Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review
Abstract
1. Introduction
2. Commonly Explored Models in Mushroom Research
2.1. In Vitro Studies
2.2. In Vivo Studies
2.2.1. Mammalian Models
2.2.2. Danio Rerio
2.2.3. Drosophila melanogaster
2.2.4. Caenorhabditis elegans
2.2.5. Clinical Trials
3. Mushrooms against Aging- and Lifestyle-Related Disorders
3.1. Neuroprotective Activity
3.2. Antidiabetic Activity
3.3. Cardiovascular Protection
3.4. Cosmeceuticals and Nutricosmetics
4. Toxicity
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahi, D.K.; Malik, D. Diversity of mushrooms and their metabolites of nutraceutical and therapeutic significance. J. Mycol. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Cheung, P.C. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef] [PubMed]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A critical review on submerged production of mushroom and their bioactive metabolites. 3 Biotech. 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.W.; Han, C. Medicinal and edible fungi as an alternative medicine for treating age-related disease. Evid. Based Complement. Alternat Med. 2014, 2014, 638561. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S. Anti-HIV-1 protease activity of the crude extracts and isolated compounds from Auricularia polytricha. BMC Complement. Altern. Med. 2019, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech. 2019, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Bielecka, J.; Mielcarek, K.; Grabia, M.; Socha, K. Treasures from the forest: Evaluation of mushroom extracts as anti-cancer agents. Biomed. Pharmacother. 2021, 143, 112106. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S.; Tencomnao, T. Neuroprotective effects against Glutamate-Induced HT-22 hippocampal cell damage and Caenorhabditis elegans lifespan/healthspan enhancing activity of Auricularia polytricha mushroom extracts. Pharmaceuticals 2021, 14, 1001. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An updated review of bioactive peptides from mushrooms in a well-defined molecular weight range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, medicinal, and cosmetic value of bioactive compounds in button mushroom (Agaricus bisporus): A review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Graudejus, O.; Ponce Wong, R.D.; Varghese, N.; Wagner, S.; Morrison, B. Bridging the gap between in vivo and in vitro research: Reproducing in vitro the mechanical and electrical environment of cells in vivo. Front. Cell. Neurosci. 2018, 12, 00069. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, K.; Akimoto, K.; Ikehara, Y.; Inafuku, K.; Okubo, A.; Yamazaki, S. Secretion of TNF-α, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Struct. Funct. 2001, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bruggemann, R.; Matsuo Orlandi, J.; Benati, F.J.; Faccin, L.C.; Mantovani, M.S.; Nozawa, C.; Linhares, R.E. Antiviral activity of Agaricus blazei Murrill ss. Heinem extract against human and bovine herpesviruses in cell culture. Braz. J. Microbiol. 2006, 37, 561–565. [Google Scholar] [CrossRef][Green Version]
- Faccin, L.C.; Benati, F.; Rincão, V.P.; Mantovani, M.S.; Soares, S.A.; Gonzaga, M.L.; Nozawa, C.; Carvalho Linhares, R.E. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007, 45, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Beppu, H.; Akiyama, H.; Wakamatsu, K.; Ito, S.; Kawamoto, Y.; Shimpo, K.; Sumiya, T.; Koike, T.; Matsui, T. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 669–673. [Google Scholar] [CrossRef]
- de Sousa Cardozo, F.T.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.; de Mendonça, M.M.; Simões, C.M. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Misgiati, M.; Widyawaruyanti, A.; Raharjo, S.J.; Sukardiman, S. Ergosterol isolated from Agaricus blazei Murill n-hexane extracts as potential anticancer MCF-7 activity. Pharmacogn. J. 2021, 13, 418–426. [Google Scholar] [CrossRef]
- Adams, L.S.; Chen, S.; Phung, S.; Wu, X.; Ki, L. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr. Cancer 2008, 60, 744–756. [Google Scholar] [CrossRef]
- Jeong, S.C.; Koyyalamudi, S.R.; Jeong, Y.T.; Song, C.H.; Pang, G. Macrophage immunomodulating and antitumor activities of polysaccharides isolated from Agaricus bisporus white button mushrooms. J. Med. Food 2012, 15, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Grzywacz, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory potential of in vitro cultures of the white button mushroom, Agaricus bisporus (Agaricomycetes), in Caco-2 cells. Int. J. Med. Mushrooms 2018, 20, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.R.; Ruthes, A.C.; van Arkel, J.; Chanput, W.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complement. Altern. Med. 2011, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, L.; Xue, B.; Zhao, D.; Zhang, Y.; Yan, X. A new lectin from Auricularia auricula inhibited the proliferation of lung cancer cells and improved pulmonary Flora. BioMed Res. Int. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Hikam, A.R.; Ekowati, N.; Hernayanti, H. The Cytotoxic and Apoptosis Effects of Chloroform Extracts of Auricularia auricula on Cervical Cancer Cells. Biosaintifika J. Biol. Biol. Educ. 2019, 11, 32–38. [Google Scholar] [CrossRef][Green Version]
- Kang, M.A.; Jeon, Y.K.; Nam, M.J. Auricularia auricula increases an apoptosis in human hepatocellular carcinoma cells via a regulation of the peroxiredoxin1. J. Food Biochem. 2020, 44, e13373. [Google Scholar] [CrossRef]
- Shahar, O.; Pereman, I.; Khamisie, H.; Ezov, N.; Danay, O.; Khattib, A.; Khatib, S.; Mahajna, J. Compounds originating from the edible mushroom Auricularia auricula-judae inhibit tropomyosin receptor kinase B activity. Heliyon 2023, 9, e13756. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Li, T.; Liu, Y.; Zhang, Z.; Zhang, Y. Purification, characterization and in vitro antioxidant activity of a polysaccharide AAP-3-1 from Auricularia auricula. Int. J. Biol. Macromol. 2020, 162, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, R.; Zhao, Z.; Wang, Y. Auricularia polytricha polysaccharides induce cell cycle arrest and apoptosis in human lung cancer A549 cells. Int. J. Biol. Macromol. 2014, 68, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Res. Int. 2022, 157, 111433. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Mongkolpobsin, K.; Chuchawankul, S.; Tencomnao, T.; Baek, S.J. Neuroprotective effects of ergosterol against TNF-α-induced HT-22 hippocampal cell injury. Biomed. Pharmacother. 2022, 154, 113596. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, C.S.; Chang, W.H.; Lu, F.J.; Lai, Y.C.; Chen, C.C.; Hseu, T.H.; Kuo, C.T.; Hseu, Y.C. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by Antrodia camphorata. Cancer Lett. 2006, 231, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Kuo, Y.H.; Tsai, C.T.; Huang, Y.T.; Chen, S.C.; Chang, H.W.; Lin, E.; Lin, W.H.; Hseu, Y.C. Anti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem. Toxicol. 2011, 49, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Wu, F.Y.; Wu, J.J.; Chen, J.Y.; Chang, W.H.; Lu, F.J.; Lai, Y.C.; Yang, H.L. Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int. Immunopharmacol. 2005, 5, 1914–1925. [Google Scholar] [CrossRef]
- Yeh, C.T.; Rao, Y.K.; Yao, C.J.; Yeh, C.F.; Li, C.H.; Chuang, S.E.; Luong, J.H.; Lai, G.M.; Tzeng, Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009, 285, 73–79. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, X.; Qiao, X.; Ji, S.; Liu, K.; Yeh, C.T.; Tzeng, Y.M.; Guo, D.; Ye, M. Antcamphins A–L, ergostanoids from Antrodia camphorata. J. Nat. Prod. 2014, 77, 118–124. [Google Scholar] [CrossRef]
- Rao, Y.K.; Wu, A.T.; Geethangili, M.; Huang, M.T.; Chao, W.J.; Wu, C.H.; Deng, W.P.; Yeh, C.T.; Tzeng, Y.M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef]
- Yeh, C.T.; Huang, W.C.; Rao, Y.K.; Ye, M.; Lee, W.H.; Wang, L.S.; Tzeng, D.T.; Wu, C.H.; Shieh, Y.S.; Huang, C.Y.; et al. A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis 2013, 34, 2918–2928. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chen, K.C.; Peng, R.Y.; Su, C.H.; Hsieh-Li, H.M. Human urinary bladder cancer T24 cells are susceptible to the Antrodia camphorata extracts. Cancer Lett. 2006, 243, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Chu, F.H.; Wang, Y.S.; Chien, S.C.; Chang, S.T.; Shaw, J.F.; Chen, C.Y.; Hsiao, W.W.; Kuo, Y.H.; Wang, S.Y. Antrocamphin A, an anti-inflammatory principal from the fruiting body of Taiwanofungus camphoratus, and its mechanisms. J. Agric. Food Chem. 2010, 58, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Shiao, Y.J.; Lin, R.D.; Shao, Y.Y.; Lai, M.N.; Lin, C.C.; Ng, L.T.; Kuo, Y.H. Neuroprotective Diterpenes from the Fruiting Body of Antrodiac amphorata. J. Nat. Prod. 2006, 69, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Lee, T.H.; Hsu, C.H.; Chang, Y.J.; Chang, M.S.; Wang, Y.C.; Ho, Y.S.; Wen, W.C.; Lin, R.K. Antroquinonol D, isolated from Antrodia camphorata, with DNA demethylation and anticancer potential. J. Agric. Food Chem. 2014, 62, 5625–5635. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, W.C.; Hseu, Y.T.; Lee, C.Y.; Yech, Y.J.; Chen, P.C.; Chen, J.Y.; Yang, H.L. Protection of oxidative damage by aqueous extract from Antrodia camphorata mycelia in normal human erythrocytes. Life Sci. 2002, 71, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.M.; Lin, H.W.; Wang, Y.J.; Chen, L.C.; Yang, D.Y.; Lai, Y.Y.; Ho, Y.S. Inhibition of anchorage-independent proliferation and G0/G1 cell-cycle regulation in human colorectal carcinoma cells by 4, 7-dimethoxy-5-methyl-l, 3-benzodioxole isolated from the fruiting body of Antrodia camphorate. Evid.-Based Complement. Altern. Med. 2011, 2011, 984027. [Google Scholar] [CrossRef]
- Zou, X.G.; Xu, M.T.; Dong, X.L.; Ying, Y.M.; Guan, R.F.; Wu, W.C.; Yang, K.; Sun, P.L. Solid-state-cultured mycelium of Antrodia camphorata exerts potential neuroprotective activities against 6-hydroxydopamine-induced toxicity in PC12 cells. J. Food Biochem. 2022, 46, e14208. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.K.; Cardoso, C.; Nunes, F.H.; Marques, G.; Pożarowski, P.; Rzeski, W. Boletus edulis biologically active biopolymers induce cell cycle arrest in human colon adenocarcinoma cells. Food Funct. 2013, 4, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.K.; Ribeiro, M.; Alves, H.G.; Marques, G.; Nunes, F.M.; Rzeski, W. Boletus edulis ribonucleic acid–a potent apoptosis inducer in human colon adenocarcinoma cells. Food Funct. 2016, 7, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yu, S.S.; Ji, H.Y.; Xu, X.M.; Liu, A.J. A novel acid polysaccharide from Boletus edulis: Extraction, characteristics and antitumor activities in vitro. Glycoconj. J. 2021, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Li, Z.H.; Dong, Z.J.; Su, J.; Li, Y.; Liu, J.K. Non-isoprenoid botryane sesquiterpenoids from basidiomycete Boletus edulis and their cytotoxic activity. Nat. Prod. Bioprospecting 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Bovi, M.; Cenci, L.; Perduca, M.; Capaldi, S.; Carrizo, M.E.; Civiero, L.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. BEL β-trefoil: A novel lectin with antineoplastic properties in king bolete (Boletus edulis) mushrooms. Glycobiology 2013, 23, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L. Isolation, structure identification and cytotoxicity evaluation of three steroids from Boletus edulis. Appl. Mech. Mater. 2014, 675, 1670–1673. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Tosun, N.G.; Özgür, A.; Tayhan, S.E.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641. [Google Scholar] [CrossRef]
- Yu, S.; Ma, R.; Dong, X.; Ji, H.; Liu, A. A novel polysaccharide from Boletus edulis: Extraction, purification, characterization and immunologic activity. Ind. Crops Prod. 2022, 186, 115206. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsiao, Y.M.; Wu, M.F.; Ou, C.C.; Lin, Y.W.; Lue, K.H.; Ko, J.L. Interruption of lung cancer cell migration and proliferation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. J. Agric. Food Chem. 2013, 61, 12044–12052. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bhartiya, P.; Kaushik, N.; Nhat Nguyen, L.; Wahab, R.; Bekeschus, S.; Choi, E.H.; Kaushik, N.K. Plasma-treated Flammulina velutipes-derived extract showed anticancer potential in human breast cancer cells. Appl. Sci. 2020, 10, 8395. [Google Scholar] [CrossRef]
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, characterization and anti-proliferation activity of polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2012, 88, 474–480. [Google Scholar] [CrossRef]
- Ukaegbu, C.I.; Shah, S.R.; Hamid, H.A.; Alara, O.R.; Sarker, M.Z. Phenolic compounds of aqueous and methanol extracts of Hypsizygus tessellatus (brown and white var.) and Flammulina velutipes caps: Antioxidant and antiproliferative activities. Pharm. Chem. J. 2020, 54, 170–183. [Google Scholar] [CrossRef]
- Chen, G.T.; Fu, Y.X.; Yang, W.J.; Hu, Q.H.; Zhao, L.Y. Effects of polysaccharides from the base of Flammulina velutipes stipe on growth of murine RAW264. 7, B16F10 and L929 cells. Int. J. Biol. Macromol. 2018, 107, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Zhong, H.; Tong, S.; Cao, X.; Firempong, C.K.; Liu, H.; Fu, M.; Yang, Y.; Feng, Y.; Zhang, H.; et al. Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. Int. J. Nanomed. 2012, 7, 5067–5078. [Google Scholar]
- Stajic, M. Se effect on biological activity of Flammulina velutipes. Ital. J. Food Sci. 2015, 27, 57–63. [Google Scholar]
- Tabuchi, A.; Fukushima-Sakuno, E.; Osaki-Oka, K.; Futamura, Y.; Motoyama, T.; Osada, H.; Ishikawa, N.K.; Nagasawa, E.; Tokimoto, K. Productivity and bioactivity of enokipodins A–D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020, 84, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.N.; Sung, T.J.; Chou, C.H.; Liu, K.L.; Hsieh, L.P.; Hsieh, C.W. Characterization and antioxidant activities of yellow strain Flammulina velutipes (Jinhua mushroom) polysaccharides and their effects on ROS content in L929 cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lv, K.; Zhang, D.; Fan, W.; Tsopmejio, I.S.; Jin, Z.; Song, H. Effect of Flammulina velutipes polysaccharides on endoplasmic reticulum stress-mediated apoptosis by activating PLC–IP3 pathway in HepG2 cells. J. Food Sci. 2023, 88, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Feng, J.; Zhang, J.; Lin, C.C.; Wang, W.; Chen, H.G. Structural characteristics of the novel polysaccharide FVPA1 from winter culinary-medicinal mushroom, Flammulina velutipes (Agaricomycetes), capable of enhancing natural killer cell activity against K562 tumor cells. Int. J. Med. Mushrooms 2017, 19, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yu, J.; Yang, W.; Kimatu, B.M.; Fang, Y.; Ma, N.; Pei, F. Identification of flavonoids from Flammulina velutipes and its neuroprotective effect on pheochromocytoma-12 cells. Food Chem. 2016, 204, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Sliva, D.; Labarrere, C.; Slivova, V.; Sedlak, M.; Lloyd, F.P., Jr.; Ho, N.W. Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem. Biophys. Res. Commun. 2002, 298, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ye, G.; Fu, G.; Cheng, J.; Yang, B.B.; Peng, C. Ganoderma lucidum exerts anti-tumor effects on ovarian cancer cells and enhances their sensitivity to cisplatin. Int. J. Oncol. 2011, 38, 1319–1327. [Google Scholar]
- Hu, H.; Ahn, N.S.; Yang, X.; Lee, Y.S.; Kang, K.S. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 2002, 102, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Wang, C.Z.; Wicks, S.; Yin, J.J.; Kong, J.; Li, J.; Li, Y.C.; Yuan, C.S. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp. Oncol. 2006, 28, 25–29. [Google Scholar] [PubMed]
- Yuen, J.W.; Gohel, M.D.; Au, D.W. Telomerase-associated apoptotic events by mushroom ganoderma lucidum on premalignant human urothelial cells. Nutr. Cancer 2007, 60, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, J.W.; Zhao, W.M.; Wei, D.Z.; Zhong, J.J. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006, 80, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Dudhgaonkar, S.; Thyagarajan, A.; Sliva, D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, A.; Mendonca, P.; Soliman, K.F. Involvement of NFƙB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. J. Neuroimmunol. 2020, 345, 577269. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dunkin, D.; Lai, J.; Song, Y.; Ceballos, C.; Benkov, K.; Li, X.M. Anti-inflammatory effects of Ganoderma lucidum triterpenoid in human crohn’s disease associated with downregulation of NF-κB signaling. Inflamm. Bowel Dis. 2015, 21, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Peng, X.R.; Dong, J.R.; Yan, H.; Kong, Q.H.; Shi, Q.Q.; Li, D.S.; Zhou, L.; Li, Z.R.; Qiu, M.H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti-inflammatory activities. Fitoterapia 2019, 134, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Tae, N.; Lee, S.; Ryoo, S.; Min, B.S.; Lee, J.H. Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264. 7 cells. Toxicol. Appl. Pharmacol. 2014, 280, 434–442. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, Y.; Su, H.; Gao, Y.; Peng, X.; Zhou, L.; Li, X.; Qiu, M. C28 steroids from the fruiting bodies of Ganoderma resinaceum with potential anti-inflammatory activity. Phytochemistry 2019, 168, 112109. [Google Scholar] [CrossRef]
- Cho, J.Y.; Sadiq, N.B.; Kim, J.C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; Kim, H.Y. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar]
- Kou, R.W.; Xia, B.; Wang, Z.J.; Li, J.N.; Yang, J.R.; Gao, Y.Q.; Yin, X.; Gao, J.M. Triterpenoids and meroterpenoids from the edible Ganoderma resinaceum and their potential anti-inflammatory, antioxidant and anti-apoptosis activities. Bioorganic Chem. 2022, 121, 105689. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Lian, C.L.; Hu, T.Y.; Wang, C.F.; Xu, Y.; Xiao, L.; Liu, Z.Q.; Qiu, S.Q.; Cheng, B.H. Two new farnesyl phenolic compounds with anti-inflammatory activities from Ganoderma duripora. Food Chem. 2018, 263, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.Q.; Zuo, F.J.; Duan, X.Y.; Wang, Y.N.; Li, J.R.; Qian, C.Z.; Xiao, J.P. Ergosterols from Ganoderma sinense and their anti-inflammatory activities by inhibiting NO production. Phytochem. Lett. 2019, 32, 177–180. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264. 7. Int. J. Biol. Macromol. 2017, 105, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Tsai, C.L.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhou, C.; Liu, T.; Dai, Y.; Huang, H. A novel Hericium erinaceus polysaccharide: Structural characterization and prevention of H2O2-induced oxidative damage in GES-1 cells. Int. J. Biol. Macromol. 2020, 154, 1460–1470. [Google Scholar] [CrossRef]
- Atay, S.; Ak, H.; Kalmis, E.; Kayalar, H.; Aydin, H.H. Transcriptome-Wide Analysis Reveals the Molecular Mechanism of Tumoricidal Effects of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), on MCF-7 Breast Cancer Cells. Int. J. Med. Mushrooms 2021, 23, 91–106. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xiao, X.; Xu, D.; Gao, Y.; Gao, Q. A polysaccharide isolated from mycelia of the lion’s mane medicinal mushroom Hericium erinaceus (Agaricomycetes) induced apoptosis in precancerous human gastric cells. Int. J. Med. Mushrooms 2017, 19, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Cui, F.; Li, Y.; Yang, Y.; Wu, D.; Sun, W.; Ping, L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2015, 76, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Yang, H.L.; Pan, J.H.; Korivi, M.; Pan, J.Y.; Hsieh, M.C.; Chao, P.M.; Huang, P.J.; Tsai, C.T.; Hseu, Y.C. Hericium erinaceus inhibits TNF-α-induced angiogenesis and ROS generation through suppression of MMP-9/NF-κB signaling and activation of Nrf2-mediated antioxidant genes in human EA. hy926 endothelial cells. Oxidative Med. Cell. Longevit 2016, 2016, 8257238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011, 2, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, R.N.; Tang, Q.J.; Zhang, J.S.; Yang, Y.; Shang, X.D. A new diterpene from the fungal mycelia of Hericium erinaceus. Phytochem. Lett. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, H.; Zhang, X. Erinacerins, Novel Glioma Inhibitors from Hericium erinaceus, Induce Apoptosis of U87 Cells through Bax/Capase-2 Pathway. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2020, 20, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Amen, Y.; Allam, A.E.; Kudo, T.; Nagata, M.; Ohnuki, K.; Shimizu, K. New isoindolinones from the fruiting bodies of the fungus Hericium erinaceus. Phytochem. Lett. 2019, 32, 10–14. [Google Scholar] [CrossRef]
