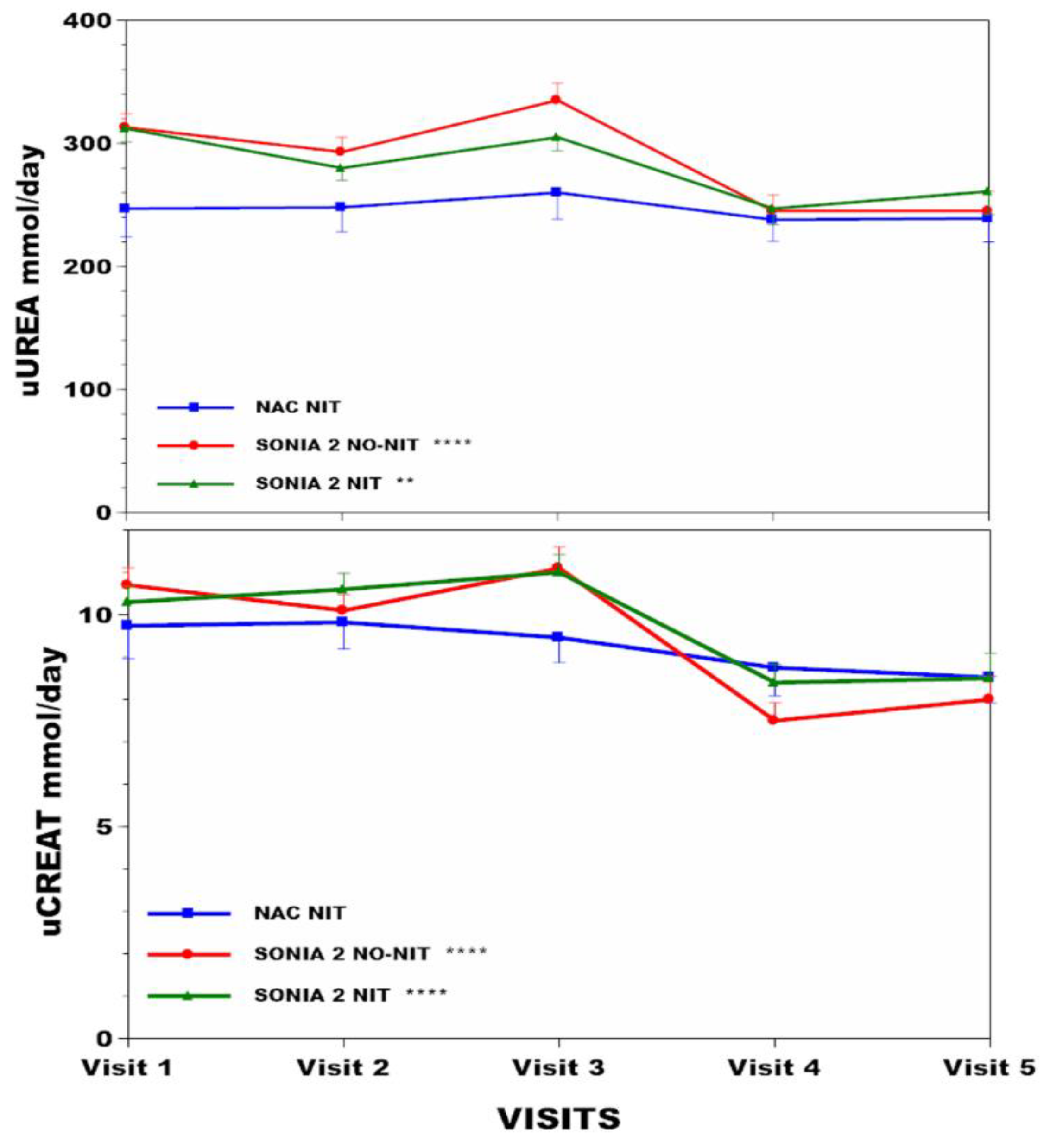
3.2. Change in Anthropometric and Metabolic Data in the NAC, SN−, and SN+ Groups over Time (Table 1)
| Age (y) | Weight kg | BMI kg/m2 | sTYR µmol/L | Corneal Keratopathy % | sPHE µmol/L | uCREAT mmol/day | uUREA mmol/day | ||
|---|---|---|---|---|---|---|---|---|---|
| NAC (n = 63) | Visit 1 | 47.1 (14.7) | 73.2 (13.9) *** | 26.4 (4.2) * | 62 (39) *** | 63.6 (11.5) * | 9.74 (3.51) ¥ | 247 (97) | |
| Visit 2 | 74.8 (14.9) | 26.7 (4.2) | 702 (194) | 4.1 | 58.5 (8.4) | 9.82 (2.81) | 248 (85) | ||
| Visit 3 | 76.1 (15.5) | 27 (4.2) | 729 (200) | 57.9 (9.6) | 9.46 (2.65) | 260 (91) | |||
| Visit 4 | 76 (15.2) | 27.3 (4.5) | 811 (153) | 59.8 (11.2) | 8.75 (2.94) | 238 (74) | |||
| Visit 5 | 76.9 (15) | 27.3 (4.5) | 801 (185) | 65.1 (11) | 8.52 (2.68) | 239 (81) | |||
| SN2 N− (n = 69) | Visit 1 | 47.6 (10.1) | 74.4 (14.2) | 26.3 (4.4) * | 65 (16) | 56.5 (9.5) *** | 10.7 (3.4) *** | 313 (92) *** | |
| Visit 2 | 74.3 (14.5) | 26.4 (4.6) | 63 (21) | 0 | 54.5 (10.8) | 10.1 (3) | 293 (97) | ||
| Visit 3 | 75.4 (15.3) | 26.9 (4.9) | 65 (14) | 62.2 (9.9) | 11.1 (3.8) | 335 (110) | |||
| Visit 4 | 75.1 (14.5) | 26.9 (4.7) | 74 (58) | 64.3 (8.8) | 7.5 (3.3) | 245 (102) | |||
| Visit 5 | 74.7 (14.9) | 26.9 (4.8) | 64 (16) | 65.3 (11.9) | 8 (4) | 245 (102) | |||
| SN2 N+ (n = 69) | Visit 1 | 49.0 (11.3) | 76.3 (15.1) *** | 27.3 (4.4) *** | 65 (15) *** | 56.8 (9.5) *** | 10.3(3) *** | 312 (94) ** | |
| Visit 2 | 78.6 (15.8) | 28.2 (4.7) | 915 (204) | 14.5 | 58.4 (11.7) | 10.6(3.1) | 280 (83) | ||
| Visit 3 | 79 (16) | 28.4 (4.6) | 872 (246) | 64.4 (10.9) | 11(3.4) | 305 (92) | |||
| Visit 4 | 79 (16.3) | 28.6 (4.8) | 897 (283) | 66.6 (11.9) | 8.4(3.3) | 247 (100) | |||
| Visit 5 | 79.1 (15.9) | 28.7 (4.7) | 848 (338) | 68.8 (10) | 8.5(4.4) | 261 (137) |
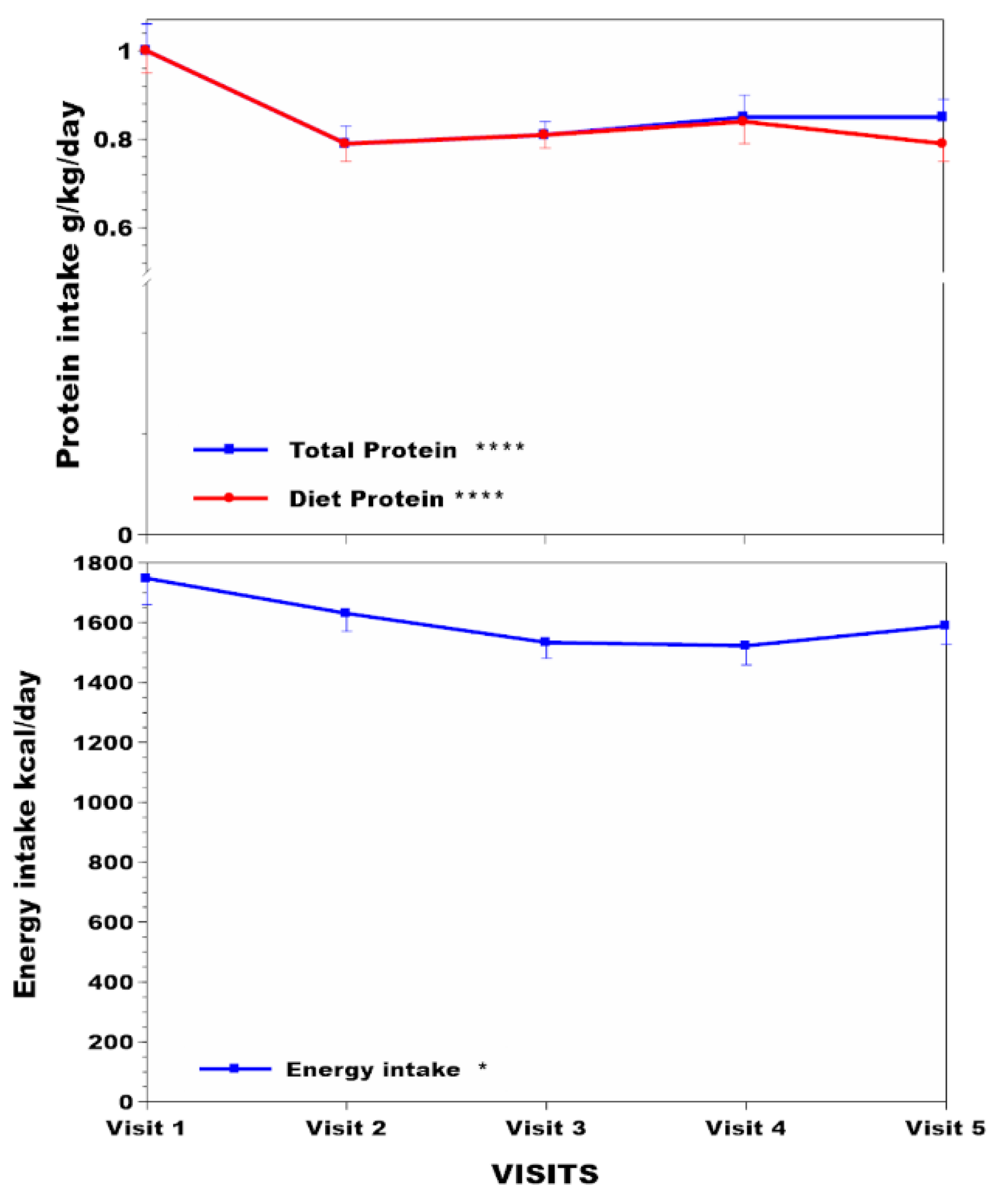
3.3. Body Compositional Analysis in the NAC over Time
3.4. Linear Regression Analysis of Relationships between sTYR, Total Protein Intake, and uUREA with Other Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrod, A.E. The incidence of alkaptonuria: A study in chemical individuality. Lancet 1902, 2, 1616–1620. [Google Scholar] [CrossRef]