- Youn, M.J.; Kim, J.K.; Park, S.Y.; Kim, Y.; Park, C.; Kim, E.S.; Park, K.I.; So, H.S.; Park, R. Potential anticancer properties of the water extract of Inontus obliquus by induction of apoptosis in melanoma B16-F10 cells. J. Ethnopharmacol. 2009, 121, 221–228. [Google Scholar] [CrossRef]
- Arata, S.; Watanabe, J.; Maeda, M.; Yamamoto, M.; Matsuhashi, H.; Mochizuki, M.; Kagami, N.; Honda, K.; Inagaki, M. Continuous intake of the Chaga mushroom (Inonotus obliquus) aqueous extract suppresses cancer progression and maintains body temperature in mice. Heliyon 2016, 2, E00111. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.S.; Yun, J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother. Res. 2009, 23, 1784–1789. [Google Scholar] [CrossRef]
- Baek, J.; Roh, H.S.; Baek, K.H.; Lee, S.; Lee, S.; Song, S.S.; Kim, K.H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 2018, 224, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.C.; Lee, J.S.; Lee, S.; Son, Y.K.; Park, J.E.; Song, J.E.; Ha, S.J.; Hong, E.K. Effects of polysaccharides isolated from Inonotus obliquus against hydrogen peroxide-induced oxidative damage in RINm5F pancreatic β-cells. Mol. Med. Rep. 2016, 14, 4263–4270. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Tong, S.; Wang, Z.; Liu, P. Chemical characterization and hypoglycaemic activities in vitro of two polysaccharides from Inonotus obliquus by submerged culture. Molecules 2018, 23, 3261. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hui, J.; Kou, W.; Xin, R.; Jia, F.; Wang, N.; Hu, F.; Zhang, H.; Liu, H. Identification of Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr. Microbiol. 2008, 57, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Cui, Z.; Liu, J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Hsu, T.H.; Lee, C.H. Extracellular polysaccharopeptides from fermented Turkey Tail medicinal mushroom, Trametes versicolor (Agaricomycetes), mitigate oxidative stress, hyperglycemia, and hyperlipidemia in rats with type 2 diabetes mellitus. Int. J. Med. Mushrooms 2020, 22, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.Y.; Kong, B.H.; Yap, C.S.; Ong, K.C.; Zain, R.B.; Tan, S.H.; Zaini, Z.M.; Ng, S.T.; Tan, C.S.; Fung, S.Y. Immunomodulatory Effect and an Intervention of TNF Signalling Leading to Apoptotic and Cell Cycle Arrest on ORL-204 Oral Cancer Cells by Tiger Milk Mushroom, Lignosus rhinocerus. Food Technol. Biotechnol. 2022, 60, 80–88. [Google Scholar] [CrossRef]
- Lee, M.L.; Tan, N.H.; Fung, S.Y.; Tan, C.S.; Ng, S.T. The antiproliferative activity of sclerotia of Lignosus rhinocerus (Tiger Milk Mushroom). Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Yap, H.Y.; Tan, N.H.; Ng, S.T.; Tan, C.S.; Fung, S.Y. Molecular attributes and apoptosis-inducing activities of a putative serine protease isolated from Tiger Milk mushroom (Lignosus rhinocerus) sclerotium against breast cancer cells in vitro. PeerJ 2018, 6, e4940. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.; Luo, Q.; Phan, C.W.; Huo, Y.; Li, P.; Li, Q.; Jin, X.; Huang, W. Neuroprotective effects of a novel peptide from Lignosus rhinocerotis against 6-hydroxydopamine-induced apoptosis in PC12 cells by inhibiting NF-κB activation. Food Sci. Nutr. 2023, 11, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Lim, C.S.; Wong, K.H.; Sabaratnam, V. Cytoprotective Effects of the Tiger’s Milk Mushroom Lignosus rhinocerotis (Agaricomycetes) Sclerotia against Oxidative Stress in PC12 Cells. Int. J. Med. Mushrooms 2022, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Seow, S.L.; Eik, L.F.; Naidu, M.; David, P.; Wong, K.H.; Sabaratnam, V. Lignosus rhinocerotis (Cooke) Ryvarden mimics the neuritogenic activity of nerve growth factor via MEK/ERK1/2 signaling pathway in PC-12 cells. Sci. Rep. 2015, 5, 16349. [Google Scholar] [CrossRef] [PubMed]
- Kittimongkolsuk, P.; Roxo, M.; Li, H.; Chuchawankul, S.; Wink, M.; Tencomnao, T. Extracts of the Tiger Milk Mushroom (Lignosus rhinocerus) Enhance Stress Resistance and Extend Lifespan in Caenorhabditis elegans via the DAF-16/FoxO Signaling Pathway. Pharmaceuticals 2021, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Tan, J.B.L.; Lim, L.W.; Tan, K.O.; Heng, B.C.; Lim, W.L. Human embryonic stem cell-derived neural lineages as in vitro models for screening the neuroprotective properties of Lignosus rhinocerus (Cooke) Ryvarden. BioMed Res. Int. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement. Altern. Med. 2013, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Eik, L.F.; Naidu, M.; David, P.; Wong, K.H.; Tan, Y.S.; Sabaratnam, V. Lignosus rhinocerus (Cooke) Ryvarden: A medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 320308. [Google Scholar] [CrossRef] [PubMed]
- Faris Taufeq, F.Y.; Habideen, N.H.; Rao, L.N.; Podder, P.K.; Katas, H. Potential Hemostatic and Wound Healing Effects of Thermoresponsive Wound Dressing Gel Loaded with Lignosus rhinocerotis and Punica granatum Extracts. Gels 2023, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Seino, T.; Inobe, M.; Jutanom, M.; Matsumoto, S.; Kinoshita, M. Polar lipid fraction from golden oyster mushrooms (Pleurotus citrinopileatus) suppresses colon injuries from inflammatory stresses in vivo and in vitro. J. Oleo Sci. 2020, 69, 751–757. [Google Scholar] [CrossRef]
- Younis, A.M.; Abdel-Aziz, M.M.; Yosri, M. Evaluation of some biological applications of Pleurotus citrinopileatus and Boletus edulis fruiting bodies. Curr. Pharm. Biotechnol. 2019, 20, 1309–1320. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Pi, X.; Zhao, S.; Liu, W. In vitro hepatoprotective and human gut microbiota modulation of polysaccharide-peptides in Pleurotus citrinopileatus. Front. Cell. Infect. Microbiol. 2022, 553. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Ma, C.Y.; Tsai, P.F.; Wang, Y.T.; Wu, J.S. In vitro antitumor and immunomodulatory effects of the protein PCP-3A from mushroom Pleurotus citrinopileatus. J. Agric. Food Chem. 2010, 58, 12117–12122. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.Q.; Ma, K.; Bao, L.; Wang, K.; Han, J.J.; Wang, W.Z.; Zhang, J.X.; Huang, C.Y.; Liu, H.W. Sesquiterpenoids with PTP1B inhibitory activity and cytotoxicity from the edible mushroom Pleurotus citrinopileatus. Planta Medica 2016, 82, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W. Activity-guided isolation and structural identification of immunomodulating substances from Pleurotus eryngii byproducts. Int. Immunopharmacol. 2017, 51, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Park, J.W.; Park, S.H.; Park, J.B.; Rho, Y.H.; Ryu, Y.B.; Lee, K.S.; Park, K.H.; Bae, Y.S. Apoptotic cell death of human leukaemia U937 cells by ubiquinone-9 purified from Pleurotus eryngii. Nat. Prod. Res. 2009, 23, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, K.K.; Son, B.Y.; Nam, S.W.; Shin, P.G.; Kim, G.D. The Anti-Adipogenic Activity of a New Cultivar, Pleurotus eryngii var. ferulae’Beesan No. 2’, through Down-Regulation of PPAR γ and C/EBP α in 3T3-L1 Cells. J. Microbiol. Biotechnol. 2016, 26, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Hwang, Y.P.; Kim, H.G.; Choi, J.H.; Im, J.H.; Yang, J.H.; Lee, H.U.; Chun, S.S.; Chung, Y.C.; Jeong, H.G. Inhibitory effect of Pleurotus eryngii extracts on the activities of allergic mediators in antigen-stimulated mast cells. Food Chem. Toxicol. 2011, 49, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A.; et al. Immunomodulating activity of Pleurotus eryngii mushrooms following their in vitro fermentation by human fecal microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Gong, P.; Long, H.; Guo, Y.; Wang, S.; Chen, F.; Chen, X. Isolation, Structural Characterization, and Hypoglycemic Activities In vitro of Polysaccharides from Pleurotus eryngii. Molecules 2022, 27, 7140. [Google Scholar] [CrossRef] [PubMed]
- Ellefsen, C.F.; Wold, C.W.; Wilkins, A.L.; Rise, F.; Samuelsen, A.B. Water-soluble polysaccharides from Pleurotus eryngii fruiting bodies, their activity and affinity for Toll-like receptor 2 and dectin-1. Carbohydr. Polym. 2021, 264, 117991. [Google Scholar] [CrossRef] [PubMed]
- Mariga, A.M.; Yang, W.J.; Mugambi, D.K.; Pei, F.; Zhao, L.Y.; Shao, Y.N.; Hu, Q. Antiproliferative and immunostimulatory activity of a protein from Pleurotus eryngii. J. Sci. Food Agric. 2014, 94, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.S.; Al-Sheikh, H. Biosynthesis and characterization of silver nanoparticles produced by Pleurotus ostreatus and their anticandidal and anticancer activities. World J. Microbiol. Biotechnol. 2014, 30, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Jedinak, A.; Sliva, D. Pleurotus ostreatus inhibits proliferation of human breast and colon cancer cells through p53-dependent as well as p53-independent pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar] [PubMed]
- Wang, L.; Li, K.; Cui, Y.; Peng, H.; Hu, Y.; Zhu, Z. Preparation, structural characterization and neuroprotective effects to against H2O2-induced oxidative damage in PC12 cells of polysaccharides from Pleurotus ostreatus. Food Res. Int. 2023, 163, 112146. [Google Scholar] [CrossRef] [PubMed]
- Hamad, D.; El-Sayed, H.; Ahmed, W.; Sonbol, H.; Ramadan, M.A. GC-MS analysis of potentially volatile compounds of Pleurotus ostreatus polar extract: In vitro antimicrobial, cytotoxic, immunomodulatory, and antioxidant activities. Front. Microbiol. 2022, 13, 834525. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, D.; Bennett, L.; Shanmugam, K.; King, K.; Williams, R.; Zabaras, D.; Head, R.; Ooi, L.; Gyengesi, E.; Münch, G. Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-γ activated murine macrophages. Food Chem. 2014, 148, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.L.; Simon, J.; Sliva, D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011, 10, 52. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Liu, H.; Liu, M.; Yang, Y.; Zhong, S. The Main Structural Unit Elucidation and Immunomodulatory Activity In vitro of a Selenium-Enriched Polysaccharide Produced by Pleurotus ostreatus. Molecules 2022, 27, 2591. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Liu, M.; Yang, Y.; Zhong, S. A natural selenium polysaccharide from Pleurotus ostreatus: Structural elucidation, anti-gastric cancer and anti-colon cancer activity in vitro. Int. J. Biol. Macromol. 2022, 201, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Chen, C.H.; Chang, W.H.; Chung, K.T.; Liu, Y.W.; Lu, F.J.; Chen, C.H. Anti-cancer effects of protein extracts from Calvatia lilacina, Pleurotus ostreatus and Volvariella volvacea. Evid.-Based Complement. Altern. Med. 2011, 2011, 982368. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, J.J.; Cheung, P.C. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-induced PI3K/AKT signaling pathway. PLoS ONE 2012, 7, e34406. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Berruguilla, E.; Romero, I.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Ünyayar, A.; Demirbilek, M.; Turkoglu, M.; Celik, A.; Mazmanci, M.A.; Erkurt, E.A.; Ünyayar, S.; Cekic, Ö.; Atacag, H. Evaluation of cytotoxic and mutagenic effects of Coriolus versicolor and Funalia trogii extracts on mammalian cells. Drug Chem. Toxicol. 2006, 29, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Yue, G.G.; Ko, C.H.; Lee, J.K.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Bik-San Lau, C. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Yang, X.; Wan, J.M. The culture duration affects the immunomodulatory and anticancer effect of polysaccharopeptide derived from Coriolus versicolor. Enzym. Microb. Technol. 2006, 38, 14–21. [Google Scholar] [CrossRef]
- Sekhon, B.K.; Sze, D.M.; Chan, W.K.; Fan, K.; Li, G.Q.; Moore, D.E.; Roubin, R.H. PSP activates monocytes in resting human peripheral blood mononuclear cells: Immunomodulatory implications for cancer treatment. Food Chem. 2013, 138, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Cell growth and gene modulatory activities of Yunzhi (Windsor Wunxi) from mushroom Trametes versicolor in androgen-dependent and androgen-insensitive human prostate cancer cells. Int. J. Oncol. 2001, 18, 81–89. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly used animal models. Princ. Anim. Res. Grad. Undergrad. Stud. 2017, 117–175. [Google Scholar] [CrossRef]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Motoi, M.; Yadomae, T. Antitumor β-glucan from the cultured fruit body of Agaricus blazei. Biol. Pharm. Bull. 2001, 24, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kido, T.; Takaku, T.; Sumiyoshi, M.; Baba, K. Isolation of an anti-angiogenic substance from Agaricus blazei Murill: Its antitumor and antimetastatic actions. Cancer Sci. 2004, 95, 758–764. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pinto, A.V.; Martins, P.R.; Romagnoli, G.G.; Campanelli, A.P.; Terezan, A.P.; Rodrigues Filho, E.; da Eira, A.F.; Kaneno, R. Polysaccharide fraction of Agaricus brasiliensis avoids tumor-induced IL-10 production and changes the microenvironment of subcutaneous Ehrlich adenocarcinoma. Cell. Immunol. 2009, 256, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fukuwatari, Y.; Okumura, K.; Takeda, K.; Ishibashi, K.I.; Furukawa, M.; Ohno, N.; Mori, K.; Gao, M.; Motoi, M. Immunomodulating activity of Agaricus brasiliensis KA21 in mice and in human volunteers. Evid.-Based Complement. Altern. Med. 2008, 5, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Ellertsen, L.K.; Hetland, G. An extract of the medicinal mushroom Agaricus blazei Murill can protect against allergy. Clin. Mol. Allergy 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Komura, D.L.; Carbonero, E.R.; Gracher, A.H.; Baggio, C.H.; Freitas, C.S.; Marcon, R.; Santos, A.R.; Gorin, P.A.; Iacomini, M. Structure of Agaricus spp. fucogalactans and their anti-inflammatory and antinociceptive properties. Bioresour. Technol. 2010, 101, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kawai, J.; Ouchi, K.; Kikuchi, H.; Osima, Y.; Hidemi, R. Agarol, an ergosterol derivative from Agaricus blazei, induces caspase-independent apoptosis in human cancer cells. Int. J. Oncol. 2016, 48, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, D.; Mori, H.; Chen, S. White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft. J. Nutr. Biochem. 2021, 89, 108580. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, H.; Wang, Z. Auricularia auriculajudae polysaccharide-cisplatin complexes conjugated with folic acid as new tumor targeting agents. Int. J. Biol. Macromol. 2018, 120, 966–974. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Zhang, L.; Zhang, Y.; Ding, K. Evaluation of water soluble β-D-glucan from Auricularia auricular-judae as potential anti-tumor agent. Carbohydr. Polym. 2010, 80, 977–983. [Google Scholar] [CrossRef]
- Liu, Q.; An, X.; Chen, Y.; Deng, Y.; Niu, H.; Ma, R.; Zhao, H.; Cao, W.; Wang, X.; Wang, M. Effects of Auricularia auricula polysaccharides on gut microbiota and metabolic phenotype in mice. Foods 2022, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Liu, X.; Wu, X.; Zheng, M.; Fu, J. Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo. Food Sci. Hum. Wellness 2021, 10, 514–522. [Google Scholar] [CrossRef]
- Chen, G.; Luo, Y.C.; Ji, B.P.; Li, B.; Guo, Y.; Li, Y.; Su, W.; Xiao, Z.L. Effect of polysaccharide from Auricularia auricula on blood lipid metabolism and lipoprotein lipase activity of ICR mice fed a cholesterol-enriched diet. J. Food Sci. 2008, 73, H103–H108. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Skin wound-healing potential of polysaccharides from medicinal mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Y.; Zhu, Y.; Li, S.; Liu, M.; Song, X.; Zhao, H.; Liu, W.; Zhang, J.; Wang, S.; et al. Hepatoprotective effects of Auricularia cornea var. Li. polysaccharides against the alcoholic liver diseases through different metabolic pathways. Sci. Rep. 2018, 8, 7574. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, X.; Qing, Y.; Luo, X.; Yang, Z.; Zheng, L. Isolation of an anti-tumor polysaccharide from Auricularia polytricha (jew’s ear) and its effects on macrophage activation. Eur. Food Res. Technol. 2009, 228, 477–485. [Google Scholar] [CrossRef]
- Song, X.; Pang, H.; Cui, W.; Zhang, J.; Li, J.; Jia, L. Renoprotective effects of enzyme-hydrolyzed polysaccharides from Auricularia polytricha on adenine-induced chronic kidney diseases in mice. Biomed. Pharmacother. 2021, 135, 111004. [Google Scholar] [CrossRef] [PubMed]
- Tsopmejio, I.S.; Yuan, J.; Diao, Z.; Fan, W.; Wei, J.; Zhao, C.; Li, Y.; Song, H. Auricularia polytricha and Flammulina velutipes reduce liver injury in DSS-induced Inflammatory Bowel Disease by improving inflammation, oxidative stress, and apoptosis through the regulation of TLR4/NF-κB signaling pathways. J. Nutr. Biochem. 2023, 111, 109190. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chen, S.C.; Chen, H.C.; Liao, J.W.; Yang, H.L. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 2680–2688. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef]
- Tu, S.H.; Wu, C.H.; Chen, L.C.; Huang, C.S.; Chang, H.W.; Chang, C.H.; Lien, H.M.; Ho, Y.S. In vivo antitumor effects of 4, 7-dimethoxy-5-methyl-1, 3-benzodioxole isolated from the fruiting body of Antrodia camphorata through activation of the p53-mediated p27/Kip1 signaling pathway. J. Agric. Food Chem. 2012, 60, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chern, C.M.; Liou, K.T.; Kuo, Y.H.; Shen, Y.C. Ergostatrien-7, 9 (11), 22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019, 10, 4725–4738. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, S.Q.; Wu, W.Z.; Yang, S.L.; Tan, J.M. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, G.; Yang, R.; Cui, Y. Anti-inflammatory effects of Boletus edulis polysaccharide on asthma pathology. Am. J. Transl. Res. 2016, 8, 4478. [Google Scholar] [PubMed]
- Zhang, Y.; Zhou, R.; Liu, F.; Ng, T.B. Purification and characterization of a novel protein with activity against non-small-cell lung cancer in vitro and in vivo from the edible mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 174, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, G.; Feng, S.; Wong, J.H.; Zhao, Y.; Chen, X.; Wang, H.; Ng, T.B. Boletus edulis nitrite reductase reduces nitrite content of pickles and mitigates intoxication in nitrite-intoxicated mice. Sci. Rep. 2015, 5, 14907. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Luo, A.; Huang, J.; Fan, Y. Purification, characterization and antioxidant activities in vitro and in vivo of the polysaccharides from Boletus edulis bull. Molecules 2012, 17, 8079–8090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ji, Y.; Chen, X.; Hu, Q.; Zhao, L. Polysaccharide from Flammulina velutipes attenuates markers of metabolic syndrome by modulating the gut microbiota and lipid metabolism in high fat diet-fed mice. Food Funct. 2021, 12, 6964–6980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, Q.; Ma, G.; Su, A.; Xie, M.; Li, X.; Chen, G.; Zhao, L. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J. Funct. Foods 2019, 56, 255–264. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, Q.; Hao, X.; Wang, J.; Ma, C.; Xi, X.; Kang, W. The effect of Flammulina velutipes polysaccharide on immunization analyzed by intestinal flora and proteomics. Front. Nutr. 2022, 9, 35. [Google Scholar] [CrossRef]
- Yuan, F.; Gao, Z.; Liu, W.; Li, H.; Zhang, Y.; Feng, Y.; Song, X.; Wang, W.; Zhang, J.; Huang, C.; et al. Characterization, antioxidant, anti-aging and organ protective effects of sulfated polysaccharides from Flammulina velutipes. Molecules 2019, 24, 3517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Ren, P.; Che, Y.; Zhou, J.; Wang, W.; Yang, Y.; Guan, L. Polysaccharide from Flammulina velutipes residues protects mice from Pb poisoning by activating Akt/GSK3β/Nrf-2/HO-1 signaling pathway and modulating gut microbiota. Int. J. Biol. Macromol. 2023, 230, 123154. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Fu, H.; Chen, W. Effects of Flammulina velutipes polysaccharides on quality improvement of fermented milk and antihyperlipidemic on streptozotocin-induced mice. J. Funct. Foods 2021, 87, 104834. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, B.; He, X.; Tang, J.; Peng, W.; Zhou, J.; Wang, Y. Flammulina velutipes Polysaccharides Modulate Gut Microbiota and Alleviate Carbon Tetrachloride-Induced Hepatic Oxidative Injury in Mice. Front. Microbiol. 2022, 13, 941. [Google Scholar]
- Chang, H.H.; Hsieh, K.Y.; Yeh, C.H.; Tu, Y.P.; Sheu, F. Oral administration of an Enoki mushroom protein FVE activates innate and adaptive immunity and induces anti-tumor activity against murine hepatocellular carcinoma. Int. Immunopharmacol. 2010, 10, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Liu, C.M.; Qin, Z.H.; Jiang, J.H.; Sun, Y.Z. Ganoderma applanatum terpenes protect mouse liver against benzo (α) pyren-induced oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2011, 31, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.Y.; Zhang, H.X.; Di, Q.Q.; Wang, Y.; Yan, Y.M.; Chen, W.L.; Cheng, Y.X. Ganoderma cochlear metabolites as probes to identify a COX-2 active site and as in vitro and in vivo anti-inflammatory agents. Org. Lett. 2020, 22, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Chen, M.L.; Chiang, B.L.; Lin, B.F. Ganoderma tsugae supplementation alleviates bronchoalveolar inflammation in an airway sensitization and challenge mouse model. Int. Immunopharmacol. 2006, 6, 241–251. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Rosario-Acevedo, R.; Aguilar-Perez, A.; Clemente, P.L.; Cubano, L.A.; Serrano, J.; Schneider, R.J.; Martínez-Montemayor, M.M. Anti-tumor effects of Ganoderma lucidum (reishi) in inflammatory breast cancer in in vivo and in vitro models. PLoS ONE 2013, 8, e57431. [Google Scholar] [CrossRef]
- Na, K.; Li, K.; Sang, T.; Wu, K.; Wang, Y.; Wang, X. Anticarcinogenic effects of water extract of sporoderm-broken spores of Ganoderma lucidum on colorectal cancer in vitro and in vivo. Int. J. Oncol. 2017, 50, 1541–1554. [Google Scholar] [CrossRef]
- Jedinak, A.; Thyagarajan-Sahu, A.; Jiang, J.; Sliva, D. Ganodermanontriol, a lanostanoid triterpene from Ganoderma lucidum, suppresses growth of colon cancer cells through ss-catenin signaling. Int. J. Oncol. 2011, 38, 761–767. [Google Scholar] [PubMed]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H.; Chen, S.N. Ganoderma lucidum beta 1, 3/1, 6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.; Ajith, T.A.; Sheena, N.; Gunapalan, N.; Janardhanan, K.K. Antiperoxidative, anti-inflammatory, and antimutagenic activities of ethanol extract of the mycelium of Ganoderma lucidum occurring in South India. Teratog. Carcinog. Mutagen. 2003, 23 (Suppl. S1), 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef]
- Kim, S.P.; Nam, S.H.; Friedman, M. Hericium erinaceus (Lion’s Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-tansplanted mice. J. Agric. Food Chem. 2013, 61, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Huang, Y.; Song, Y.; Lin, D.; Li, J.; Ma, Y.; Wu, X.; Qiu, F.; Xiao, Q. Selenizing Hericium erinaceus polysaccharides induces dendritic cells maturation through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2017, 97, 287–298. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, J.E.; Jeong, K.H.; Lim, S.C.; Kim, S.Y.; Cho, K.O. The neuroprotective effect of Hericium erinaceus extracts in mouse hippocampus after pilocarpine-induced status epilepticus. Int. J. Mol. Sci. 2019, 20, 859. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.J.; Kan, W.C.; Chang, C.M.; Peng, Y.J.; Wang, H.Y.; Yu, W.C.; Cheng, Y.H.; Jhang, Y.R.; Liu, H.W.; Chuu, J.J. Renal protective effects of low molecular weight of Inonotus obliquus polysaccharide (LIOP) on HFD/STZ-induced nephropathy in mice. Int. J. Mol. Sci. 2016, 17, 1535. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xin, C.; Yang, J.; Dong, L.; Mei, H.; Dai, X.; Wang, Q. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sang, R.; Yu, Y.; Li, J.; Ge, B.; Zhang, X. The polysaccharide from Inonotus obliquus protects mice from Toxoplasma gondii-induced liver injury. Int. J. Biol. Macromol. 2019, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, G.; Wang, X.; Lv, C.; Tian, Y. Inonotus obliquus polysaccharide ameliorates serum profiling in STZ-induced diabetic mice model. BMC Chem. 2021, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ouyang, F.; Teng, C.; Qu, J. Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep. Biochem. Biotechnol. 2021, 51, 852–859. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zheng, J.J.; Qu, C.; Xiao, Y.; Li, F.F.; Jin, Q.X.; Li, H.H.; Meng, F.P.; Jin, G.H.; Jin, D. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif. Cells Nanomed. Biotechnol. 2019, 47, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.W.; Jeong, S.C.; Lee, D.H.; Park, J.S.; Lee, J.S. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006, 27, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, H. Anti-tumor effect of Inonotus obliquus in xenograft animals with EBV+ human gastric carcinoma. Korean J. Microbiol. 2016, 52, 482–486. [Google Scholar] [CrossRef][Green Version]
- Tanaka, K.; Matsui, Y.; Ishikawa, S.; Kawanishi, T.; Harada, M. Oral ingestion of Lentinula edodes mycelia extract can restore the antitumor T cell response of mice inoculated with colon-26 cells into the subserosal space of the cecum. Oncol. Rep. 2012, 27, 325–332. [Google Scholar]
- Tanaka, K.; Ishikawa, S.; Matsui, Y.; Tamesada, M.; Harashima, N.; Harada, M. Oral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppression. Cancer Sci. 2011, 102, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Johnathan, M.; Muhamad, S.A.; Gan, S.H.; Stanslas, J.; Mohd Fuad, W.E.; Hussain, F.A.; Wan Ahmad, W.A.; Nurul, A.A. Lignosus rhinocerotis Cooke Ryvarden ameliorates airway inflammation, mucus hypersecretion and airway hyperresponsiveness in a murine model of asthma. PLoS ONE 2021, 16, e0249091. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, S.A.; Muhammad, N.S.; Ismail, N.D.; Mohamud, R.; Safuan, S.; Nurul, A.A. Intranasal administration of Lignosus rhinocerotis (Cooke) Ryvarden (Tiger Milk mushroom) extract attenuates airway inflammation in murine model of allergic asthma. Exp. Ther. Med. 2019, 17, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.J.; Kong, B.H.; Teoh, K.H.; Yap, Y.H.; Ng, S.T.; Tan, C.S.; Razif, M.F.; Fung, S.Y. In vivo anti-tumor activity of Lignosus rhinocerus TM02® using a MCF7-xenograft NCr nude mice model. J. Ethnopharmacol. 2023, 304, 115957. [Google Scholar] [CrossRef]
- Hu, T.; Huang, Q.; Wong, K.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, G.; Li, H.; Zhuang, C.; Mizuno, T.; Ito, H.; Suzuki, C.; Okamoto, H.; Li, J. Antitumor polysaccharides from a Chinese mushroom,“yuhuangmo” the fruiting body of Pleurotus citrinopileatus. Biosci. Biotechnol. Biochem. 1994, 58, 1195–1201. [Google Scholar] [CrossRef][Green Version]
- Meng, M.; Sun, Y.; Bai, Y.; Xu, J.; Sun, J.; Han, L.; Sun, H.; Han, R. A polysaccharide from Pleurotus citrinopileatus mycelia enhances the immune response in cyclophosphamide-induced immunosuppressed mice via p62/Keap1/Nrf2 signal transduction pathway. Int. J. Biol. Macromol. 2023, 228, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, L.L.; Liu, H.P.; Wu, Y.R.; Li, M.Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 51–57. [Google Scholar] [CrossRef]
- Li, X.; Jin, Q.; Zhang, Y.; Wu, Y.L.; Jin, C.M.; Cui, B.W.; Li, Y.; Jin, M.J.; Shang, Y.; Jiang, M.; et al. Inhibition of P2X7R–NLRP3 Inflammasome Activation by Pleurotus citrinopileatus: A Possible Protective Role in Alcoholic Hepatosteatosis. J. Agric. Food Chem. 2018, 66, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-obesity effect of DKB-117 through the inhibition of pancreatic lipase and α-amylase activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, X.; Chen, W. Antioxidant and Immunostimulatory Activities of Fermented Sour Soybean Milk Added With Polypeptides From Pleurotus eryngii. Front. Microbiol. 2022, 13, 750039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, J.; Fu, Q.; Fu, X.; Shu, T.; Bi, Y.; Song, B. Antitumor activity of a polysaccharide from Pleurotus eryngii on mice bearing renal cancer. Carbohydr. Polym. 2013, 95, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Hu, Q.; Han, Y.; Du, H.; Yang, W.; Pan, C.; Cao, X.; Kimatu, B.M.; Pei, F.; Xiao, H. Inhibitory effects of β-type glycosidic polysaccharide from Pleurotus eryngii on dextran sodium sulfate-induced colitis in mice. Food Funct. 2021, 12, 3831–3841. [Google Scholar] [CrossRef]
- Boulaka, A.; Mantellou, P.; Stanc, G.-M.; Souka, E.; Valavanis, C.; Saxami, G.; Mitsou, E.; Koutrotsios, G.; Zervakis, G.I.; Kyriacou, A.; et al. Genoprotective activity of the Pleurotus eryngii mushrooms following their in vitro and in vivo fermentation by fecal microbiota. Front. Nutr. 2022, 9, 988517. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.-J.; Ghim, J.; Kim, J.; Lee, H.; Lee, T.G.; Kim, J.-I.; Kim, Y.; Byun, J.W.; Min, B.S.; Son, J.S.; et al. Water extract of Pleurotus eryngii var. ferulae prevents high-fat diet-induced obesity by inhibiting pancreatic Lipase. J. Med. Food 2019, 22, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, A.; Wang, Y.; Qu, Y.; Gong, H.; Mayo, K.H.; Zhou, Y.; Cheng, H. Heterogalactan WPEP-Nb from Pleurotus eryngii enhances immunity in immunocompromised mice. Int. J. Biol. Macromol. 2023, 225, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.P.; Roy, B.; Patra, P.; Sahoo, B.; Islam, S.S.; Maiti, T.K. Characterization and lectin microarray of an immunomodulatory heteroglucan from Pleurotus ostreatus mycelia. Carbohydr. Polym. 2013, 94, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, S.; Akuzawa, Y.; Endo, F. Effects of Lentinus edodes, Grifola frondosa and Pleurotus ostreatus administration on cancer outbreak, and activities of macrophages and lymphocytes in mice treated with a carcinogen, N-butyl-N-butanolnitrosoamine. Immunopharmacol. Immunotoxicol. 1997, 19, 175–183. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Sheng, Y.; Liu, J.; Li, H.; Guo, M.; Xu, W.; Luo, Y.; Huang, K.; He, X. Pleurotus ostreatus Ameliorates Obesity by Modulating the Gut Microbiota in Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1868. [Google Scholar] [CrossRef]
- Ravi, B.; Renitta, R.E.; Prabha, M.L.; Issac, R.; Naidu, S. Evaluation of antidiabetic potential of oyster mushroom (Pleurotus ostreatus) in alloxan-induced diabetic mice. Immunopharmacol. Immunotoxicol. 2013, 35, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jedinak, A.; Dudhgaonkar, S.; Jiang, J.; Sandusky, G.; Sliva, D. Pleurotus ostreatus inhibits colitis-related colon carcinogenesis in mice. Int. J. Mol. Med. 2010, 26, 643–650. [Google Scholar] [PubMed]
- Sarangi, I.; Ghosh, D.; Bhutia, S.K.; Mallick, S.K.; Maiti, T.K. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int. Immunopharmacol. 2006, 6, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Llauradó, G.; Morris, H.J.; Lebeque, Y.; Venet, G.; Fong, O.; Marcos, J.; Fontaine, R.; Cos, P.; Bermúdez, R.C. Oral administration of an aqueous extract from the oyster mushroom Pleurotus ostreatus enhances the immunonutritional recovery of malnourished mice. Biomed. Pharmacother. 2016, 83, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Yatsuzuka, R.; Nakano, Y.; Jiang, S.; Ueda, Y.; Kishi, Y.; Suzuki, Y.; Yokota, E.; Rahman, A.; Ono, R.; Kohno, I.; et al. Effect of usuhiratake (Pleurotus pulmonarius) on sneezing and nasal rubbing in BALB/c mice. Biol. Pharm. Bull. 2007, 30, 1557–1560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmad Tarmizi, N.A.; Kushairi, N.; Phan, C.W.; Sabaratnam, V.; Naidu, M.; David, P. β-Glucan-Rich Extract of Gray Oyster Mushroom, Pleurotus pulmonarius, Improves Object Recognition Memory and Hippocampus Morphology in Mice Fed a High-Fat Diet. J. Med. Food 2022, 25, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Levinson, D.; Peri, I.; Tekoah, Y.; Hadar, Y.; Schwartz, B. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Appl. Microbiol. Biotechnol. 2010, 85, 1977–1990. [Google Scholar] [CrossRef]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the edible mushroom Pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef]
- Mao, X.W.; Archambeau, J.O.; Gridley, D.S. Immunotherapy with low-dose interleukin-2 and a polysaccharopeptide derived from Coriolus versicolor. Cancer Biother. Radiopharm. 1996, 11, 393–403. [Google Scholar] [CrossRef]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Yue, G.G.; Gao, S.; Luo, K.W.; Siu, W.S.; Shum, W.T.; Shiu, H.T.; Lee, J.K.; Li, G.; Leung, P.C.; et al. Evaluation of the combined use of metronomic zoledronic acid and Coriolus versicolor in intratibial breast cancer mouse model. J. Ethnopharmacol. 2017, 204, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.; Or, P.M. Effects of polysaccharide peptides from COV-1 strain of Coriolus versicolor on glutathione and glutathione-related enzymes in the mouse. Food Chem. Toxicol. 2007, 45, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Jumes, F.M.; Lugarini, D.; Pereira, A.L.; de Oliveira, A.; Christoff, A.D.; Linde, G.A.; do Valle, J.S.; Colauto, N.B.; Acco, A. Effects of Agaricus brasiliensis mushroom in Walker-256 tumor-bearing rats. Can. J. Physiol. Pharmacol. 2010, 88, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hong, U.P.; Kim, J.S.; Kim, C.H.; Lee, K.W.; Choi, S.E.; Park, K.H.; Lee, M.W. Antidiabetic effect of Auricularia auricula mycelia in streptozotocin-induced diabetic rats. Nat. Prod. Sci. 2007, 13, 390–393. [Google Scholar]
- Kyakulaga, A.H.; Ogwang, P.E.; Obua, C.; Nakabonge, G.; Mwavu, E.N. Immunomodulatory effects of aqueous extracts of auricularia sp and pleurotus sp mushrooms in cyclophosphamide-immunosuppressed wistar rats. Br. J. Pharm. Res. 2013, 3, 662–670. [Google Scholar] [CrossRef]
- Li, Z.; Yao, X.; Liu, B.; Reheman, H.N.; Yang, G.; Zhan, S.; Qi, M.A. Auricularia auricular-judae polysaccharide attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and inflammation. Biomed. Rep. 2015, 3, 478–482. [Google Scholar] [CrossRef] [PubMed]
- KChellappan, D.; Ganasen, S.; Batumalai, S.; Candasamy, M.; Krishnappa, P.; Dua, K.; Chellian, J.; Gupta, G. The protective action of the aqueous extract of Auricularia polytricha in paracetamol induced hepatotoxicity in rats. Recent Pat. Drug Deliv. Formul. 2016, 10, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Budinastiti, R.; Sunoko, H.R.; Widiastiti, N.S. The effect of cloud ear fungus (Auricularia polytricha) on serum total cholesterol, LDL and HDL levels on Wistar rats induced by reused cooking oil. In E3S Web of Conferences 2018; EDP Sciences: Les Ulis, France, 2018; Volume 31, p. 06006. [Google Scholar]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in rats. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef]
- Sheu, J.R.; Geraldine, P.; Yen, M.H. Bioactives and traditional herbal medicine for the treatment of cardiovascular/cerebrovascular diseases 2015. Evid.-Based Complement. Altern. Med. 2015, 2015, 320545. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid β-protein-induced neurotoxicity and memory impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Cheng, K.C.; Wang, H.T.; Hsieh, C.W.; Lai, Y.J. Extracts of Antrodia cinnamomea mycelium as a highly potent tyrosinase inhibitor. J. Cosmet. Dermatol. 2021, 20, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Lei, A.; Chen, Y.; Yu, Q.; Xie, J.; Yang, Y.; Yuan, T.; Su, D. The protective effects of the Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food Funct. 2021, 12, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.W.; Liao, C.W.; Lin, C.H.; Tseng, C.Y. Immunomodulatory protein from ganoderma microsporum protects against oxidative damages and cognitive impairments after traumatic brain injury. Mol. Cell. Neurosci. 2022, 120, 103735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Tang, Y.P.; Xiang, J.; Wua, P.; Jin, H.M.; Wang, Z.; Mori, M.; Cai, D.F. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J. Ethnopharmacol. 2010, 131, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Li, X.; Yap, A.C.; Cheung, P.C.; Tan, C.S.; Ng, S.T.; Roberts, R.; Ting, K.N.; Fung, S.Y. Airway relaxation effects of water-soluble sclerotial extract from Lignosus rhinocerotis. Front. Pharmacol. 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lim, K.H.; Millns, P.; Mohankumar, S.K.; Ng, S.T.; Tan, C.S.; Then, S.M.; Mbaki, Y.; Ting, K.N. Bronchodilator effects of Lignosus rhinocerotis extract on rat isolated airways is linked to the blockage of calcium entry. Phytomedicine 2018, 42, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Seow, S.L.; Naidu, M.; Sabaratnam, V.; Vidyadaran, S.; Wong, K.H. Tiger’s milk medicinal mushroom, Lignosus rhinocerotis (Agaricomycetes) sclerotium inhibits nitric oxide production in LPS-stimulated BV2 microglia. Int. J. Med. Mushrooms 2017, 19, 405–418. [Google Scholar] [CrossRef]
- Lee, S.S.; Tan, N.H.; Fung, S.Y.; Sim, S.M.; Tan, C.S.; Ng, S.T. Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the Tiger Milk mushroom. BMC Complement. Altern. Med. 2014, 14, 359. [Google Scholar] [CrossRef]
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Yamanaka, M.; Gyokusen, M.; Kaneko, S.; Tsutsui, M.; Sato, J.; Sato, I.; Sato, M.; Kondo, R. Estrogen-like activity and prevention effect of bone loss in calcium deficient ovariectomized rats by the extract of Pleurotus eryngii. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Zhan, T.; Yu, X.L.; He, Q.A.; Huang, W.J.; Lin, L.Z.; Du, Y.T.; Pan, Y.T. Therapeutic effect of Pleurotus eryngii cellulose on experimental fatty liver in rats. Genet. Mol. Res. 2016, 15, 15017805. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Q.; Lin, Z.; Liu, S.; Su, Q.; Pan, Y. Therapeutic effects of chitin from Pleurotus eryngii on high-fat diet induced obesity in rats. Acta Sci. Polonorum. Technol. Alimentaria. 2020, 19, 279–289. [Google Scholar]
- Yang, Q.; Huang, B.; Li, H.; Zhang, C.; Zhang, R.; Huang, Y.; Wang, J. Gastroprotective activities of a polysaccharide from the fruiting bodies of Pleurotus ostreatus in rats. Int. J. Biol. Macromol. 2012, 50, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Olufemi, A.E.; Terry, A.O.; Kola, O.J. Anti-leukemic and immunomodulatory effects of fungal metabolites of Pleurotus pulmonarius and Pleurotus ostreatus on benzene-induced leukemia in Wister rats. Korean J. Hematol. 2012, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Balaji, P.; Madhanraj, R.; Rameshkumar, K.; Veeramanikandan, V.; Eyini, M.; Arun, A.; Thulasinathan, B.; Al Farraj, D.A.; Elshikh, M.S.; Alokda, A.M.; et al. Evaluation of antidiabetic activity of Pleurotus pulmonarius against streptozotocin-nicotinamide induced diabetic wistar albino rats. Saudi J. Biol. Sci. 2020, 27, 913–924. [Google Scholar] [CrossRef]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Dadar, M.; Khalili, M. Powder of the white bottom mushroom, Agaricus bisporus (Agaricomycetes), improved immunomodulatory and health-promoting effects of Lactobacillus casei in zebrafish (Danio rerio). Int. J. Med. Mushrooms 2018, 20, 695–704. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Pang, L.; Len, B.; Lin, Z.; Huang, J.; ShangGuan, Z.; Pan, Y. Agaricus bisporus-derived β-glucan prevents obesity through PPAR γ downregulation and autophagy induction in zebrafish fed by chicken egg yolk. Int. J. Biol. Macromol. 2019, 125, 820–828. [Google Scholar] [CrossRef]