- LaDu, B.N.; Zannoni, V.G.; Laster, L.; Seegmiller, J.E. The nature of the defect in tyrosine metabolism in alcaptonuria. J. Biol. Chem. 1958, 230, 251–260. [Google Scholar] [CrossRef]
- Virchow, R. Ein Fall von allgemeiner Ochronose der Knorpel und knorpelähnlichen 806 26 Theile. Arch. Pathol. Anat. Physiol. Klin. Med. 1866, 37, 212–219. [Google Scholar]
- Ranganath, L.R.; Norman, B.P.; Gallagher, J.A. Ochronotic pigmentation is caused by homogentisic acid and is the key event in Alkaptonuria leading to the destructive consequences of the disease—A review. J. Inherit. Metab. Dis. 2019, 42, 776–792. [Google Scholar] [CrossRef]
- O’Brien, W.M.; La Du, B.N.; Bunim, J.J. Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: Review of world literature (1584–1962). Am. J. Med. 1963, 34, 813–838. [Google Scholar] [CrossRef]
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural history of Alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef]
- Judd, S.; Khedr, M.; Milan, A.M.; Davison, A.S.; Hughes, A.T.; Needham, A.; Psarelli, E.E.; Shenkin, A.; Ranganath, L.R. The Nutritional Status of People with Alkaptonuria—An exploratory analysis suggests a protein/energy dilemma. JIMD Rep. 2020, 53, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Dutton, J.J.; Fitzgerald, R.; Briggs, M.C.; Bygott, H.; Psarelli, E.E.; Cox, T.F.; Gallagher, J.A.; et al. Suitability of nitisinone in alkaptonuria 1 (SONIA 1): An international, multicentre, randomised, open label, no treatment controlled, parallel group, dose response study to investigate the effect of once daily nitisinone on 24h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann. Rheum. Dis. 2016, 75, 362–367. [Google Scholar]
- Ranganath, L.R.; Psarelli, E.E.; Arnoux, J.-B.; Braconi, D.; Briggs, M.; Bröijersén, A.; Loftus, N.; Bygott, H.; Cox, T.F.; Davison, A.S.; et al. Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2)—A randomised study on the efficacy and safety of nitisinone in alkaptonuria. Lancet Diab Endocrinol. 2020, 8, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Ellis, M.K.; Gaskin, P.; Robinson, M.; Auton, T.R.; Provan, W.M.; Smith, L.L.; Prisbylla, M.P.; Mutter, L.C.; Lee, D.L. From toxicological problem to therapeutic use: The discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J. Inherit. Metab. Dis. 1998, 21, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.M.; Briggs, M.C.; Jarvis, J.C.; Gallagher, J.A.; Ranganath, L. Reversible keratopathy due to hypertyrosinaemia following intermittent low-dose nitisinone in alkaptonuria—A case report. JIMD Rep. 2014, 17, 1–6. [Google Scholar]
- Ranganath, L.R.; Khedr, M.; Milan, A.M.; Davison, A.S.; Hughes, A.T.; Usher, J.L.; Taylor, S.; Loftus, N.; Daroszewska, A.; West, E.; et al. Nitisinone arrests ochronosis and decreases rate of progression of alkaptonuria: Evaluation of the effect of nitisinone in the United Kingdom National Alkaptonuria Centre. Mol. Genet. Metab. 2018, 125, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Olsson, B.; Rudebeck, M.; Imrich, R.; Norman, B.P.; Bou-Gharios, G.; Gallagher, J.A.; et al. Temporal adaptations in the phenylalanine/tyrosine pathway and related factors during nitisinone-induced tyrosinaemia in alkaptonuria. Mol. Genet. Metab. 2022. online ahead of print. [Google Scholar]
- Hughes, A.T.; Milan, A.M.; Christensen, P.; Ross, G.; Davison, A.S.; Gallagher, J.A.; Dutton, J.J.; Ranganath, L.R. Urine homogentisic acid and tyrosine: Simultaneous analysis by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 963, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.T.; Milan, A.M.; Davison, A.S.; Christensen, P.; Ross, G.; Gallagher, J.A.; Dutton, J.J.; Ranganath, L.R. Serum markers in alkaptonuria: Simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 2015, 52, 597–605. [Google Scholar] [CrossRef]
- Kida, Y.; Ueda, H.; Tanaka, H.; Ichinose, M. Estimation of protein intake using urinary urea nitrogen in patients with early-stage liver cirrhosis. Hepatol. Int. 2007, 1, 382–386. [Google Scholar] [CrossRef][Green Version]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef]