- Zakariaee, H.; Sudagar, M.; Hosseini, S.S.; Paknejad, H.; Baruah, K. In vitro Selection of Synbiotics and in vivo Investigation of Growth Indices, Reproduction Performance, Survival, and Ovarian Cyp19α Gene Expression in Zebrafish Danio rerio. Front. Microbiol. 2021, 12, 758758. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, W.; Wu, Q.; Cai, S.; Jia, T.; Sun, J.; Lin, Z.; Alitongbieke, G.; Chen, Y.; Su, Y.; et al. Agaricus bisporus-derived glucosamine hydrochloride facilitates skeletal injury repair through Bmp signaling in zebrafish osteoporosis model. J. Nat. Prod. 2021, 84, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Unravelling anti-melanogenic potency of edible mushrooms laetiporus sulphureus and agaricus silvaticus in vivo using the zebrafish model. J. Fungi 2021, 7, 834. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, L.; Wang, S.; Yang, L.; Li, P. Deacetyl Ganoderic Acid F Inhibits LPS-Induced Neural Inflammation via NF-κB Pathway Both In vitro and In vivo. Nutrients 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, L.; Zhang, L.; Hu, B.; Wang, Q.; Liang, L. Antioxidant Properties of Triterpenoids Isolated from Bagasse-Cultivated Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), at Different Developmental Stages. Int. J. Med. Mushrooms 2022, 24, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Rowan, N. Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. Int. J. Mol. Sci. 2021, 22, 1675. [Google Scholar] [CrossRef] [PubMed]
- Valu, M.V.; Soare, L.C.; Ducu, C.; Moga, S.; Negrea, D.; Vamanu, E.; Balseanu, T.A.; Carradori, S.; Hritcu, L.; Boiangiu, R.S. Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment. J. Fungi 2021, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.I.; Das, B. Molecular insights and cell cycle assessment upon exposure to Chaga (Inonotus obliquus) mushroom polysaccharides in zebrafish (Danio rerio). Sci. Rep. 2020, 10, 7406. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.I.; Al-Tuwaijri, M.M.; Mohanty, S.; Das, B. Chaga mushroom (Inonotus obliquus) polysaccharides exhibit genoprotective effects in UVB-exposed embryonic zebrafish (Danio rerio) through coordinated expression of DNA repair genes. Heliyon 2021, 7, e06003. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Lee, S.; Lee, J.; Jeong, W.T.; Lim, H.B.; Hyun, T.K.; Yi, S.J.; Kim, K. Ethyl acetate fraction of aqueous extract of lentinula edodes inhibits osteoclastogenesis by suppressing nfatc1 expression. Int. J. Mol. Sci. 2020, 21, 1347. [Google Scholar] [CrossRef]
- Mahmood, I.; Azfaralariff, A.; Mohamad, A.; Airianah, O.B.; Law, D.; Dyari, H.R.; Lim, Y.C.; Fazry, S. Mutated Shiitake extracts inhibit melanin-producing neural crest-derived cells in zebrafish embryo. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2021, 245, 109033. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Gu, J.Y.; Kim, K.M.; Lee, J.J.; Kim, T.H.; Kang, S.C. Ultraviolet B-irradiated mushroom supplementation increased the Ca++ uptake and ameliorated the LPS-induced inflammatory responses in zebrafish larvae. J. Food Biochem. 2021, 45, e13742. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lai, T.C.; Chen, L.; Kwok, H.F.; Lau, C.B.; Cheung, P.C. Antioxidant and antiangiogenic properties of phenolic extract from Pleurotus tuber-regium. J. Agric. Food Chem. 2014, 62, 9488–9498. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Lan, Y.; Yuan, J. Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium. Foods 2022, 11, 2881. [Google Scholar] [CrossRef]
- Panchal, K.; Tiwari, A.K. Drosophila melanogaster “a potential model organism” for identification of pharmacological properties of plants/plant-derived components. Biomed. Pharmacother. 2017, 89, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Pratomo, A.R.; Salim, E.; Hori, A.; Kuraishi, T. Drosophila as an Animal Model for Testing Plant-Based Immunomodulators. Int. J. Mol. Sci. 2022, 23, 14801. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Kuo, Y.H.; Cheng, J.; Chang, L.Z.; Chang, M.S.; Su, L.W.; Chuang, T.N.; Lin, W.Y. Ergosta-7,9,22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress. Molecules 2021, 26, 5338. [Google Scholar] [CrossRef]
- Ma, W.W.; Tao, Y.; Wang, Y.Y.; Peng, I.F. Effects of Gardenia jasminoides extracts on cognition and innate immune response in an adult Drosophila model of Alzheimer’s disease. Chin. J. Nat. Med. 2017, 15, 899–904. [Google Scholar] [CrossRef]
- Li, I.C.; Lee, L.Y.; Chen, Y.J.; Chou, M.Y.; Wang, M.F.; Chen, W.P.; Chen, Y.P.; Chen, C.C. Erinacine A-enriched Hericium erinaceus mycelia promotes longevity in Drosophila melanogaster and aged mice. PLoS ONE 2019, 14, e0217226. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, S.C.; Chang, J.C.; Lin, W.Y.; Chen, C.C.; Li, C.C.; Hsieh, M.; Chen, H.W.; Chang, T.Y.; Liu, C.S.; et al. The protective effect of erinacine A-enriched Hericium erinaceus mycelium ethanol extract on oxidative Stress-Induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type 3. Free Radic. Biol. Med. 2023, 195, 1–12. [Google Scholar] [CrossRef]
- Matjuskova, N.; Azena, E.; Serstnova, K.; Muiznieks, I. The influence of the hot water extract from shiitake medicinal mushroom, Lentinus edodes (higher Basidiomycetes) on the food intake, life span, and age-related locomotor activity of Drosophila melanogaster. Int. J. Med. Mushrooms 2014, 16, 605–6015. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Falade, A.O. Pleurotus ostreatus and Lentinus subnudus supplemented diets restore altered acetylcholinesterase and butyrylcholinesterase activities and improve antioxidant status in transgenic Drosophila melanogaster model. J. Diet. Suppl. 2021, 18, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Kylyc, A.; Yesilada, E. Preliminary results on antigenotoxic effects of dried mycelia of two medicinal mushrooms in Drosophila melanogaster somatic mutation and recombination test. Int. J. Med. Mushrooms 2013, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Miranda-Vizuete, A.; Sánchez-Hernández, E.; Romero, M.R.; Dueñas, M.; Santos-Buelga, C.; González-Paramás, A.M. Exploring target genes involved in the effect of quercetin on the response to oxidative stress in Caenorhabditis elegans. Antioxidants 2019, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Fang, Z.; Chen, Y.; Chen, Y.; Xiao, B.; Guo, L.; Xu, Y.; Wang, G.; Wang, W.; Zhang, Y. Hypoglycemic Effect of the Degraded Polysaccharides from the Wood Ear Medicinal Mushroom Auricularia auricula-judae (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xiao, B.; Chen, S.; Zeng, J.; Yao, J.; Tan, J.; Wang, G.; Wang, W.; Zhang, Y. Effect of Enzyme-Assisted Extraction on the Chemical Properties and Antioxidant Activities of Polysaccharides Obtained from the Wood Ear Mushroom, Auricularia auricula (Agaricomycetes). Int. J. Med. Mushrooms 2022, 24, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, M.; Wang, X.; Shan, X.; Ji, L.; Zhang, Y. Preparation of Wood Ear Medicinal Mushroom, Auricularia auricula-judae (Agaricomycetes), Melanin and Its Antioxidant Properties: Evaluation In vitro and In vivo. Int. J. Med. Mushrooms 2021, 23, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Jiang, K. Antioxidant activity in vitro and in vivo of the polysaccharides from different varieties of Auricularia auricula. Food Funct. 2016, 7, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, Q.; Liu, J.; Ye, Z.; Feng, T.; Wang, G.; Wang, W.; Zhang, Y. Ultrasonic-assisted extraction of polysaccharides from Auricularia auricula and effects of its acid hydrolysate on the biological function of Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 167, 423–433. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, S.; Huang, Q.; Tan, J.; Zeng, J.; Yao, J.; Feng, T.; Wang, G.; Zhang, Y. The lipid lowering and antioxidative stress potential of polysaccharide from Auricularia auricula prepared by enzymatic method. Int. J. Biol. Macromol. 2021, 187, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, Y.; Wang, G.; Feng, T.; Shen, M.; Xiao, B.; Gu, J.; Wang, W.; Li, J.; Zhang, Y. Evaluation of the antioxidant effects of acid hydrolysates from Auricularia auricular polysaccharides using a Caenorhabditis elegans model. Food Funct. 2019, 10, 5531–5543. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Lin, L.; Cui, H.; Wang, T.; Chen, M.; Wang, C. Cellulase-assisted extraction and anti-ultraviolet activity of polysaccharides from the root of Flammulina velutipes on Caenorhabditis elegans. Pak. J. Pharm. Sci. 2018, 31, 2487–2495. [Google Scholar] [PubMed]
- Chuang, M.H.; Chiou, S.H.; Huang, C.H.; Yang, W.B.; Wong, C.H. The lifespan-promoting effect of acetic acid and Reishi polysaccharide. Bioorg Med. Chem. 2009, 17, 7831–7840. [Google Scholar] [CrossRef] [PubMed]
- Cuong, V.T.; Chen, W.; Shi, J.; Zhang, M.; Yang, H.; Wang, N.; Yang, S.; Li, J.; Yang, P.; Fei, J. The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol. 2019, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.H.; Wu, C.Y.; Hsiao, Y.C.; Martel, J.; Ke, P.Y.; Chiu, C.Y.; Liau, J.C.; Chang, I.T.; Su, Y.H.; Ko, Y.F.; et al. Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells. Aging 2021, 13, 13474–13495. [Google Scholar] [CrossRef]
- Wu, F.; Jia, X.; Yin, L.; Cheng, Y.; Miao, Y.; Zhang, X. The Effect of Hemicellulose and Lignin on Properties of Polysaccharides in Lentinus edodes and Their Antioxidant Evaluation. Molecules 2019, 24, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kittimongkolsuk, P.; Pattarachotanant, N.; Chuchawankul, S.; Wink, M.; Tencomnao, T. Neuroprotective Effects of Extracts from Tiger Milk Mushroom Lignosus rhinocerus Against Glutamate-Induced Toxicity in HT22 Hippocampal Neuronal Cells and Neurodegenerative Diseases in Caenorhabditis elegans. Biology 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Grinde, B.; Hedand, G.; Johnson, E. Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. Int. Immunopharmacol. 2006, 6, 1311–1314. [Google Scholar] [CrossRef]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Tangen, J.M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Troseid, A.M.S.; Wang, J.B.; Tjonnfjord, G.E.; Hetland, G. Immunomodulatory Effects of the Agaricus blazei Murrill-Based Mushroom Extract AndoSan in Patients with Multiple Myeloma Undergoing High Dose Chemotherapy and Autologous Stem Cell Transplantation: A Randomized, Double Blinded Clinical Study. Biomed. Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef] [PubMed]
- Valadares, F.; Garbi Novaes, M.R.; Canete, R. Effect of Agaricus sylvaticus supplementation on nutritional status and adverse events of chemotherapy of breast cancer: A randomized, placebo-controlled, double-blind clinical trial. Indian J. Pharmacol. 2013, 45, 217–222. [Google Scholar]
- Fortes, R.C.; Recova, V.L.; Melo, A.L.; Novaes, M.R.C.G. Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: A randomized, double-blind, placebo-controlled clinical study. Nutr. Hosp. 2008, 23, 591–598. [Google Scholar] [PubMed]
- Tsai, M.Y.; Hung, Y.C.; Chen, Y.H.; Chen, Y.H.; Huang, Y.C.; Kao, C.W.; Su, Y.L.; Chiu, H.H.; Rau, K.M. A preliminary randomised controlled study of short-term Antrodia cinnamomea treatment combined with chemotherapy for patients with advanced cancer. BMC Complement. Altern. Med. 2016, 16, 322. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes versicolor in Women with Breast Cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.W.; Choo, S.P. Coriolus versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore Powder of Ganoderma lucidum Improves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evid. Based Complement. Alternat. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.T.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate Specific-Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. Aphase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Wesa, K.M.; Cunningham-Rundles, S.; Klimek, V.M.; Vertosick, E.; Coleton, M.I.; Yeung, K.S.; Lin, H.; Nimer, S.; Cassileth, B.R. Maitake mushroom extract in myelodysplastic syndromes (MDS): A phase II study. Cancer Immunol. Immun. 2015, 64, 237–247. [Google Scholar] [CrossRef]
- Griessmayr, P.C.; Gauthier, M.; Barber, L.G.; Cotter, S.M. Mushroom-derived Maitake PET fraction as single agent for the treatment of lymphoma in dogs. J. Vet. Intern. Med. 2007, 21, 1409–1412. [Google Scholar] [PubMed]
- Docherty, S.; Doughty, F.L.; Smith, E.F. The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Nutrients 2023, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and Safety of Orally Administered Lentinula edodes Mycelia Extract for Patients Undergoing Cancer Chemotherapy: A Pilot Study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef] [PubMed]
- White, R.W.D.; Hackman, R.M.; Soares, S.E.; Beckett, L.A.; Sun, B.X. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology 2002, 60, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, Y.; Hashine, K.; Kakehi, Y.; Yoshimura, K.; Satou, T.; Kuruma, H.; Namiki, S.; Shinohara, N. Dietary Administration of Mushroom Mycelium Extracts in Patients with Early-Stage Prostate Cancers Managed Expectantly: A Phase II Study. Jpn. J. Clin. Oncol. 2010, 40, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Urushima, H.; Sakaue, M.; Yukawa, S.; Honda, H.; Hirai, K.; Igura, T.; Hayashi, N.; Maeda, K.; Kitagawa, T.; et al. Reduction of Adverse Effects by a Mushroom Product, Active Hexose Correlated Compound (AHCC) in Patients with Advanced Cancer During Chemotherapy-The Significance of the Levels of HHV-6 DNA in Saliva as a Surrogate Biomarker during Chemotherapy. Nutr. Cancer 2014, 66, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Meera, C.R.; Janardhanan, K.K. Antitumor Activity of a Polysaccharide-Protein Complex Isolated from a Wood-Rotting Polypore Macro Fungus Phellinus rimosus (Berk) Pilat. J. Environ. Pathol. Tox. 2012, 31, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Ellinger, S. Effect of the Intake of Oyster Mushrooms (Pleurotus ostreatus) on Cardiometabolic Parameters-A Systematic Review of Clinical Trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cha, H.J. Poria cocos Wolf extracts represses pigmentation in vitro and in vivo. Cell Mol. Biol. 2018, 64, 80–84. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Zhang, M.; Jiang, Y.F.; Li, X.L.; He, Y.L.; Zeng, P.J.; Guo, Z.H.; Chang, Y.J.; Luo, H.; Liu, Y.; et al. Lentinan as an immunotherapeutic for treating lung cancer: A review of 12 years clinical studies in China. J. Cancer Res. Clin. 2018, 144, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Morita, S.; Oba, K.; Matsui, T.; Kobayashi, M.; Nakazato, H.; Ohashi, Y. Meta-Analysis Group of the Japanese Society for Cancer of the Colon, R. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: A meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol. Immunother. 2006, 55, 404–411. [Google Scholar] [PubMed]
- Taguchi, T.; Furue, H.; Kimura, T.; Kondo, T.; Hattori, T.; Itoh, T.; Osawa, N. End point result of a randomized controlled study of the treatment of gastrointestinal cancer with a combination of lentinan and chemotherapeutic agents. Excerpta Med. 1985, 40, 151–165. [Google Scholar]
- Taguchi, T.; Furue, H.; Kimura, T.; Kondo, T.; Hattori, T.; Itoh, T.; Osawa, N. End point results of phase III study of lentinan. Jpn. J. Cancer Chemother. 1985, 12, 366–380. [Google Scholar]
- Furue, H.; Kitoh, I. Phase 111-study on Lentinan. Jpn. J. Cancer Chemother. 1981, 8, 944–960. [Google Scholar]
- Daba, A.S.; Ezeronye, Q.U. Anti-cancer effect of polysaccharides isolated from higher basidiomycetes mushrooms. Afr. J. Biotechnol. 2003, 2, 672–678. [Google Scholar]
- Kimura, Y.; Mizuno, H.; Satake, K.; Tahara, H.; Tsukuda, M. Clinical evaluation of Sizofiran an assistant immunotherapy in treatment of head and neck cancer. Acta Oto-Laryngol. 1994, 511, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Suzuki, M.; Chihara, T.; Fujiwara, A.; Fukuda, T.; Goto, S.; Ichinohe, K.; Jimi, S.; Kasamatsu, T.; Kawai, N.; et al. Clinical-Evaluation of Schizophyllan Combined with Irradiation in Patients with Cervical-Cancer—A Randomized Controlled Study. Cancer 1986, 58, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Kinukawa, T.; Tsumura, Y.; Otani, T.; Itoh, T.; Kobayashi, H.; Matsuura, O.; Kobayashi, M.; Fukutsu, T.; Ohshima, S. Adjuvant immunochemotherapy: Two randomized controlled studies of patients with cervical cancer. Biomed. Pharmacother. 1989, 43, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Mizutani, H.; Katabuchi, H.; Fukuma, K.; Fujisaki, S.; Okamura, H. Activated (Hla-Dr+) T-Lymphocyte Subsets in Cervical-Carcinoma and Effects of Radiotherapy and Immunotherapy with Sizofiran on Cell-Mediated-Immunity and Survival. Gynecol. Oncol. 1995, 56, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Li, I.C.; Lin, T.W.; Chang, H.L.; Lin, W.H.; Shen, Y.C. Efficacy and safety of oral Antrodia cinnamomea mycelium in mildly hypertensive adults: A randomized controlled pilot clinical study. Eur. J. Integr. Med. 2016, 8, 654–660. [Google Scholar] [CrossRef]
- Yen, Y.T.; Park, J.H.; Kang, S.W.; Su, T.; Cheng, H.; Wen, W.C.; Lin, S.Y.; Tai, Y.; Chen, P.N.; Tsai, S.C. Clinical benefits of golden-Antrodia camphorata containing antroquinonol in liver protection and liver fat reduction after alcoholic hepatitis. Front. Pharmacol. 2022, 13, 757494. [Google Scholar] [CrossRef] [PubMed]
- Dosychev, E.A.; Bystrova, V.N. Treatment of psoriasis using “Chaga” fungus preparations. Vestnik Dermatologii i Venerologii 1973, 47, 79–83. [Google Scholar] [PubMed]
- Kim, Y.R. Immunomodulatory activity of the water extract from medici nal mushroom Inonotus obliquus. Mycobiology 2005, 33, 158–162. [Google Scholar] [CrossRef]
- Fedotov, A.A.; Yu, I.R. Effect of Befungin on the central nervous system in patients with peptic ulcers. Klin. Meditsina 1981, 59, 22–25. [Google Scholar]
- Ku, Y.H.; Kang, J.H. Efficacy of Phellinus linteus extract on immunity enhancement: A CONSORT-randomized, double-blind, placebo controlled pilot trial. Medicine 2022, 101, 30829. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.Y.; Lee, H.; Kang, J.H. A pilot clinical study of the efficacy and safety of Phellinus Linteus (Sanghuang) extract treatment for knee osteoar thritis. J. Acupuncture Res. 2022, 39, 115–121. [Google Scholar] [CrossRef]
- Sung, H.L.; Ho, K.H.; Chang, M.K.; Woo, J.L. Potential impact of Phellinus linteus on adherence to adjuvant treatment after curative resection of pancreatic ductal adenocarcinoma: Outcomes of a propensity score matched analysis. Integr. Cancer Ther. 2018, 18, 1534735418816825. [Google Scholar]
- Tee, P.Y.E.; Krishnan, T.; Cheong, X.T.; Maniam, S.A.P.; Looi, C.Y.; Ooi, Y.Y.; Chua, C.L.L.; Fung, S.Y.; Chia, A.Y.Y. A review on the cultivation, bioactive compounds, health-promoting factors and clinical trials of medicinal mushrooms Taiwanofungus camphoratus, Inonotus obliquus and Tropicoporus linteus. Fungal Biol. Biotechnol. 2024, 11, 7. [Google Scholar] [CrossRef]
- Muddapu, V.R.; Dharshini, S.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative diseases–is metabolic deficiency the root cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Obara, Y.; Nakahata, N. The signaling pathway of neurotrophic factor biosynthesis. Drug News Perspect. 2002, 15, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kolotushkina, E.V.; Moldavan, M.G.; Voronin, K.Y.; Skibo, G.G. The influence of Hericium erinaceus extract on myelination process in vitro. Fiziol Zh 2003, 49, 38–45. [Google Scholar]
- Lai, P.L.; Naidu, M.; Sabaratnam, V.; Wong, K.H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N.A. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The neuroprotective properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Kanagasabapathy, G.; Bakar, R.; Phan, C.W.; Sabaratnam, V. Restoration of sensory dysfunction following peripheral nerve injury by the polysaccharide from culinary and medicinal mushroom, Hericium erinaceus (Bull.: Fr.) Pers. through its neuroregenerative action. Food Sci. Technol. 2015, 35, 712–721. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid β (25–35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Aysun, E.; Hüseyin, O.; Emre, B.B.; Cenap, E.; Senay, D.; Engin, D. Neuroprotective Effects of Ganoderma lucidum on Spinal Cord Injury. Int. J. Morphol. 2018, 36, 175–179. [Google Scholar]