- National Diet & Nutrition Survey. Results from Years 5-6 of the Rolling Programme, 2012/13, 2013/14; Public Health England: London, UK, 2016; Gateway Number 2016248. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organ Tech Rep Ser. 894:i-xii; WHO: Geneva, Switzerland, 2000; pp. 1–253. [Google Scholar] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Imrich, R.; Rudebeck, M.; Olsson, B.; Norman, B.P.; Bou-Gharios, G.; et al. Comparing the phenylalanine/tyrosine pathway and related factors between keratopathy and no-keratopathy groups as well as between genders in alkaptonuria during nitisinone treatment. Metabolites 2022, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Pencharz, P.B.; Hsu, J.W.-C.; Ball, R.O. Aromatic amino acid requirements in healthy human subjects. J. Nutr. 2007, 137, 1576S–1578S, discussion 1597S–1598S. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.; Khedr, M.; Evans, L.A.; Bygott, H.; Luangrath, E.; West, E. Vitiligo, alkaptonuria, and nitisinone—A report of four cases and review of the literature. JIMD Rep. 2021, 61, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.; Khedr, M.; Milan, A.M.; Davison, A.S.; Norman, B.P.; Janssen, M.C.H.; Lock, E.; Bou-Gharios, G.; Gallagher, J.A. Increased prevalence of Parkinson’s disease in Alkaptonuria—Report of five new cases and a review of the literature. J. Inherit. Metab. Dis. Rep. 2023, 64, 282–292. [Google Scholar]
- Ahmad, M.S.Z.; Ahmed, M.; Khedr, M.; Borgia, A.; Madden, A.; Ranganath, L.R.; Kaye, S. Association of alkaptonuria and low dose nitisinone therapy with cataract formation in a large cohort of patients. JIMD Rep. 2022, 63, 351–360. [Google Scholar] [CrossRef]
- Lock, E.A.; Gaskin, P.; Ellis, M.K.; Provan, W.M.; Robinson, M.; Smith, L.L.; Prisbylla, M.P.; Mutter, L.C. Tissue distribution of 2-(2 nitro-4-trifluromethylbenzoyl)-1,3-cyclohexanedione (NTBC): Effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol. Appl. Pharmacol. 1996, 141, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B.; Williams, D.L. Enhancement of rat brain catecholamine synthesis by administration of small doses of tyrosine and evidence for substrate inhibition of tyrosine hydroxylase activity by large doses of the amino acid. Biochem. J. 1982, 206, 165–168. [Google Scholar] [CrossRef]
- Norman, B.P.; Davison, A.S.; Hickton, B.; Ross, G.A.; Milan, A.M.; Hughes, A.T.; Wilson, P.J.M.; Sutherland, H.; Hughes, J.H.; Roberts, N.B.; et al. Comprehensive biotransformation analysis of phenylalanine-tyrosine metabolism reveals alternative routes of metabolite clearance in nitisinone-treated alkaptonuria. Metabolites 2022, 12, 927. [Google Scholar] [CrossRef]
- Masud, T.; Young, V.R.; Chapman, T.; Maroni, B.J. Adaptive responses to very low protein diets: The first comparison of ketoacids to essential amino acids. Kidney Int. 1994, 45, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Gaudichon, C.; Calvez, J. Determinants of amino acid bioavailability from ingested protein in relation to gut health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.H.; Wilson, P.J.M.; Sutherland, H.; Judd, S.; Hughes, A.T.; Milan, A.M.; Jarvis, J.C.; Bou-Gharios, G.; Ranganath, L.R.; Gallagher, J.A. Dietary restriction of tyrosine and phenylalanine lowers nitisinone-induced tyrosinaemia in the alkaptonuria mouse model. J. Inherit. Metab. Dis. 2020, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Norman, B.P.; Bou-Gharios, G.; Gallagher, J.A.; Imrich, R.; Arnoux, J.B.; et al. Determinants of tyrosinaemia during nitisinone therapy in alkaptonuria. Sci. Rep. 2022, 12, 16083. [Google Scholar] [CrossRef]
- Rudebeck, M.; Scott, C.; Rhodes, N.P.; van Kan, C.; Olsson, B.; Al-Sbou, M.; Hall, A.K.; Sireau, N.; Ranganath, L.R. Clinical development innovation in rare diseases: Lessons learned and best practices from the DevelopAKUre consortium. Orphanet J. Rare Dis. 2021, 16, 510. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine—From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Oterdoom, H.; Gansevoort, R.T.; Schouten, J.P.; de Jong, P.E.; Gans, R.O.; Bakker, S.J. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 2009, 207, 534–540. [Google Scholar] [CrossRef]
- Joachim, H.; de Boer, I.H.; Wassel, C.L.; Criqui, M.H.; Shlipak, M.G.; Whooley, M.A. Urinary Creatinine Excretion Rate and Mortality in Persons with Coronary Artery Disease: The Heart and Soul Study. Circulation 2010, 121, 1295–1303. [Google Scholar]