- Divanlıoğlu, D.; Salihoğlu, E.M.; Korkmaz, M.; Seçen, A.E.; Öcal, Ö.; Günerhan, G.; Belen, D.; Dalgıç, A. Biochemical analysis for neuroprotective effects of ganoderma lucidum in experimental rat spinal cord trauma model. J. Turk. Spinal Surg. 2021, 32, 116–122. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, Z.Q.; Zeng, Y.S.; Lin, Y.K.; Li, Y.; Chung, P.; Wong, R.; Hägg, U. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp. Toxicol. Pathol. 2012, 64, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Z.; Liao, Y.; Li, W.; Guo, L.M. Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Neural Regen. Res. 2017, 12, 953. [Google Scholar]
- Sharma, P.; Tulsawani, R. Ganoderma lucidum aqueous extract prevents hypobaric hypoxia induced memory deficit by modulating neurotransmission, neuroplasticity and maintaining redox homeostasis. Sci. Rep. 2020, 10, 8944. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, X.J.; Zhou, S.; Sun, D.X.; Wang, H.; Cheng, P.F.; Ma, X.R.; Liu, L.; Liu, J.X.; Wang, F.F.; et al. Intervention effects of ganoderma lucidum spores on epileptiform discharge hippocampal neurons and expression of neurotrophin-4 and N-cadherin. PLoS ONE 2013, 8, e61687. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Li, X.J.; Qiu, H.B.; Jiang, Z.M.; Simon, M.; Ma, X.R.; Liu, L.; Liu, J.X.; Wang, F.F.; Liang, Y.F.; et al. Anti-epileptic effect of Ganoderma lucidum polysaccharides by inhibition of intracellular calcium accumulation and stimulation of expression of CaMKII α in epileptic hippocampal neurons. PLoS ONE 2014, 9, e102161. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Pan, Z.; Leng, Y.; Lv, J.; Li, B. The effects of herbal medicine on epilepsy. Oncotarget 2017, 8, 48385. [Google Scholar] [CrossRef]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.S.; Lee, E.N.; Seo, G.S.; Lee, J.S. Screening and optimal extraction of a new antidementia β-secretase inhibitor-containing mushroom. Mycobiology 2008, 36, 195–197. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Nan, S.; Fan, J.; Chen, Q.; Zhang, Y. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Biol. Macromol. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Gunjima, K.; Tomiyama, R.; Takakura, K.; Yamada, T.; Hashida, K.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3, 4-dihydroxybenzalacetone protects against Parkinson’s disease-related neurotoxin 6-OHDA through Akt/Nrf2/glutathione pathway. J. Cell. Biochem. 2014, 115, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.X.; Wang, X.B.; Lv, T.M.; Hou, Z.L.; Lin, B.; Huang, X.X.; Song, S.J. Flavan derivative enantiomers and drimane sesquiterpene lactones from the Inonotus obliquus with neuroprotective effects. Bioorganic Chem. 2020, 96, 103588. [Google Scholar] [CrossRef] [PubMed]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Jin, C.Y.; Kim, G.Y.; Lee, J.D.; Park, C.; Kim, G.D.; Kim, W.J.; Jung, W.K.; Seo, S.K.; Choi, I.W.; et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, A.; He, Y.; Si, Z.; Xu, S.; Zhang, S.; Wang, K.; Wang, D.; Liu, Y. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull. 2016, 127, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wang, K.; Luo, C.; Huang, Y.; Misilimu, D.; Wen, H.; Jin, P.; Li, C.; Gong, Y.; Gao, Y. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J. Neuroinflammation 2021, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Pagiotti, R.; Maltese, F.; Mwankie GN, O.M.; Hoshino, T.; Obara, Y.; Nakahata, N. Cyathane diterpenes from Sarcodon cyrneus and evaluation of their activities of neuritegenesis and nerve growth factor production. Bioorganic Med. Chem. 2007, 15, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Obara, Y.; Hoshino, T.; Marcotullio, M.C.; Pagiotti, R.; Nakahata, N. A novel cyathane diterpene, cyrneine A, induces neurite outgrowth in a Rac1-dependent mechanism in PC12 cells. Life Sci. 2007, 80, 1669–1677. [Google Scholar] [CrossRef]
- Shi, X.W.; Liu, L.; Gao, J.M.; Zhang, A.L. Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and their neurite outgrowth-promoting activity. Eur. J. Med. Chem. 2011, 46, 3112–3117. [Google Scholar] [CrossRef]
- Cao, C.Y.; Zhang, C.C.; Shi, X.W.; Li, D.; Cao, W.; Yin, X.; Gao, J.M. Sarcodonin G derivatives exhibit distinctive effects on neurite outgrowth by modulating NGF signaling in PC12 cells. ACS Chem. Neurosci. 2018, 9, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, X.W.; Zong, S.C.; Tang, J.J.; Gao, J.M. Scabronine M, a novel inhibitor of NGF-induced neurite outgrowth from PC12 cells from the fungus Sarcodon scabrosus. Bioorganic Med. Chem. Lett. 2012, 22, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A mechanistic review on medicinal mushrooms-derived bioactive compounds: Potential mycotherapy candidates for alleviating neurological disorders. Planta Medica 2020, 86, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; Wong, W.L.; David, P.; Naidu, M.; Sabaratnam, V. Pleurotus giganteus (Berk.) Karunarathna & KD Hyde: Nutritional value and in vitro neurite outgrowth activity in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2012, 12, 102. [Google Scholar]
- Phan, C.W.; David, P.; Wong, K.H.; Naidu, M.; Sabaratnam, V. Uridine from Pleurotus giganteus and its neurite outgrowth stimulatory effects with underlying mechanism. PLoS ONE 2015, 10, e0143004. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflammation 2013, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Aramabašić Jovanović, J.; Mihailović, M.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidaković, M. The effects of major mushroom bioactive compounds on mechanisms that control blood glucose level. J. Fungi 2021, 7, 58. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and plant-derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). J. Endocrinol. 1998, 157, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Wang, H.; Li, C.; Qi, P.; Bao, J. Agaricus bisporus lectins mediates islet β-cell proliferation through regulation of cell cycle proteins. Exp. Biol. Med. 2012, 237, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhan, Y.; Chen, B.; Xie, B.; Fang, T.; Ravishankar, S.; Jiang, Y. Assessment of antioxidant and antidiabetic properties of Agaricus blazei Murill extracts. Food Sci. Nutr. 2020, 8, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.H.; Cho, J.H.; Park, K.I.; Kim, K.; Kim, T.H. Enzymatic transformation of esculetin as a potent class of α-glucosidase inhibitors. Bioorganic Med. Chem. Lett. 2023, 88, 129302. [Google Scholar]
- Lee, S.K.; Ryu, S.H.; Turk, A.; Yeon, S.W.; Jo, Y.H.; Han, Y.K.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Characterization of α-glucosidase inhibitory constituents of the fruiting body of lion’s mane mushroom (Hericium erinaceus). J. Ethnopharmacol. 2020, 262, 113197. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Philippe, E.; Everard, A.; Kassis, N.; Rouch, C.; Denom, J.; Takeda, Y.; Uchiyama, S.; Delzenne, N.M.; Cani, P.D.; et al. Dietary supplementation with Agaricus blazei Murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity 2013, 21, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, K.H.; Choi, H.J.; Lee, D.S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, C.; Wang, X.; Jia, D.; Lu, W.; Sun, X.; Liu, Y.; Yuan, L. Hpyerglycemic and anti-diabetic nephritis activities of polysaccharides separated from Auricularia auricular in diet-streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017, 13, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Fan, Z.; Qiu, J.; Rumbani, M.; Yang, X.; Zhang, H.; Wang, Z. Effects of polysaccharide from the fruiting bodies of Auricularia auricular on glucose metabolism in 60Co-γ-radiated mice. Int. J. Biol. Macromol. 2019, 135, 887–897. [Google Scholar] [CrossRef]
- Wu, N.J.; Chiou, F.J.; Weng, Y.M.; Yu, Z.R.; Wang, B.J. In vitro hypoglycemic effects of hot water extract from Auricularia polytricha (wood ear mushroom). Int. J. Food Sci. Nutr. 2014, 65, 502–506. [Google Scholar] [CrossRef]
- Kuang, Y.; Chai, Y.; Su, H.; Lo, J.Y.; Qiao, X.; Ye, M. A network pharmacology-based strategy to explore the pharmacological mechanisms of Antrodia camphorata and antcin K for treating type II diabetes mellitus. Phytomedicine 2022, 96, 153851. [Google Scholar] [CrossRef]
- Senthil, K.K.; Gokila, V.M.; Wang, S.Y. Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells. Oncotarget 2017, 8, 96568. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cui, F.; Zhang, J.; Gao, X.; Zhou, M.; Xu, N.; Zhao, H.; Liu, M.; Zhang, C.; Jia, L.E. Antioxidative and renoprotective effects of residue polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2016, 146, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.H.; Chiu, Y.C.; Yar, N.; Chen, Y.C.; Cheng, C.H.; Liu, Y.C.; Chang, C.Y.; Chuu, J.J. Renoprotective Impacts of Inonotus obliquus Ethanol-Ethyl Acetate Extract on Combined Streptozotocin and Unilateral Nephrectomy-Induced Diabetic Nephropathy in Mice. Int. J. Mol. Sci. 2023, 24, 4443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tie, L. Preventive and therapeutic effect of Ganoderma (Lingzhi) on diabetes. Ganoderma Health Pharmacol. Clin. Appl. 2019, 1182, 201–215. [Google Scholar]
- Yi, Z.; Shao-Long, Y.; Ai-Hong, W.; Zhi-Chun, S.; Ya-Fen, Z.; Ye-Ting, X.; Yu-Ling, H. Protective effect of ethanol extracts of Hericium erinaceus on alloxan-induced diabetic neuropathic pain in rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 595480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci. Rep. 2017, 7, 10847. [Google Scholar] [CrossRef]
- Miletić, D.; Turło, J.; Podsadni, P.; Sknepnek, A.; Szczepańska, A.; Lević, S.; Nedović, V.; Nikšić, M. Turkey tail medicinal mushroom, Trametes versicolor (Agaricomycetes), crude exopolysaccharides with antioxidative activity. Int. J. Med. Mushrooms 2020, 22, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lo, H.C.; Hsu, T.H.; Lin, F.Y.; Lin, Y.S.; Wang, Z.J.; Chen, S.T.; Shen, C.L. Polysaccharides of Trametes versicolor improve bone properties in diabetic rats. J. Agric. Food Chem. 2015, 63, 9232–9238. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G. Cardiovascular disease. N. Engl. J. Med. 2003, 349, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol. Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [PubMed]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Uffelman, C.N.; Chan, N.I.; Davis, E.M.; Wang, Y.; McGowan, B.S.; Campbell, W.W. An assessment of mushroom consumption on cardiometabolic disease risk factors and morbidities in humans: A systematic review. Nutrients 2023, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mago, P.; Sharma, R.; Hafeez, I.; Nawaz, I.; Joshi, M.; Mehrotra, R. Mushroom based Cosmeceuticals: An Upcoming Biotechnology Sector. Biosci. Biotechnol. Res. Asia 2023, 20, 381–394. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.H.; Li, J.; Yang, H.; Shin, H.J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Srivastava, A.; Attri, B.L.; Sharma, V.P. Status report on mushroom based cosmetic products in market. Mushroom Res. 2020, 29. [Google Scholar] [CrossRef]
- Wennig, R.; Eyer, F.; Schaper, A.; Zilker, T.; Streichert, H.A. Mushroom Poisoning. Dtsch. Arztebl. Int. 2020, 117, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Mustonen, A.M. Toxic Potential of Traditionally Consumed Mushroom Species-A Controversial Continuum with Many Unanswered Questions. Toxins 2020, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Tagwireyi, C.; Musiyiwa, K.; Chipurura, B.; Nyamangara, J.; Sanganyado, E.; Chaukura, N. Occurrence, behavior, and human exposure and health risks of potentially toxic elements in edible mushrooms with focus on Africa. Env. Monit. Assess. 2021, 193, 302. [Google Scholar] [CrossRef]
- Parulska, D.S.; Falandysz, J. A Review of the Occurrence of Alpha-Emitting Radionuclides in Wild Mushrooms. Int. J. Env. Res. Public. Health 2020, 17, 8220. [Google Scholar] [CrossRef]
- Amobonye, A.; Lalung, J.; Awasthi, M.K.; Pillai, S. Fungal mycelium as leather alternative: A sustainable biogenic material for the fashion industry. Sustain. Mater. Technol. 2023, 38, e00724. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-Based Composite: The Future Sustainable Biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.J.; Murugan, M.; Jeyaraj, M.; Rathinam, N.K.; Sangiliyandi, G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli & B. subtilis. J. Ind. Eng. Chem. 2013, 5, 2325–2331. [Google Scholar]
- Afshar, P.; Sedaghat, S. Bio-synthesis of silver nanoparticles using water extracts of Satureja hortensis L and evaluation of the antibacterial properties. Curr. Nanosci. 2016, 12, 90–93. [Google Scholar] [CrossRef]
- Owaid, M.N.; Barish, A.; Shariati, M.A. Cultivation of Agaricus bisporus (button mushroom) and its usages in the biosynthesis of nanoparticles. Open Agric. 2017, 2, 537–543. [Google Scholar] [CrossRef]
- Paul, S.; Sasikumari, C.S.; Singh, A.R. Fabrication of silver nanoparticles synthesized from Ganodermalucidum intothecottonfabric and its antimicrobial property. Int. J. Pharm. Pharm. Sci. 2015, 7, 53–56. [Google Scholar]
- Adebayo, E.A.; Azeez, M.A.; Alao, M.B.; Oke, M.A.; Aina, D.A. Mushroom Nanobiotechnology: Concepts, Developments and Potentials. In Materials Horizons: From Nature to Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 257–285. [Google Scholar] [CrossRef]
- Gudikandula, K.; Vadapally, P.; Charya, M.A.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. Open Nano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Baymiller, M.; Huang, F.; Rogelj, S. Rapid one-step synthesis of gold nanoparticles using the ubiquitous coenzyme, NADH. Matters 2017, 3, e201705000007. [Google Scholar] [CrossRef]
- Hietzschold, S.; Walter, A.; Davis, C.; Taylor, A.A.; Sepunaru, L. Does nitrate reductase play a role in silver nanoparticle synthesis? Evidence for NADPH as the sole reducing agent. ACS Sustain. Chem. Eng. 2019, 7, 8070–8076. [Google Scholar] [CrossRef]
- Baker, S.; Rakshith, D.; Kavitha, K.S.; Santosh, P.; Kavitha, H.U.; Rao, Y.; Satish, S. Plants: Emerging as nanofactories towards facile route in the synthesis of nanoparticles. Bio Impacts 2013, 3, 111–117. [Google Scholar]
- Liu, K.; Liu, Y.; Lu, J.; Liu, X.; Hao, L.; Yi, J. Nanoparticles prepared by polysaccharides extracted from Biyang floral mushroom loaded with resveratrol: Characterization, bioactivity and release behavior under in vitro digestion. Food Chem. 2023, 426, 136612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, M.; Wu, D.; Li, W.; Chen, W.; Liu, P.; Zhang, H.; Yang, Y. Resveratrol-loaded sulfated Hericium erinaceus β-glucan-chitosan nanoparticles: Preparation, characterization and synergistic anti-inflammatory effects. Carbohydr Polym. 2024, 332, 121916. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Nanotechnology in Food and Plant Science: Challenges and Future Prospects. Plants 2023, 12, 2565. [Google Scholar] [CrossRef]


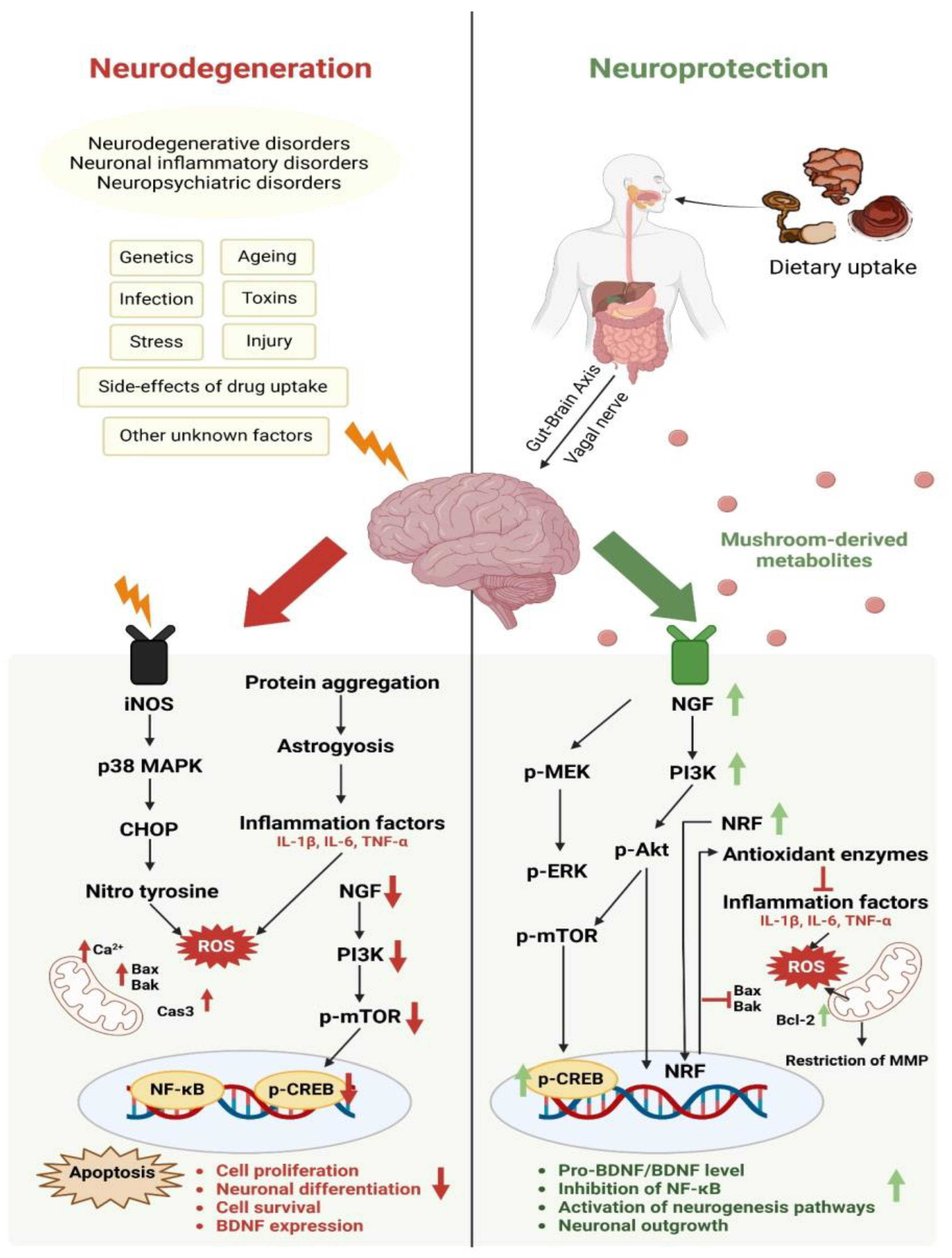
| Mushroom Name | Cell Line Used | Extract/ Compound | Protective Effects | Mechanism | References |
|---|---|---|---|---|---|
| Agaricus blazei Murill | primary macrophage from rat bone marrow | Aqueous extract | - | Induced macrophage activity via promoting cytokine and NO secretion | [17] |
| HEP-2 cells | Antiviral activity against herpes simplex type 1 (HSV-1) and bovine herpes type 1 (BoHV-1) | - | [18] | ||
| Isolated polysaccharide | Antiviral activity against poliovirus infection, Poliovirus type 1-induced infection | - | [19] | ||
| U937, MOLT4, HL60 and K562 | Agaritine, β-N-(γ-l(+)-glutamyl)-4-(hydroxymethyl) phenylhydrazine | Antitumor activity | Inhibited tumor cell proliferation | [20] | |
| Vero cells | Sulfated polysaccharides | Antiviral activity against Herpes simplex virus, HSV-1 KOS and 29 R, and HSV-2 333-induced infection | - | [21] | |
| MCF-7 | Ergosterol | Anticancer activity | Induced cancer cell apoptosis and inhibit cell growth | [22] | |
| Agaricus bisporus | LNCaP, PC3, and DU145 cell lines | Conjugated linoleic acid | Anticancer activity | Inhibited prostate cancer cell proliferation | [23] |
| Murine J774A.1 macrophages, murine Sarcoma 180 cells, and human cancer cell lines (MCF-7, HT29, DU145 and CRL-1739) | Polysaccharides | Anticancer activity | Induced macrophage activation and inhibit cancer cell growth | [24] | |
| Caco-2 cells | Anti-inflammatory and antioxidant activity, LPS- and TNF-α-induced inflammation | - | [25] | ||
| THP-1 cells | Immunomodulatory effect | Activation of pro-inflammatory cytokine production | [26] | ||
| Auricularia auricula-judae | A549 cells | Lectin (Ammonium-sulfate fractionation) | Antitumor | Modulation of JUN, TLR4, and MYD88 expression | [27] |
| HeLa cells | Chloroform extract | Anticancer activity | - | [28] | |
| HCT-15, huh-7, SK-MEL-5, SNU-213, and SNU-484 | Polysaccharide | Anticancer activity | Anticancer cell proliferation and anti-oxidative enzyme. Inhibition of the peroxiredoxin 1 pathway | [29] | |
| Ba/F3 TPR-Met and Ba/F3 TPR-TrkB cell lines | Ethyl acetate extract | Anticancer activity | Inhibition of cancer cell growth via suppressing tropomyosin receptor kinase B activity. Obstruction of auto-phosphorylation of TrkB | [30] | |
| HepG2 cells | Auricularia auricula polysaccharide-3-1 | Antioxidant activity | Reduction of reactive oxygen species production, malondialdehyde, and increased activities of superoxide dismutase, glutathione peroxidase, and catalase | [31] | |
| Auricularia polytricha | A549 and 3T3-L1 cells | Polysaccharides (Ethanol extraction) | Anticancer activity | Inhibition of cancer cell proliferation and induced cell apoptosis. Increased p53 and p21 levels and decreased cyclin A, cyclin D, and CDK2 expression | [32] |
| BV2 and HT-22 cells | Ergosterol (Hexane and ethanol extraction) | Anti-inflammatory and antioxidant activities | Increased SOD-1 expression and modulation of the NF-κB signaling pathway in BPA-induced inflammation | [33,34] | |
| Antrodia camphorate | MCF-7 and HBL100 cells | Fermented culture broth | Anticancer activity | Inhibition of cell viability, chromatin condensation, inter-nucleosomal DNA fragmentation, and sub-G1 phase accumulation | [35] |
| MDA-MB-231 cells | Antimetastatic activities | Inhibition of MMP-9, MMP-2, uPA, uPA receptor (uPAR), and vascular endothelial growth factor (VEGF) and suppression of phosphorylation of ERK1/2, p38, and JNK1/2 | [36] | ||
| HER-2/Neu-overexpressing SKOV-3 cells and human ovarian surface epithelial (IOSE) cells | Anticancer activity | Inhibition of HER-2/Neu activity, tyrosine phosphorylation, activation of PI3K/Akt and their downstream effector β-catenin | [37] | ||
| RAW 264 cells (LPS induced) | Anti-inflammatory activity | Reduced tumor necrosis factor (TNF-α) and interleukin (IL)-1β levels and decreased cytokine, iNOS, and COX-2 expression by blocking NF-κB activation | [37] | ||
| HT-29 cells | Five lanostanes (2, 3, 4, 6, and 8) and three ergostane types (1, 5, and 7) triterpenes. (Chloroform extraction) | Anticancer activity | Induced cytotoxicity and sub-G1 cell cycle arrest | [38] | |
| MDA-MB-23, A549, and HS68 cells | Twelve ergostanoids, named antcamphins A–L (1–12), together with 20 known triterpenoids. (95% ethanol extraction). | Anticancer activity | Induction of cytotoxicity | [39] | |
| MCF-7, MDA-MB-231, MCF10A, and HS-68 cells | Antrocin | Anticancer activity | Induction of cleavage of caspase-3 and poly (ADP-ribose) polymerase, reduced Bcl-2 and Bcl-xL, and suppressed phosphorylation of Akt and the activities of its downstream effectors mTOR, GSK-3β, and NF-κB | [40] | |