- Morell, P.; Fiszman, S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocoll. 2017, 68, 199–210. [Google Scholar] [CrossRef]
- Cook, A.; Pryer, J.; Shetty, P. The problem of accuracy in dietary surveys. Analysis of the over 65 UK National Diet and Nutrition Survey. J. Epidemiol. Community Health 2000, 54, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Khedr, M.; Norman, B.P.; Alsbou, M.; Imrich, R.; Gornall, M.; Sireau, N.; Gallagher, J.A.; et al. Comparing nitisinone 2 mg and 10 mg in the treatment of alkaptonuria—An approach using statistical modelling. JIMD Rep. 2021, 63, 80–92. [Google Scholar] [CrossRef]

| sTYR | Total Protein Intake | uUREA |
|---|---|---|
| Vs uUREA (R 0.08) | Vs BMI (R −0.53) **** | Vs uCREAT (R 0.69) **** |
| Vs Weight (R −0.57) **** | ||
| Vs sPHE (R 0.18) *** | Vs Muscle mass (R −0.39) **** | |
| Vs Diet protein intake (R 0.04) | Vs MUAC (R −0.48) **** | |
| Vs Total protein intake (R 0.1) | Vs %Body Fat (R −0.29) **** | |
| Vs L Hand grip (R −0.14) * | ||
| Vs R Hand grip (R −0.12) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganath, L.R.; Khedr, M.; Milan, A.M.; Davison, A.S.; Hughes, A.T.; Norman, B.P.; Bygott, H.; Luangrath, E.; Judd, S.; Soulsby, C.; et al. Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria. Nutrients 2024, 16, 2722. https://doi.org/10.3390/nu16162722
Ranganath LR, Khedr M, Milan AM, Davison AS, Hughes AT, Norman BP, Bygott H, Luangrath E, Judd S, Soulsby C, et al. Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria. Nutrients. 2024; 16(16):2722. https://doi.org/10.3390/nu16162722
Chicago/Turabian StyleRanganath, L. R., M. Khedr, A. M. Milan, A. S. Davison, A. T. Hughes, B. P. Norman, H. Bygott, E. Luangrath, S. Judd, C. Soulsby, and et al. 2024. "Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria" Nutrients 16, no. 16: 2722. https://doi.org/10.3390/nu16162722
APA StyleRanganath, L. R., Khedr, M., Milan, A. M., Davison, A. S., Hughes, A. T., Norman, B. P., Bygott, H., Luangrath, E., Judd, S., Soulsby, C., Olsson, B., & Imrich, R. (2024). Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria. Nutrients, 16(16), 2722. https://doi.org/10.3390/nu16162722