| CL1-0, CL1-5, A549, H1975, H441, PC9, and BEAS-2B cells | Anticancer activity | Increased expression of caspase-3, increased Bax/Bcl2 ratio, and downregulated the JAK/STAT signaling pathway | [41] | ||
| HTB-4 cell line | Phosphate-buffered saline (PBS) at the ratio of 1:25 (w/v) | Anticancer and anti-metastatic activities | Inhibited MMP-9 and induced phase G2M arrest | [42] | |
| Huh7, HepG2, and Hep3B cell lines | Antcin A, antcin C, and methyl antcinate A | Anticancer activity | Induced sub-G1 population, DNA fragmentation, TUNEL-positive cells, and caspase activation | [43] | |
| LNCaP, PC-3, and MEF cell lines | Ethanol extraction | Anticancer activity | Induced G1/S phase arrest, inhibited cyclin D1 activity, and prevented pRb phosphorylation | [44] | |
| MCF7, T47D, MDA-MB-231, MCF10A, and IMR-90 cell lines | Antroquinonol D | Anticancer activity | Inhibited DNMT1 methyltransferase activity | [45] | |
| Human erythrocytes | Aqueous extraction | Antioxidant activity | Increased glutathione (GSH) and ATP levels in peroxyl radical [2,2′-Azobis(2-amidinopropane) dihydrochloride, AAPH]-induced oxidation | [46] | |
| COLO 205 cell line | 5-Substituted 4,7-dimethoxy-1,3-benzodioxoles (compounds 1–9) (Ethyl acetate extraction) | Anticancer activity | Induced G0/G1 cell cycle arrest and increased p53, p21, and p27 levels | [47] | |
| PC12 cell line (6-hydroxydopamine-induced) | 95% ethanol extraction | Neuroprotective | Reduced the loss of dopaminergic neurons. Increased tyrosine hydroxylase (TH) and dopamine transporter (DAT) levels and reduced α-synuclein levels | [48] | |
| Boletus edulis | LS180 and CCD 841 CoTr cell lines | Biopolymers (polysaccharides and glycoproteins) | Anticancer activity | Induced G0/G1-phase arrest and inhibited the p16/cyclin D1/CDK4-6/pRb pathway | [49] |
| Ribonucleic acid | Anticancer activity | Increased Bax, TP53, and CDKN1A levels | [50] | ||
| MDA- MB-231 and Ca761 | Cold water-soluble polysaccharide (BEP); galactose, glucose, xylose, mannose, glucuronic, and galacturonic acid | Anticancer activity | Induced Bax/Bcl-2 ratios, the release of cytochrome C, and activated the expression of caspase-3 and caspase-9 | [51] | |
| MCF-7, SMMC-7721,hHL-60, SW480, and A549 cells | Non-isoprenoid botryane sesquiterpenoids, named boledulins A-C (1–3) | Anticancer activity | - | [52] | |
| MCF-7, HepG-2, CaCo, CFPAC, Hela, U-87, HT-29, SK-MEL-28, and A549 cells | Boletus edulis lectin (BEL) β-trefoil (Aqueous extraction) | Antineoplastic properties | - | [53] | |
| BEL-7402, HT-299, SPC-A1, and U-251 cells | 5-Cholestene-2,3-oxide, β-sitosterol, and stigmasterol | Anticancer activity | Induced cytotoxicity | [54] | |
| MCF7, HT-29, HUH-7, and L929 cells | Aqueous extraction | Anticancer, antimicrobial, and wound-healing activities | - | [55] | |
| RAW264.7 cells | Polysaccharide (BEP) | Immunomodulatory activity | Induced phagocytosis and NO, IL-6, and TNF-α secretion | [56] | |
| Flammulina velutipes | A549 | Fungal immunomodulatory protein-five (FIP-fve) | Anticancer activity | Induced p53 and p21 expression and inhibited EGF-induced activation of Rac1 via decreasing RACGAP1 mRNA and protein levels | [57] |
| MCF7 and MDA-MB231 cells | 5% ethanol extraction | Anticancer activity | Induced DNA damage (γ-H2AX foci formation), G2/M phase arrest, cytochrome c release, and caspase cleavage activity | [58] | |
| BGC-823 and A549 cells | Polysaccharide (FVP-1 and FVP-2) (Ultrasonic-assisted extraction) | Anticancer activity and antiproliferative activity | - | [59] | |
| MCF-10a, MCF-7, and MDA-MB-23 cells | Aqueous and methanol extraction | Antiproliferative and antioxidant activities | - | [60] | |
| RAW264.7, L929, and B16F10 cells | Polysaccharide (FVSP-1, FVSP-2 and FVSP-3) (Ultrasonic-assisted extraction) | Anticancer activity and antiproliferative activity | - | [61] | |
| SGC and LoVo cells | Ergosterol and 22,23-dihydroergosterol | Induced cytotoxicity | [62] | ||
| HeLa and LS174 cells | Ethanol extraction | Antioxidant activity | - | [63] | |
| HL-60, HeLa, rc ts-NRK, and FM3A cells. | Enokipodins A, B, C, and D | Anticancer activity | - | [64] | |
| L929 cells | Polysaccharides | Antioxidant activity | Reduced reactive oxygen species (ROS) production | [65] | |
| HepG2 and L02 cells | Anticancer activity | Induced ER stress via increasing intracellular Ca2+ concentrations by activating the phospholipase C–inositol-1,4,5-triphosphate (PLC-IP3) pathway | [66] | ||
| K562 cells | FVPA1 (a novel polysaccharide) | Immunomodulatory activity | Induced natural killer cell activity | [67] | |
| PC12 cells (H2O2 induced) | Flavonoids (arbutin, epicatechin, phillyrin, apigenin, kaempferol, and formononetin) | Antioxidant properties | Increased cell viability, glutathione levels, and superoxide dismutase activity | [68] | |
| Ganoderma lucidum | MDA-MB-231 and PC-3 cells | Aqueous extraction | Anticancer activity | Induced AP-1 and NF-κB expression and inhibited urokinase-type plasminogen activator (uPA) expression | [69] |
| IOSE-398, OV2008, C13*, A2780s, A2780-cp, and SKOV-3 cell line. | Anticancer activity | Induced cell cycle arrest at the G2/M phase and activated caspase-3 and p53 levels | [70] | ||
| MCF-7 cell line | ethanol extraction | Anticancer activity | Induced cell cycle arrest and apoptosis via increasing p21/Waf1 and decreasing cyclin D1 levels | [71] | |
| SW 480 cell line | polysaccharides and triterpenoid | Anticancer activity and antioxidant activity | Inhibited cell proliferation | [72] | |
| HUC-PC model | 95% ethanol extraction | Induced cell apoptosis, inhibited telomerase activity, and increased oxidative stress | [73] | ||
| 95-D, SMMC7721, KB-A-1, KB-3-1, and HeLa cells and human normal cell lines HLF and L-02 | Ganoderic acid T (GA-T), a lanostane triterpenoid | Anticancer activity | Induced cell cycle arrest at the G1 phase and increased p53 and Bax levels, while decreasing the Bcl-2/Bax ratio | [74] | |
| RAW264.7 cells | Triterpene | Anti-inflammatory activity | Decreased tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), inflammatory mediator nitric oxide (NO), and prostaglandin E2 (PGE2) levels | [75] | |
| BV-2 cells | Ganoderenic acid C, ganoderic acid C2, ganoderic acid G, ganoderenic acid B, ganoderic acid B, ganoderic acid A, ganoderic acid H, ganoderenic acid D, ganoderic acid D, ganoderic acid F, and polysaccharides | Anti-inflammatory activity | Decreased G-CSF, IL1-α, MCP-5, and MIP3α, with a higher effect on MIP3α levels, and reduced mRNA expression of CHUK, NFκB1/p150, and IKBKE (NFƙB signaling) | [76] | |
| RAW 264.7 cell line, inflamed colonic biopsy specimens of patients with Crohn’s disease and Human Peripheral Blood Mononuclear Cells | Triterpene ganoderic acid C1 (GAC1) | - | Reduced TNF-α, IFN-γ, and IL-17A production and inhibited the NF-κB signaling pathway | [77] | |
| PC12 (corticosterone-induced) and RAW264.7 cells (LPS induced) | Aromatic compounds (lucidumins A-D and lucidimine E) | Neuroprotective and anti-inflammatory activities | - | [78] | |
| RAW264.7 cells | Triterpenes (butyl lucidenateE2, butyl lucidenateD2 (GT-2), butyl lucidenate P, butyl lucidenate Q, Ganoderiol F, methyl ganodenate J, and butyl lucidenate N) | Anti-inflammatory activity | Induced HO-1 expression via the PI3K/AKT-Nrf2 pathway and decreased tumor necrosis factor-α, interleukin-6, nitric oxide synthase, and cyclooxygenase-2 expression | [79] | |
| Ergostane-type steroids, C28 steroids | Anti-inflammatory activity | Reduced nitric oxide production | [80] | ||
| HaCaT cells | Ganoderic acids | Antioxidant, antidiabetic, and anti-inflammatory activities | Decreased the response to inflammation-related cytokines at the mRNA level | [81] | |
| Ganoderma resinaceum | BV-2 cells | Lanostane triterpenoids (ganoresinoids A-D) and meroterpenoid (ganoresinoid E) | Anti-inflammatory, antioxidant, and anti-apoptosis activities | Reduced nitric oxide (NO), IL-1β, IL-6, and TNF-α levels and inhibited the TLR-4/NF-κB and MAPK signaling pathways | [82] |
| Ganoderma duripora | RAW 264.7 cells | Farnesyl phenolic compounds, ganoduriporols A and B | Anti-inflammatory activity | Inhibited tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and prostaglandin E2 (PGE2) through suppression of the COX-2, MAPK, and NF-κB signaling pathways | [83] |
| Ganoderma sinense | Ergosterols, ganocalidophins A–C (1–3) | Anti-inflammatory activity | Inhibited NO production | [84] | |
| Hericium erinaceus | Chago-K1 | Peptides | Anticancer activity | Scavenged free radicals, induced apoptosis, and increased caspase-3, -8, and -9 levels | [85] |
| FHC and HCT-116 cell lines | Polysaccharide | Anticancer activity | Inhibited the growth of colon cancer cells and induced S phase cell arrest | [86] | |
| RAW264.7 cell line | Immunomodulatory effects | Increased NO, IL-6, and TNF-α production | [87] | ||
| PC12 cell line | Antioxidant and neuroprotective effects | Reduced ROS production and increased mitochondrial membrane potentials in amyloid beta-induced neurotoxicity | [88] | ||
| GES-1 cell line | Antioxidant activity | Induced cell proliferation, inhibited cell necrosis, reduced ROS levels, regulated mitochondrial membrane potential, and maintained mitochondrial membrane permeability in H2O2-induced oxidative damage | [89] | ||
| MCF-7 cell line | Aqueous extraction | Anticancer activity | Induced cell apoptosis and G1 cell cycle arrest and changed the expression of a total of 362 transcripts | [90] | |
| GES-1 and MC cell lines | Polysaccharide (EP-1) | Anticancer activity | Induced cell apoptosis and cell cycle arrest at the G0/G1 phase and regulated Bax, Bcl-2, and caspase-3 levels | [91] | |
| SGC-7901 cell line | Polysaccharide-protein HEG-5 | Anticancer activity | Reduced Bcl2, PI3K, and AKT1 levels and increased caspase-8, caspase-3, p53, CDK4, Bax, and Bad expression | [92] | |
| EA hy926 cell line | Ethanol extraction | Anticancer activity | Inhibition of antiangiogenic and antioxidant potentials and regulated the MMP-9/NF-κB and Nrf2-antioxidant signaling pathways | [93] | |
| U937 cell line | Hot water (HWE), microwaved 50% ethanol (MWE), acidic (ACE), and alkaline (AKE) extracts | Anticancer activity | Induced the activation of mitochondria-mediated caspase-3 and caspase-9 | [94] | |
| K562, LANCAP, and HEP2 cell lines | Diterpene, 4-chloro-3,5-dimethoxybenzyl alcohol, 7α, 8β, 11-trihydroxydrimane, 3-acetyl-4-methoylbenzoic acid, 1-(5-chloro-2-hydroxyphenyl)-3-methyl-1-butanone, and 4-chloro-3, 5-dimethoxybenzoic acid | Antibacterial activity and anticancer activity | Inhibited the growth of Helicobacter pylori | [95] | |
| U87 cell line | Erinacerin O and erinacerin P, novel glioma inhibitors | Antitumor activity | Induced the apoptosis rate, reduced DNA replication, and regulated the Bax/capase-3 pathway | [96] | |
| SH-SY5Y, 1321N1, Caco-2, HCT-116, OVK18, and HeLa cells | Isoindolin-1-ones, named erinacerins (M–N) | Anticancer activity | - | [97] | |
| Inonotus obliquus | B16–F10 cell line | Water extract | Antiproliferation | G0/G1 cell cycle arrest. Reduction of cyclin E/D1 and Cdk 2/4 expression | [98] |
| 3LL cell line | Hot water extract | Anticancer activity | - | [99] | |
| HT-29 cell line | Antitumor activity | Induced apoptosis, inhibited tumor growth by regulation of Bcl-2, Bax, and caspase-3 | [100] | ||
| A549, H1264, H1299, and Calu cell lines | Chagabusone A and triterpenoids | Anticancer activity | - | [101] | |
| PC3 and MDA-MB-231 cell lines | Ethyl acetate | Antiproliferation | - | [102] | |
| RINm5F cells | Polysaccharides | Antioxidant activity | Inhibited insulin secretion, regulation of caspase-3, Bax, Bcl-2, NF-κB, and MAPKs | [103] | |
| HepG2 cells | Hypoglycemic activity | - | [104] | ||
| SMMC7721, and Jurkat cell lines | Antitumor activity | - | [105,106] | ||
| Lentinula edodes | MRC-5 and A549 cell lines | Polysaccharides: branched α-(1,4)-glucan (L10 | - | Induced THP-1 differentiation, increased superoxide and interleukin-8, RANTES production, decreased angiogenin and vascular endothelial growth factor, regulation of TLR-4/MyD88/ IKK/NFkB | [107] |
| Lignosus rhinoceros | ORL-204 cell line | High-molecular-mass fraction (HMM) | Anti-oral cancer activity | Modulated tumor necrosis factor (TNF) cell signaling leading to apoptosis, proliferation inhibition (cell cycle arrest), and immunomodulation | [108] |
| MCF-7 and A549 cell line | Antiproliferative activity | - | [109] | ||
| MCF-7 cell line | Protein fraction termed F5 | Anti-breast cancer activity | Increased extrinsic and intrinsic apoptotic pathways | [110] | |
| PC12 cell line | Peptides (Thr-Leu-Ala-Pro-Thr-Phe-Leu-Ser-Ser-Leu-Gly-Pro-Cys-Leu-Leu) (Crude protein extract) | Neuroprotective effects | Neuroprotective effects against 6-hydroxydopamine-induced toxicity. Enhanced cellular antioxidant activity and inhibited nuclear factor-kappa B (NF-κB) activation | [111] | |
| Water and ethanol extracts | Cytoprotective effects | Cytoprotective effects against hydrogen peroxide (H2O2)-induced oxidative stress. Decreased apoptosis via inhibition of caspase-3/7 activities | [112] | ||
| Hot water and ethanol extracts, and isolated polysaccharide | Neuroprotective effects | Neurite outgrowth stimulatory effect. Upregulated the MEK/ERK1/2 signaling pathway | [113] | ||
| HT22 cell line | Ethanol extract | Antioxidant activity | Protective effects against glutamate-induced toxicity Increased antioxidant-related genes, including catalase (CAT), superoxide dismutase (SOD1 and SOD2), and glutathione peroxidase (GPx) | [114] | |
| (hESC)-derived neural lineages | Methanol extract | - | Protection against dexamethasone (DEX)-induced toxicity. Inhibited apoptosis and reduction of phospho-Akt (pAkt) levels | [115] | |
| Neuro-2a and PC-12 cell line | Water extract | - | Neurite outgrowth stimulatory effect | [116,117] | |
| NHDF cell line | F5 thermoresponsive gel | Wound-healing activity | - | [118] | |
| Pleurotus citrinopileatus | Caco-2 cells | Sphingolipids | - | Colon injury suppression. Reduced LPS-induced cell death by inhibiting apoptosis-related proteins | [119] |
| HepG2 and HeLa cell lines | Methanol extract | Antitumor activity | Induced apoptosis and cell cycle arrested at the G1 and G2/M phases | [120] | |
| HepG2 cell line | Crude polysaccharide-peptides | Hepatoprotection | Reduced high-fat diet-induced hepatocyte injury by inhibiting lipid accumulation and enhancing SOD enzymatic activity and the adiponectin pathway | [121] | |
| U937 cell line | A non-lectin glycoprotein | Antitumor activity and immune modulation | Induced a shift of T helper cells toward Th 1 response by activating TNF-R, IFN-γ, and IL-2 secretion and indirect inhibition of cell growth | [122] | |
| K562, HCT116, DU-145, and PC-3 cell lines | Sesquiterpenoids | Antidiabetic and antitumor activities. | Reduced cancer cell viability and inhibited the protein tyrosine phosphatase 1B enzymatic activity | [123] | |
| Pleurotus eryngii | Ana-1 cell line | Adenosine | Immunomodulatory activity | Increased the proliferative rate of Aha-1 cells and elevated TNF-α and IL-6 levels | [124] |
| U937 cell line | Ubiquinone-9 | Antitumor activity | Induced apoptosis by inhibiting DNA topoisomerase I activity | [125] | |
| 3T3-L1 cell line | Chloroform extract | Anti-adipogenic activity | Lipid accumulation was inhibited in adipocytes by targeting PI3K/Akt/mTOR signaling, which resulted in PPAR γ and C/EBP suppression | [126] | |
| RBL-2H3 cell line | Ethanol extract | Allergic prevention and anti-inflammation activity | Decreased pro-inflammatory cytokines and histamine levels by inhibiting NFAT-, NF-кB-, and FceRI-mediated signaling | [127] | |
| PBMCs and U937 cell lines | Powder | Immunomodulatory activity | Increased activated Th2 cells, dendritic cells, and macrophages in PBMCs and regulated TNF-α and IL-10 levels | [128] | |
| RAW 264.7 cell line | Polysaccharides | Immunomodulatory activity | Produced nitric oxide, TNF-α, IL-1, and IL-6 through the p38, JNK, and MAPK signaling pathways | [129] | |
| HepG2 cell line | Antitumor activity | - | [130] | ||
| Hypoglycemic activity. Restored insulin resistance by activation of the PI3K-Akt signaling pathway | [131] | ||||
| Mouse-derived dendritic cells | Immunomodulatory activity | Immunomodulation by increasing the production of nitric oxide and TNF-α levels. Cytokines were activated through TLR2/TLR6 and dectin-1 receptors induced by β-glucan | [132] | ||
| A549, BGC-823, HepG2, HGC-27, and RAW264.7 cell lines | Protein fractions | Antitumor activity and immunostimulant activity | Induced cytotoxicity against cancer cells and stimulated lysosome activity, pinocytosis, and nitric oxide production in RAW264.7 cells with no toxicity against normal liver cells | [133] | |
| Pleurotus ostreatus | MCF-7 cell line | Silver nanoparticles (Biomass) | Antitumor activity | Inhibition of cell growth | [134] |
| MDA-MB-231, MCF-7, MCF-10A, HCT-116, HT-29, and FHC cell lines | Methanol extract | Antitumor activity | Inhibited the cell cycle at the G0/G1 phase of breast and colon cancer cells via p53-dependent as well as p53-independent pathways | [135] | |
| PC12 cell line | Polysaccharides | Neuroprotective effects | Ameliorated PC12 cells from H2O2-induced oxidative damage through the PI3K/Akt signaling pathway and inhibited apoptosis-related pathway proteins | [136] | |
| Vero, MCF-7, HepG2, CaCo-2, and HeLa cell lines | Polar extract | Antitumor activity | Induced cell cycle arrest in the sub-G1 stage and apoptosis. Expression of TNF-α was increased while IL-6 expression was decreased | [137] | |
| RAW264.7 cell line | Ultrasonication and heating | Anti-inflammation activity | Inhibited nitric oxide and TNF-α production in LPS-induced inflammation macrophages | [138] | |
| Water extract | Anti-inflammation activity | Suppressed LPS-induced secretion of tumor necrosis factor-a (TNF-a, interleukin-6 (IL-6), and IL-12p40 | [139] | ||
| Selenium-enriched polysaccharides | Immunomodulation | Induced macrophages to produce proinflammatory cytokines (NO, ROS, TNF-α, IL-1β, and IL-6) by activating the NF-κB pathway | [140] | ||
| HepG2, MCF-7, SKOV3, HeLa, and PC-3, L02, MCF-10A, and IOSE cell lines | Antitumor activity | Induced apoptosis by disrupting the Bax/Bcl-2 protein ratio and inhibiting the epithelial-to-mesenchymal transition | [141] | ||
| SW480 cell line | Water-soluble protein extract | Antitumor activity | Induced apoptosis in cells partially through ROS production, GSH depletion, and mitochondrial dysfunction | [142] | |
| Pleurotus pulmonarius | Huh7, Hep3B, and SMMC-7721 cell lines | Polysaccharide-protein complex | Antitumor activity | Inhibited cell growth, colony formation, and invasion by suppressing the PI3K-Akt signaling pathway | [143] |
| Trametes versicolor | HeLa and Jukart cell lines | PSK | Antiproliferation | G0/G1 cell cycle arrest, direct cytotoxicity. Increased lymphocyte proliferation (synergistic with IL-2) | [144] |
| AGS, A549, B16, and Ando-2 cell lines | - | Induced apoptosis by regulating caspase-3 | |||
| HeLa | Cold buffer extract | Anticancer, antiproliferation | - | [145] | |
| 4T1 cell line | Polysaccharides | Antitumor, Antimetastasis, Immunomodulation | - | [146] | |
| MOLT4 cell line | PSP | Antitumor | Inhibited cancer cell growth, S phase arrest, and induced apoptosis | [147] | |
| PBMC cell line | - | Increased proliferation, IL-1β, TNF-α, and IFN-γ | |||
| - | Increased monocyte numbers by possible regulation of TLR2/6/4 and Dectin-1 | [148] | |||
| LN-CaP cell line | Ethanol extract | Antiproliferation | Reduced secretion of prostate-specific antigen (PSA) in an androgen receptor-independent manner | [149] | |
| PC-3 and DU-145 cell lines | Modest antiproliferation |
| Mammalian Model | Mushroom Name | Extract/ Compound | Protective Effects | Mechanism | References |
|---|---|---|---|---|---|
| Mice | Agaricus blazei Murill | 1,6-beta-glucan | Antitumor activity in sarcoma 180-bearing ICR mice | - | [151] |
| Agaricus brasiliensis | Sodium pyroglutamate | Anti-angiogenic and antimetastatic activity | Inhibited tumor growth. Increased CD8+ T and natural killer cell levels and von Willebrand factor expression | [152] | |
| β-glucan | Antitumor activity | Inhibited tumor growth, reduced IL-10 levels, and induced IFN-gamma production | [153] | ||
| Hot water and cold water extracts | Antitumor activity | Antitumor effect, and increases in CD4+ T and natural killer cells were observed. Reductions in cholesterol levels and blood glucose levels were also observed in sarcoma 180-bearing ICR mice and LPS and Concanavalin A-induced inflammation and hepatic injury, respectively, in Balb/c mice | [154] | ||
| Aqueous extraction | - | Prevention of allergy via reducing cytokine levels. Reduction of anti-OVA Ig-E levels and Th2 relative to Th1 cytokine levels in ovalbumin-induced allergy in NIH/OlaHsd, C57Bl/6, and BALB/c mice | [155] | ||
| Fucogalactan | - | Antinociceptive activity in acetic acid-induced abdominal contraction in male Swiss mice | [156] | ||
| Agarol (an ergosterol derivative | Anticancer activity | Induced cancer cell apoptosis and anti-tumor activity. Increased ROS production, AIF levels, and Bax levels, and decreased Bcl-2 levels in SCID mice | [157] | ||
| Agaricus bisporus | Aqueous extraction | Antitumor activity | Inhibited tumor growth and increased tumor cell apoptosis in BALB/c Nu-Nu athymic mice | [23] | |
| Antitumor activity | Inhibited tumor growth and induction of nuclear factor-κB with the production p50/105 heterodimers in male C57BL/6 murine sarcoma model (injected 180 cells) | [24] | |||
| Anticancer activity | Inhibition of cancer cell proliferation via suppression of androgen receptor and cell cycle progression in male intact C57BL mice and tumor-derived xenograft/PDX tumor-implanted male NSG intact mice. | [158] | |||
| Auricularia auricula-judae | Auricularia auricula judae polysaccharide-cisplatin complex (AAP-CDDP) | Anticancer activity | Upregulation of Bax, cytochrome-c, and caspase-3, downregulated Bcl-2 expression, and induced superoxide dismutase, catalase, and glutathione peroxidase activities. A decrease in mitochondria potential was also observed. (HeLa (cervical cancer) cell line-induced cancer in female BALB/c mice). | [159] | |
| β-D-glucan | Anticancer activity | Induction of apoptosis through upregulated Bax and downregulated Bcl-2 expression. (Sarcoma 180 (S-180) tumor cell-induced cancer in male BALB/c mice). | [160] | ||
| Aqueous extraction | - | Enriched the arginine biosynthesis pathway. Changed the gut microbiota composition. (C57BL/6J male mice) | [161] | ||
| Melanin | - | Inhibition of CYP2E1 and activation of Nrf 2 along with its downstream antioxidase. Reduction of ALT, AST, TG, and MDA levels and increased antioxidant enzyme levels, such as ADH, SOD, and CAT. (50% ethanol-induced acute alcoholic liver in male C57BL/6 mice) | [162] | ||
| Polysaccharide (hot water and ultrasonic-assisted extraction) | Anticholestrimic effect | Reduction of serum total cholesterol and low-density lipoprotein cholesterol levels. Increased total antioxidant capacity and lipoprotein lipase activity. (Cholesterol-enriched diet-fed male ICR mice) | [163] | ||
| Polysaccharide (methanol extraction) | - | Significantly accelerated wound closure through fibroblast and keratinocyte proliferation, migration, and invasion. Promotion of collagen synthesis and reduction of E-cadherin expression | [164] | ||
| Auricularia cornea var. Li. (an evolutionary varieties of A. auricula-judae) | Polysaccharide | - | Inhibition of aldose reductase and CYP2E1 as well as protein expression of iNOS and COX-2 in ADL mice model. Furthermore, expression of pro-inflammatory players, such as IL-1β, TNF-α, and IL-6, was found to be decreased, and SOD, GSH-Px, and CAT were found to be modulated. | [165] | |
| Auricularia polytricha | Polysaccharides (APPIIA) | Antitumor activity | Antitumor effect and induced macrophage activation. (180 cells injected in male BALB/c albino mice) | [166] | |
| Polysaccharides | - | Decreased Bax and caspase-3 expression, increased Bcl-2 expression, and anti-fibrosis effect. Protection against chronic kidney diseases. | [167] | ||
| 95% ethanol extraction | Hepatoprotective activity | Modulation of ALT and AST activities, impeded the TLR4/NF-κB and caspase signaling pathways, and induced the Keap1/Nrf2 signaling pathway to promote a hepatoprotective effect in dextran sulfate sodium-induced ulcerative colitis in specific pathogen-free ICR male mice. | [168] | ||
| Antrodia camphorate | Fermented culture broth | - | Decreased tumor size. Reduction of cell proliferation markers such as cyclin D1 and PCNA. Reduction of Bcl-2, which resulted in the promotion of apoptosis. | [169] | |
| Phosphate-buffered saline (PBS) at the ratio of 1:25 (w/v) | Hepatoprotective activity | Reduction of plasma aspartate aminotransferase (GOT) and alanine aminotransferase (GPT) levels and induced the activities of SOD, glutathione, and catalase in CCl4-induced liver toxicity in male ICR mice. | [170] | ||
| 4,7-Dimethoxy-5-methyl-1,3-benzodioxole | Anticancer activity | Induced p53-mediated p27/Kip1 protein and reduced cyclin D1, D3, and A levels in COLO-205 human colon cancer cell-injected BALB/c nu/nu mouse. | [171] | ||
| Ergostatrien-7,9(11),22-trien-3β-ol | - | Reduction of p65NF-κB and caspase-3 expression, induced PI3K/Akt, as well as inhibited GSK-3 levels in transient focal cerebral ischemia-induced male ICR mice | [172] | ||
| Boletus edulis | Polysaccharide | Antitumor activity | Real (BUN and creatin) and liver (AST and ALT) damage parameters were found to be significantly reduced in treated mice. Acted as a mitogen in tumor-bearing mice. Induction of antitumor activity through cytotoxic lymphocytes (NK and CTL cells). Increased secretion of IL-2 and TNF-α. | [173] | |
| Anti-asthmatic | Restoration of lung pathology, reduced IL-4 and IFN-γ levels, and increased CD4+CD25+FOXP3+ Treg cells in ovalbumin-induced asthma in female BALB/c mice. | [174] | |||
| Isolated protein (BEAP) | Antitumor activity | Significantly decreased PARP and caspases-3, -8, and -9. Increased the Bax/Bcl-2 ratio, implying a tumor reduction through induction of apoptosis in A549 cell-injected female BALB/c nude mice. | [175] | ||
| Fungal nitrite reductase | - | Inhibited nitrite-induced toxicity by reducing nitrite in blood, hence increasing the lifespan of sodium nitrite toxicity-induced male Kunming mice | [176] | ||
| A water-soluble polysaccharide (BEBP); BEBP-1, BEBP-2, and BEBP-3 | - | Increased SOD and decreased MDA levels in the serum of Kunming mice with D-galactose-induced oxidation. | [177] | ||
| Flammulina velutipes | Starch-free β-type glycosidic polysaccharide | Gut protection | Reduction of morphological and physiological changes in the colon. Reduction of pro-inflammatory cytokines such as TNF-α, IL-6, MCP-1, and MIP-1α and increased the relative expression of tight junction proteins such as claudin-1, occludin, and zonula occludens. Dramatic change in gut microbiota was also observed in dextran sulfate sodium (DSS)-induced colitis in C57BL/6J male mice. | [178] | |
| Polysaccharide/polysaccharides consisting of glucose linked with β-glycosidic bonds. | Gut protection | Induced production of short-chain fatty acids. Improved gut microbiota through immunomodulation of expression of TNF-α, IF-γ, IL-6, and IL-8. | [179,180] | ||
| Sulfated polysaccharides (SFPS) | Anti-aging | Decreased levels of ALT, AST, and ALP (liver toxicity index) as well as CRE, BUN, and UA (kidney toxicity index). Improved lipid metabolism and resisted aging as well as organ damage induced by d-galactose in male Kunming strain mice. | [181] | ||
| Polysaccharides | Neuroprotective activity | Reduction of IL-1β, TNF-α, IL-6, and IL-10 levels and decreased escape latency and total swimming distance in scopolamine-induced learning and memory impairment in male mice (C57BL/6). | [180] | ||
| Gut protection | Protective effect by activation of the Akt/GSK3β/Nrf-2/HO-1 signaling pathway and modulation of gut microbiota against Pb-induced toxicity in SPF-grade Kunming male mice. | [182] | |||
| Antidiabetic activity | Modulation of the PI3K/Akt signaling pathway to reduce blood glucose and insulin levels and regulate dyslipidemia. | [183] | |||
| Hepatoprotective activity | Hepatoprotection through the reduction of AST, ALT, triglyceride (TG), total cholesterol (TC), and total bile acid (TBA) contents, change in liver histopathology, and decrease in IL-6, IL-1β, and TNF-α levels in carbon tetrachloride-induced hepatic oxidative injury in male C57BL/6 mice. | [184] | |||
| Immunomodulatory protein (FVE) | Antitumor activity | Increased survival and inhibition of tumor size and angiogenesis through regulation of INF-γ in BNL hepatoma cell-injected female BALB/c mice. Increased MHC class I and II and costimulatory CD80 molecules on peripheral blood mononuclear cells. | [185] | ||
| Ganoderma applanatum | Terpenes | Hepatoprotective activity | Reduced Cu/Zn-SOD, CAT, GPx, and GST activities, decreased IL-1β and COX-2, and inhibited NF-κB translocation in male Kunming strain mice with benzo(α)pyren-induced oxidative stress and inflammation, hence providing anti-inflammatory activity, antioxidant activity, and hepatoprotective effects. | [186] | |
| Ganoderma cochlear | (±)-Dispirocochlearoids A–C (1–3), meroterpenoids with a 6/6/5/6/6/6 ring system | Anti-inflammatory activity | Inhibition of neutrophil and macrophage infiltration, decreased protein concentrations in bronchoalveolar lavage fluid, inhibition of COX-2 in lung tissue, and suppression of PEG2 and proinflammatory cytokines in LPS-induced acute lung injury mice. | [187] | |
| Ganoderma tsugae | - | Inhibition of allergic airway by reducing leukocyte influx, eotaxin levels, histamine levels, and PGE2. No significant reductions in NO and proinflammatory cytokines (IL-1β and IL-6) were observed in ovalbumin (OVA)-induced allergic asthma in female BALB/c mice. | [188] | ||
| Ganoderma lucidum | 95% ethanol extraction | - | Reduction of E-cadherin, mTOR, eIF4G, and p70S6K and activation of extracellular regulated kinase (ERK1/2) levels in SUM-149 cell-injected female severe combined immunodeficient (SCID) mice. | [189] | |
| Aqueous extraction | Antitumor activity | Decreased tumor growth and volume. Regulation of NAG-1. Relative expression of P16 and RB1 (retinoblastoma gene) was found to be significantly increased. Protein mRNA expression of FOXO3a was found to be increased, also the mRNA level of P21 was found to be increased. WEE1and E2F1 mRNA expression was found to be significantly reduced as well as reductions in cyclin D1 and B, PCNA, and Ki67. Dose-dependent reductions in anti-apoptotic gene expression, such as Bcl-2, NF-κβ, and c-FOS, were also observed in HCT116-injected male BALB/C nude mice. | [190] | ||
| Ganodermanontriol, a lanostanoid triterpene | Antitumor activity | Tumor volume and weight were found to be reduced and immunohistochemistry revealed a significant reduction in cyclin D1 in HT-29 colon cancer cell-injected male nude immunodeficient mice (nu/nu). | [191] | ||
| Triterpene acids (lucidenic acids and ganoderic acids) and Sterols (fungisterol, 5,6-dihydroergosterol, ergosterol, ergosterolperoxide, 9(11)-dehydroergosterol peroxide, and demethylincisterolA3) | Anti-inflammatory and antitumor activity | Anti-inflammatory and antitumor-promoting effects in TPA-induced ear-edema inflammation in specific pathogen-free female ICR mice and 7,12-dimethylbenzene[a]anthracene (DMBA) and TPA-induced two-stage mouse skin carcinogenesis in SENCAR mice. | [192] | ||
| Beta 1,3/1,6 glucan | - | Significant production of IgA in serum | [193] | ||
| Ethanol extract | Anti-inflammatory | Reduction of malondialdehyde levels. Inhibition of acute and chronic inflammation induced by carrageenan and formalin, respectively, in Swiss albino mice | [194] | ||
| Ganoderic acid A and ergosterol | Neuroprotective activity | Promoted motor performance and protected against loss of dopaminergic neuronal cells in MPTP-treated mice (Parkinson’s model). Protective effect through the MPK/mTOR/ULK1 and PINK1/Parkin pathways. | [195] | ||
| Hericium erinaceus | Aqueous extraction | - | The extracts were found to be more active and less toxic compared to 5-FU against HT-29, NCI-87, and Huh-7 xenografts and also significantly delayed tumor doubling time. It showed similar effects in a dose-dependent manner against HepG2 xenograft in SCID mice. Reduction of tumor growth, increased NO, elevated phagocytic activity, and angiogenesis was also significantly inhibited. | [196] | |
| Antitumor activity | Reduction of tumors. Increased NK cell activity and elevated phagocytic activity of macrophages. Inhibition of angiogenesis by inhibiting VEGF, COX-1, 5-LOX, PGE2, and LTB4 in CT-26 colon cancer cell-transplanted in BALB/c mice. | [197] | |||
| Hot water and microwaved 50% ethanol extracts | Antitumor activity | Histopathological evidence revealed reductions in tumor nodules and metastasis in lungs. Inhibition of metastasis by decreasing matrix metalloproteinases MMP-2, MMP-9, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) phosphorylation in CT-26 colon cancer cells transplanted in pathogen-free female BALB/c mice. | [197] | ||
| Polysaccharides | - | Ameliorated diarrhea, rectal bleeding, reduction in weight, and colitis in 2% DSS (w/v)-induced colitis in male C57BL/6 mice. Suppressed MDA and myeloperoxidase activities, improved T-SOD levels, and IL-β, TNF-α, and IL-6 activities were greatly reduced through the attenuation of NF-κB, AKT, and MAPK phosphorylation. iNOS and COX-2 were found to be decreased in a dose-dependent manner. | [198] | ||
| Erinacine A | Antiproliferative effect | Antiproliferative effect observed in DLD-1 cell-injected BALB/c-nu mice could be attributed to the cell cycle G1 arrest induced by the compound in DLD 1 cells (in vitro). ROS, JNK, 1/2MAPK, and mTOR pathways were also found to be involved. | [199] | ||
| Nine selenium polysaccharide derivatives, sHEP1-sHEP9 | Immunomodulatory activity | Induced DC maturation and increased MHC-II and CD86, phosphorylation of ERK, p38, and JNK, and the nuclear translocation of transcription factors p-c-Jun, p-CREB, and c-Fos in LPS-induced ICR mouse. | [200] | ||
| 70% ethanol extract | Neuroprotective activity | Significant hippocampal neuroprotection post acute seizures in pilocarpine-induced status epilepticus in male C57BL/6 mice. Increased NeuN-expressing cells. Reduced COX-2-expressing cells. | [201] | ||
| Inonotus obliquus | Polysaccharides | Antitumor activity | Tumor growth was found to be reduced 3-fold in treated (ip post-administration) in BALB/c mice. The effect could be attributed to G0/G1 phase cell arrest and activation of caspase-3-mediated apoptosis as observed in in vitro analysis. Proteins p53, pRb, and p27 were found to be decreased in a dose-dependent manner. | [98] | |
| Anti-diabetic and Nephroprotective activity | Significantly reduced fasting blood glucose levels, insulin tolerance, triglyceride levels, and elevated HDL/LDL ratio. Urine albumin-creatin levels were found to be decreased. NF-κB and TGF-β levels were found to be decreased in a dose-dependent manner in HFD/STZ-induced nephropathy in C57BL/6 mice | [202] | |||
| Anti-diabetic activity | IgA, TNF-α, and IL-6 were found to be significantly reduced in the intestine region. H&E staining showed improvement in histological structures of intestine tissue in extract-treated diabetic mice. The mRNA expression of Ki-67, ZO-1, and MUC2 genes was found to be significantly upregulated in a dose-dependent manner. | [203] | |||
| Hepatoprotective activity | ALT and AST were found to be decreased, serum SOD and liver GHS levels were found to be increased, and NO levels were found to be decreased. Serum levels of TNF-α, IFN-γ, IL-4, IL-6, and IL-1β were found to be decreased. Alleviated hepatocyte enlargement, cytoplasmic vacuolation, cellular infiltration, and necrosis. TLR-2, TLR-4, and pNF-κBp65 were found to be suppressed and p-IκBα was significantly downregulated. Nrf2 and HO-1 expression was found to be increased. | [204] | |||
| Antitumor and anticancer activity | Tumor growth regression. Enhancement of T and B cells. Anti-cancer mechanism by promoting cytokines IL-2, IL-6, IL-12, and TNF-α. Stimulation of macrophage function. Promoted apoptosis via promoting Bax-2 and inhibiting Bcl-2 expression in a concentration-dependent manner in Jurkat tumor-bearing Kunming mice. | [106] | |||
| - | Metabolic regulation, reduced blood glucose, and improved lipid metabolism. Improved serum profiling and reversed metabolites leucine and proline. | [205] | |||
| Antidiabetic activity | Effectively prevented loss of body weight, controlled blood glucose, and increased insulin sensitivity. Alleviated oxidative stress induced by hyperglycemia. The PI3K/Akt pathway plays a role in modulating metabolism in high-fat diet- and STZ-induced T2DM mice. | [206] | |||
| Immunomodulatory activity | Restoration of damaged colon by alleviating colon tissue injury and tight junction protein deficiency. Possible modulation of Th17-, Th2-, Th1-, Treg-related cytokines through the JAK-STAT (p-STAT1, p-STAT6, p-STAT3) signaling pathway in colitis-induced mice. | [207] | |||
| Hot water extract | Antitumor activity | Decreased tumor progression, vascularization, and metastasis. Maintenance of optimum body temperature. | [99] | ||
| Trp-Gly-Cys tripeptide | - | Platelet aggregation inhibitor | [208] | ||
| Ethanol extract | Anticancer activity | Increased expression of p53, p21, Bax, and caspase-9 was observed. EBV viral proteins BZLF-1 (key factor for EVB lysis reactivation) and LMP-2 (essential for EVB latency) were found to be moderately repressed in NOD/SCID mice implanted with EBV+ human gastric carcinoma (SNU719). | [209] | ||
| Lentinula edodes | Mycelia extract (hot water) | - | Lowered the increased TGF-β and IL-6 plasma levels in C57BL⁄6 (H-2b) BALB⁄c Nu⁄Nu (H-2 d) mice inoculated with C26. Inhibition of Th17 cells and myeloid-derived suppressor cells. | [210] | |
| Mycelia extract | Antitumor activity | T cell-dependent antitumor activity. Decreased Tregs and TGF-β | [211] | ||
| Lignosus rhinocerus | Hot water extract | Antiasthmatic activity | Inhibition of airway hyperresponsiveness (AHR) in asthma model in house dust mite (HDM)-induced asthma in BALB/c mice. | [212] | |
| Antiasthmatic activity | Inhibition of airway inflammation in asthma model. Attenuated IgE, Th2 cytokines, CD4+ T cell population, leukocyte infiltration, and mucus-producing goblet cells in the lung epithelium | [213] | |||
| Rhinoprolycan fraction | Antitumor activity | Airway relaxation effects. Antitumor activity in MCF7-xenograft NCr nude mice. | [214] | ||
| Polysaccharides | Immunomodulatory activity | Inhibition of immunosuppressive activity. Improved immune organs and stimulated the release of cytokines TNF-α and INF-γ in cyclophosphamide (Cy)-induced SPF Kunming mice. | [215] | ||
| Pleurotus citrinopileatus | Polysaccharides | Antitumor activity | Decreased tumor size and lowered the mortality ratio in ICR mice with Sarcoma 180 tumor-bearing. | [216] | |
| Immunomodulatory activity | Increased Nrf2, Keap1, p62, HO-1, and NQO1 expression in immunocompromised mice via the p62/Keap1/Nrf2 signaling pathway. The immune activity was activated by phagocytic activity, Th1/Th2 balance, and cytokine production in male SPF-grade Kunming mice. | [217] | |||
| Antitumor activity | Tumor weights were reduced and apoptosis increased. Cell cycle arrest at the S phase. The immunity of tumor-bearing mice was improved in the spleen index with polysaccharide treatment in male mice bearing H22 hepatoma tumors. | [218] | |||
| Anti-obesity activity | Improved body weight, lipid accumulation, and serum biochemistry parameters in high-fat diet-induced obese (DIO) C57BL/6J mice. | [219] | |||
| Lectin | Antitumor activity | The tumor size was reduced and anti-HIV-1 reverse transcriptase was inhibited in mice bearing Sarcoma 180 tumors. | [220] | ||
| Lipid fraction | - | Reduced morphological changes in the colon and reduced inflammation stress in DSS-induced chorionic crypt injury in male BALB/c mice. | [119] | ||
| Water extract | Anti-inflammatory activity | Alcoholic steatohepatitis prevention. Decreased serum lipid profiles, cellular lipid accumulation, and inflammation by activating the SIRT1–AMPK and P2X7R–NLRP3 inflammasome in ethanol-induced male C57BL/6 mice. | [221] | ||
| Pleurotus eryngii | Ethanol extract | - | Reduced lipid absorption and carbohydrate-degrading enzyme activity (α-amylase) in male C57BL/JJmsSlc mice. | [222] | |
| Polypeptides | Immunomodulatory activity | Restored the complication from cyclophosphamide-induced immunosuppression by shifting the thymus/spleen index, lymphocyte count, and gut microbiota abundances in male Kunming strain mice. | [223] | ||
| Antitumor activity | Inhibited the tumor volume in mice and enhanced NK cell and spleen activities with higher TNF-α and IL-2 serum levels in female BALB/c mice with renal-bearing cancer. | [224] | |||
| β-type glycosidic polysaccharides | Anticolitis activity and probiotic enhancement | Inhibited proinflammatory cytokines (TNF-α, INF-γ, and IL-10) via NF-κB and improved gut microbiota in dextran sodium sulfate (DSS)-induced colitis in male CD-1 (ICR) mice. | [225] | ||
| Water extract | Immunomodulatory activity | Gut and liver immunity were improved by regulating NrF2, Nfkb, DNMT1, and IL-22 genes in CD1 mice and whole peripheral blood and fecal samples collected from healthy donors. | [226] | ||
| Anti-obesity activity | Lipid absorption was inhibited by reducing pancreatic lipase enzymatic activity in high-fat diet-fed C57BL/6 male mice. | [227] | |||
| Heterogalactan | Immunomodulatory activity | Macrophages were activated by p38, JNK, and NF-κB via TLR2 and splenocyte activation was induced by the TLR4-PKC axis in cyclophosphamide (CTX)-immunocompromised BALB-C mice. | [228] | ||
| Pleurotus ostreatus | Glucans | Antitumor activity | Activated nitric oxide production in macrophages and inhibited tumor growth in Swiss albino mice with Sarcoma 180 (S-180) tumors. | [229] | |
| Polypeptides | Immunomodulatory activity | Immune cell populations (T cells, NK cells, macrophages) were increased, activating the gut microbiota to balance system immunity in female C57BL/6 mice. | [140] | ||
| Dried powder | Anticancer activity | Abolished the effect of BBN in mice by stimulating NK cell and lymphocyte activities in female ICR mice with BBN-induced carcinogenesis. | [230] | ||
| Anti-obesity activity | Improved body weight, serum lipids, blood sugar, and liver and kidney functions in high-fat diet-fed C57BL/6J male mice | [231] | |||
| Ethanol extract | Antidiabetic activity | Reduced body weight and serum lipid profiles and increased HDL cholesterol levels and antidiabetic activity in alloxan-induced diabetic BALB/C mice. | [232] | ||
| Anticancer activity | Prevented colon injury and carcinogenesis via suppression of COX-2, F4/80, Ki-67, and cyclin D1 in PhIP-induced male ICR mice. | [233] | |||
| Proteoglycans | Anticancer activity | Inhibited cell growth and arrest at the G0/G1 phase. Stimulated NK cells and macrophage functions in Swiss albino mice with Sarcoma 180 (S-180) tumors. | [234] | ||
| Water extract | Immunomodulatory activity | Immune regulation and malnutrition relief through increased total liver proteins and DNA and protein contents in gut mucosa and stimulated humoral immunity Balb/C mice. | [235] | ||
| Immunomodulatory activity | Suppressed secretion of TNF-α and IL-6 in LPS-induced inflammation and inhibited interferon-g (IFN-g), IL-2, and IL-6 in concanavalin A (ConA)-stimulated mouse splenocytes in BALB/c mice. | [139] | |||
| Pleurotus pulmonarius | Dried powder | Allergic relief | Showed no effect on antigen-induced nasal rubbing and sneezing and inhibited histamine release from rat mast cells in female BALB/c mice. | [236] | |
| b-glucan-rich fraction | Memory improvement | Suppressed histological changes and neuronal loss and reduced neuroinflammation by reducing Iba-1-positive microglial cells in high-fat diet-fed female ICR mice. | [237] | ||
| polysaccharide-protein complex | Antitumor activity | Suppressed the VEGF-induced PI3K/AKT signaling pathway in liver cancer cells in Huh7 tumor-bearing male BALB/c mice. | [143] | ||
| Glucan | Colitis prevention | Improved colon damage, decreased MPO activity levels, and decreased proinflammatory cytokines (IL-1b and TNF-α) in DSS-induced colitis female BALB/c mice. | [238] | ||
| Colitis prevention | Inhibited cell proliferation, induced apoptosis, and inhibited inflammation in DSS-induced colitis FVB/N mice. | [239] | |||
| Trametes versicolor | Polysaccharopeptide (PSP in combination with IL-2 | Antitumor activity | Decreased ROS production. Both early and late treatment induced IL-2 expression. An increase in TNF-α and reduction in TGF-β was observed in tumor cells in BALB/cByJ mice with H238. | [240] | |
| Polysaccharides | Antitumor activity | Significant decrease in tumor weight. Preservation of bone integrity (CT images). Increases in IL-2, -6, and -12 but no change in IL-10. Increases in IFN-γ and TNF-α in BALB/c mice bearing 4T1 tumors. | [146] | ||
| Immunomodulatory activity | Activation of splenocytes and selective binding to CD19+ cells and CD14+ cells. Ig class switching and IL-2 production were observed. TLR4-mediated B cell activation, p38 MAPK pathway activation, and cytosolic translocation of NF-κB p65 were observed in female BALB/c, C3H/HeJ, and C3H/HeN mice. | [241] | |||
| Aqueous extract in combination with metronomic zoledronic acid (mZOL) | Antitumor activity | Diminished tumor growth, protected bones, and inhibited metastasis in the liver and lungs in female BALB/c Nu/Nu nude mice inoculated with MDA-MB-231-TXSA. | [242] | ||
| PSP | - | Slightly increased glutathione S-transferase (GST) activity and increased blood GPX activity. Possibly P450-mediated metabolism in male C57 mice. | [243] | ||
| Rat | Agaricus blazei Murill, Agaricus brasiliensis | Aqueous, acid, and alkaline extraction | Antitumor activity | Inhibited tumor growth and increased body weight. Increased liver catalase and superoxide dismutase activities in Walker-256 tumor-bearing rats. | [244] |
| Agaricus bisporus | Powdered mushroom | Antidiabetic activity | Reduction of plasma glucose and cholesterol (LDL). No change in triglyceride levels and protected against hepatic toxicity in streptozotocin-induced type 2 diabetes in male Sprague–Dawley rats. | [24] | |
| Auricularia auricula-judae | Aqueous extract | Antidiabetic activity | Reduced plasma glucose, total cholesterol, triglyceride, GOT, and GPT levels in streptozotocin-induced diabetic male Sprague–Dawley (SD) rats. | [245] | |
| Immunomodulatory activity | Promoted immune modulatory effect by inducing total and differential WBCs in cyclophosphamide-induced immunodeficiency in Wistar rats. | [246] | |||
| Hot water and ultrasonic-assisted extraction | - | Reduction of bronchoalveolar lavage fluid and lung edema, significantly inhibited myeloperoxidase (MPO) activity and malondialdehyde (MDA) levels, decreased TNF-α and IL-6 in the blood, and alleviation of LPS-induced pathological changes in the lungs of LPS-induced inflammation in adult Sprague–Dawley rats. | [247] | ||
| Auricularia polytricha | Aqueous extract | Hepatoprotective activity | Reduced AST, ALT, ALP, LDH, TB, TG, and cholesterol levels and increased total protein levels. Hepatoprotective activity in paracetamol-induced liver toxicity in Sprague–Dawley rats. | [248] | |
| Anticholestremic activity | Decreased total cholesterol and LDL and increased HDL levels in reused cooking oil-induced hyperlipidemia in Wistar rats | [249] | |||
| Aqueous extract- soluble polysaccharide | Antihyperlipidemic effect | Decreased total cholesterol and LDL and increased HDL levels in high-fat diet-induced hypercholesterolemia in male Sprague–Dawley (SD) rats | [250] | ||
| Antrodia camphorate | Oral treatment | - | Suppression of iNOS and HO-1 expression and reduction of Bax and caspase-3 in thromboembolic cerebral tissue. Inhibition of OH• signals was also observed. | [251] | |
| Water extract and ethanol extract | Memory improvement | Enhanced long-term and short-term memory and learning ability. Significantly reduced ROS levels in hippocampus as well as p-tau and Aβ40 in Aβ-infused male Wistar rats | [252] | ||
| Flammulina velutipes | Aqueous extraction | Neuroprotective activity | Induction of cell proliferation and elongation, stimulated nerve functional recovery and axonal outgrowth, and increased growth-associated protein 43 (GAP-43) and the JAK2/STAT3 pathway in female Sprague–Dawley rats | [253] | |
| Enokitake (Flammulina velutipes) fiber | - | Reduced LDL (VLDL), intermediate-density lipoprotein (IDL), and LDL-cholesterol concentrations in cholesterol-free diet with cellulose powder-fed male F344/DuCrj rats. | [254] | ||
| Ganoderma atrum | polysaccharide | Anti-inflammatory and antioxidant activity | Reduction of 8-OHdG levels, increased superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activities and IL-10 levels, and prevented the overproduction of malondialdehyde (MDA), IL-1β, IL-6, and TNF-α in male SD rats with acrylamide-induced inflammation and oxidative damage. | [255] | |
| Ganoderma microsporum | immunomodulatory protein | Antioxidant activity | Reduction of oxidative damage and cognitive impairment. Increased superoxide dismutase 1 (SOD-1) and lowered astroglia proliferation in traumatic brain injury-induced female and male Sprague–Dawley rats | [256] | |
| Ganoderma lucidum | Neuroprotective activity | Neuroprotective effect, reduction of the cerebellar infarct area, neurological functional deficits, neuronal apoptosis, and decreased active caspase-3, -8, and -9 and Bax levels in middle cerebral artery occlusion (MCAO) in Sprague–Dawley (SD) rats and oxygen and glucose deprivation (OGD) in primary cultured rat cortical neurons | [257] | ||
| Lignosus rhinocerus | Hot water extract | Anti-asthmatic activity | Inhibition of airway inflammation in asthma model. Attenuated IgE, Th2 cytokines, leukocyte infiltration, and mucus-producing goblet cells in the lung epithelium of Sprague–Dawley rats with ovalbumin (OVA)-induced asthma. | [212] | |
| High-molecular-mass fraction (HMM) | - | Airway relaxation effects. Suppressed carbachol-, 5-hydroxytrptamine-, and calcium-induced airway contractions in Sprague–Dawley rats. | [258] | ||
| Cold water extract | - | Bronchodilator effect mediated by the calcium signaling pathway downstream of Gαq-coupled protein receptors in Sprague– Dawley rats. | [259] | ||
| Freeze-dried mushroom powder | Antioxidant activity | Antioxidant activity and amelioration of diabetic complications in streptozotocin-induced diabetic rats. | [260] | ||
| High-molecular-mass fraction (HMM) | Anti-inflammatory activity | Anti-acute inflammatory activity in carrageenan-induced paw edema in Sprague–Dawley rats. | [261] | ||
| Pleurotus citrinopileatus | Ethyl acetate and methanol extract | - | Reduced serum cholesterol and triglycerides in high-fat diet-fed mice and increased glutathione peroxidase and superoxide dismutase activities in the blood of female hamster rats. | [262] | |
| Pleurotus eryngii | Methanol extract | - | Estrogen-like activity and bone loss prevention. Trabecular bone density was increased in the extract treatment group in eleven-week-old female Sprague–Dawley rats (240–260 g) with removed ovaries. | [263] | |
| Cellulose | Hepatoprotective activity | Fatty liver prevention. Reduced ALT, AST, TC, and TG levels in the serum of high-fat diet-fed rats and decreased fatty accumulation in the liver in male SD rats | [264] | ||
| Chitin | Cardioprotective activity | Serum lipid levels decreased and ALT, AST, and SOD enzymatic activities improved. Prevented liver steatosis and aortic atherosclerosis in male Sprague–Dawley (SD) rats. | [265] | ||
| Pleurotus ostreatus | Water-soluble polysaccharide | Gastroprotective effect | Inhibited oxidative stress from acetic acid-induced gastric lesions and increased mucus synthesis. | [266] | |
| Pleurotus pulmonarius | Metabolites | Antitumor and immunomodulatory effects | Suppressed leukemia induction and elevated the phagocytic index of macrophages in leukemia-induced Wister rats. | [267] | |
| Mycelial hot water extract and (HWE) and acetone extracts (AE) | Antidiabetic activity | Reduced serum lipid profiles and elevated high-density lipoprotein cholesterol in diabetes-induced Wistar albino rats. | [268] |
| Model | Mushroom Name | Extract/ Compound | Protective Effects/ Mechanism | References |
|---|---|---|---|---|
| Danio rerio | Agaricus bisporus | Powdered mushroom + Lactobacillus casei | Upregulated the expression of growth-related (gh and igf1), mucosal immune-related (tnf-α, lyz, and il1b), and antioxidant-related (sod and cat) genes. | [270] |
| β-glucan | Reduced lipid accumulation and triglyceride levels. Lowered C/EBP α, c SREBP1, LXR α, PPAR γ, and increased LC3 II/LC3 I. Induction of autophagy. | [271] | ||
| Aqueous extract + Lactobacillus acidophilus and Lactobacillus delbrueckii subsp. Bulgaricus | Acted as a prebiotic to Lactobacillus acidophilus (La) and Lactobacillus delbrueckii subsp. Bulgaricus (Lb). Feeding La and Lb improved growth and reproduction, as well as increased cyp19a gene expression. | [272] | ||
| Glucosamine hydrochloride | Promoted larval skeletal development and caudal fin regeneration and increased the expression of bone specific markers such as col1a2, col10a1a, and col2a1a. Promoted skeletal injury repair in osteoporosis model of zebrafish larvae and adults. Activation of Bmp signaling. | [273] | ||
| Ethanol extract | Inhibited melanogenesis | [274] | ||
| Antrodia cinnamomea | Ethanol extracts | Reduced the melanin content and inhibited tyrosinase activity. | [253] | |
| Ganoderma lucidum | Deacetyl ganoderic acid F | Attenuated the increase in nitric oxide, which was induced by LPS. | [275] | |
| Triterpenoids | Decreased LPS-induced intracellular ROS | [276] | ||
| Ganoderma applanatum | Exopolysaccharides and endopolysaccharides | Non-toxic to embryos. Did not delay or alter hatching, development, and heart rate. | [277] | |
| Hericium erinaceus | Ethanol extracts | Improved locomotion pattern, reduced anxiety, and improved memory by exhibiting anti-acetylcholine esterase activity and antioxidant potential. | [278] | |
| Inonotus obliquus | Polysaccharides | Alleviated oxidative stress, reduced ROS, and reduced apoptosis in developing embryos. | [279] | |
| Ameliorated the genotoxic effects in UVB-exposed zebrafish by enhancing the expression of DNA repair genes, aiding in normal development | [280] | |||
| 2α-hydroxy-inotodiol | Alleviated H2O2-induced apoptosis in zebrafish head region. | [82] | ||
| Lentinula edodes | Ethyl acetate fraction | Reduced prednisolone-induced osteoporosis | [281] | |
| Wild type and mutant mushroom | Reduced pigmentation in embryos | [282] | ||
| UV-B exposure | Increased the hatching rate and the length of larvae along with an improved anti-inflammatory effect | [283] | ||
| Pleurotus tuber-regium | Sclerotium | Inhibited blood vessel formation and subintestinal vessel plexus. | [284] | |
| Pleurotus tuber-regium | Total triterpenes | Reduced changes in the body mass index and lipid accumulation induced by a high-fat diet. | [285] |
| Model | Mushroom Name | Extract/ Compound | Protective Effects/ Mechanism | References |
|---|---|---|---|---|
| Drosophila melanogaster | Antrodia camphorata | Ergosta-7,9(11),22-trien-3β-ol | Improved the life span, motor function, learning, and memory of the AD model. Reduced the biomarkers of microglia activation and inflammation, without affecting lipid peroxidation or catalase and SOD activities. | [288] |
| Ganoderma lucidum | The formulation (Panax notoginseng, Panax ginseng, and gardenoside) aided in memory improvement in the AD model | [289] | ||
| Hericium erinaceus | Erinacine A | Extended the lifespan of both male and female Drosophila. | [290] | |
| Improved survival and locomotion and regulated apoptosis of tert-butyl hydroperoxide-treated ELAV-SCA3tr-Q78 flies. | [291] | |||
| Lentinus edodes | Hot water extract | Increased the life span and locomotive activities of male flies but showed early mortality and decreased locomotive activity in female flies. | [292] | |
| Lentinus subnudus | Reduced the levels of acetylcholinesterase and butyrylcholinesterase, ROS and MDA, improved catalase activity, and total thiol levels | [293] | ||
| Pleurotus ostreatus | Dried mycelia | Exhibited antigenotoxic activity against mitomycin C mutagen. | [294] | |
| Trametes versicolor | Dried mycelia | Exhibited antigenotoxic activity against mitomycin C mutagen. | [294] |
| Model | Mushroom Name | Extract/ Compound | Protective Effects/ Mechanism | References |
|---|---|---|---|---|
| C. elegans | Auricularia auricula-judae | Degraded polysaccharides | Extended the lifespan under high sugar stress conditions. | [296] |
| Polysaccharides | Extended the lifespan under oxidative stress and improved antioxidant activity | [297] | ||
| Melanin | Extended the lifespan and locomotive properties | [298] | ||
| Auricularia polytricha | Ethanol extract | Extended the lifespan and improved pharyngeal pumping rate | [11] | |
| Polysaccharides | Exhibited antioxidant activity by scavenging free radicals, improving antioxidant enzymes, and reducing the level of ROS during oxidative stress | [299] | ||
| Extended the lifespan, enhanced antioxidant enzymes, and regulated the expression of skn-1, sod-1, sod-2, sod-3, and sir-2.1 | [300] | |||
| Extended the lifespan, enhanced antioxidant enzymes, and regulated the expression of daf-16 and skn-1 | [301] | |||
| Acid hydrosylates of polysaccharides | Extended the lifespan, enhanced antioxidant enzymes, and regulated the expression of daf-16, skn-1, sir, sod-1, and sod-2 | [302] | ||
| Flammulina velutipes | Polysaccharides | Exhibited anti-ultraviolet activity | [303] | |
| Ganoderma lucidum | Polysaccharides | Extended the lifespan and activate daf-16 via TIR-1 receptor and the MAPK pathway | [304] | |
| Water extract | Extended the lifespan and reduced oxidative stress and heavy metal stress. Activation of CR pathway and mTOR/S6K pathway. | [305] | ||
| Water extract and polysaccharides | Extended the lifespan and improved stress resistance by modulating autophagy | [306] | ||
| Lentinula edodes | Polysaccharides | Extended the lifespan under heat-induced stress conditions. | [307] | |
| Lignosus rhinocerus | Ethanol extract | Inhibition of Alzheimer’s and Huntington’s diseases. | [114] | |
| Ethanol, cold water, and hot water extract | Antioxidant and lifespan extension. Upregulated the DAF-16/FOXO pathway. | [308] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharika, R.; Mongkolpobsin, K.; Rangsinth, P.; Prasanth, M.I.; Nilkhet, S.; Pradniwat, P.; Tencomnao, T.; Chuchawankul, S. Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review. Nutrients 2024, 16, 2682. https://doi.org/10.3390/nu16162682
Sharika R, Mongkolpobsin K, Rangsinth P, Prasanth MI, Nilkhet S, Pradniwat P, Tencomnao T, Chuchawankul S. Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review. Nutrients. 2024; 16(16):2682. https://doi.org/10.3390/nu16162682
Chicago/Turabian StyleSharika, Rajasekharan, Kuljira Mongkolpobsin, Panthakarn Rangsinth, Mani Iyer Prasanth, Sunita Nilkhet, Paweena Pradniwat, Tewin Tencomnao, and Siriporn Chuchawankul. 2024. "Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review" Nutrients 16, no. 16: 2682. https://doi.org/10.3390/nu16162682
APA StyleSharika, R., Mongkolpobsin, K., Rangsinth, P., Prasanth, M. I., Nilkhet, S., Pradniwat, P., Tencomnao, T., & Chuchawankul, S. (2024). Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review. Nutrients, 16(16), 2682. https://doi.org/10.3390/nu16162682








