Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Study Population
2.3. Prolonged Run—Mountain Ultramarathon
2.4. Vitamin D3 Administration
2.5. Sample Collection Protocol
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasprowicz, K.; Ziemann, E.; Ratkowski, W.; Laskowski, R.; Kaczor, J.J.; Dadci, R.; Antosiewicz, J. Running a 100-km ultra-marathon induces an inflammatory response but does not raise the level of the plasma iron-regulatory protein hepcidin. J. Sports Med. Phys. Fit. 2013, 53, 533–537. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Kortas, J.; Ziemann, E.; Juszczak, D.; Micielska, K.; Kozlowska, M.; Prusik, K.; Prusik, K.; Antosiewicz, J. Iron Status in Elderly Women Impacts Myostatin, Adiponectin and Osteocalcin Levels Induced by Nordic Walking Training. Nutrients 2020, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Nyyssonen, K.; Korpela, H.; Tuomilehto, J.; Seppanen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kortas, J.; Kuchta, A.; Prusik, K.; Prusik, K.; Ziemann, E.; Labudda, S.; Cwiklinska, A.; Wieczorek, E.; Jankowski, M.; Antosiewicz, J. Nordic walking training attenuation of oxidative stress in association with a drop in body iron stores in elderly women. Biogerontology 2017, 18, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Serfass, R.C.; Mackey-Bojack, S.M.; Kelly, K.L.; Titus, J.L.; Apple, F.S. Cardiac troponin T alterations in myocardium and serum of rats after stressful, prolonged intense exercise. J. Appl. Physiol. 2000, 88, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Mieszkowski, J.; Stankiewicz, B.E.; Kochanowicz, A.; Niespodzinski, B.; Borkowska, A.E.; Sikorska, K.; Danilowicz-Szymanowicz, L.; Brzezinska, P.; Antosiewicz, J. Remote Ischemic Preconditioning Reduces Marathon-Induced Oxidative Stress and Decreases Liver and Heart Injury Markers in the Serum. Front. Physiol. 2021, 12, 731889. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes. PLoS ONE 2016, 11, e0153617. [Google Scholar] [CrossRef]
- Lindsay, A.; Lewis, J.G.; Scarrott, C.; Gill, N.; Gieseg, S.P.; Draper, N. Assessing the Effectiveness of Selected Biomarkers in the Acute and Cumulative Physiological Stress Response in Professional Rugby Union through Non-invasive Assessment. Int. J. Sports Med. 2015, 36, 446–454. [Google Scholar] [CrossRef]
- Alsufiani, H.M.; AlGhamdi, S.A.; AlShaibi, H.F.; Khoja, S.O.; Saif, S.F.; Carlberg, C. A Single Vitamin D(3) Bolus Supplementation Improves Vitamin D Status and Reduces Proinflammatory Cytokines in Healthy Females. Nutrients 2022, 14, 3963. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, P.; Voors, A.A.; Lipsic, E.; van Gilst, W.H.; van Veldhuisen, D.J. Erythropoietin in cardiovascular diseases. Eur. Heart J. 2004, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Baggish, A.L.; Chung, E.H.; George, K.; Kleiven, O.; Mingels, A.M.A.; Orn, S.; Shave, R.E.; Thompson, P.D.; Eijsvogels, T.M.H. Exercise-Induced Cardiac Troponin Elevations: From Underlying Mechanisms to Clinical Relevance. Circulation 2021, 144, 1955–1972. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-dose vitamin D(3) reduces circulating hepcidin concentrations: A pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin. Nutr. 2017, 36, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Murr, C.; Pilz, S.; Grammer, T.B.; Kleber, M.E.; Meinitzer, A.; Boehm, B.O.; Marz, W.; Fuchs, D. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chem. Lab. Med. 2012, 50, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Mieszkowski, J.; Borkowska, A.; Stankiewicz, B.; Kochanowicz, A.; Niespodzinski, B.; Surmiak, M.; Waldzinski, T.; Rola, R.; Petr, M.; Antosiewicz, J. Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Ganz, T. Erythropoietic regulators of iron metabolism. Free Radic. Biol. Med. 2019, 133, 69–74. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Weng, S.; Sprague, J.E.; Oh, J.; Riek, A.E.; Chin, K.; Garcia, M.; Bernal-Mizrachi, C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS ONE 2013, 8, e54625. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Ziolkowski, W.; Kaczor, J.J.; Herman-Antosiewicz, A. Tumor necrosis factor-alpha-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron pool. Free Radic. Biol. Med. 2007, 43, 265–270. [Google Scholar] [CrossRef]
- Borkowska, A.; Sielicka-Dudzin, A.; Herman-Antosiewicz, A.; Halon, M.; Wozniak, M.; Antosiewicz, J. P66Shc mediated ferritin degradation--a novel mechanism of ROS formation. Free Radic. Biol. Med. 2011, 51, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Boluyt, M.O.; Loyd, A.M.; Roth, M.H.; Randall, M.J.; Song, E.Y. Activation of JNK in rat heart by exercise: Effect of training. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2639–H2647. [Google Scholar] [CrossRef]
- Hur, S.J.; Kim, D.M.; Lim, K.H.; Yoon, S.H.; Chung, H.C.; Lee, J.S.; Park, J. Vitamin D levels and their relationship with cardiac biomarkers in chronic hemodialysis patients. J. Korean Med. Sci. 2009, 24 (Suppl. S1), S109–S114. [Google Scholar] [CrossRef] [PubMed]

| Variable |
Supplemented Group (n = 16) |
Placebo (Control) Group (n = 19) | p | Effect Size (η2) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age (years) | 42.40 ± 7.59 | 39.48 ± 6.89 | 0.21 | 0.04 |
| Body height (cm) | 175.20 ± 4.34 * | 179.67 ± 4.64 | 0.01 | 0.17 |
| Body mass (kg) | 72.51 ± 6.71 | 76.19 ± 5.25 | 0.07 | 0.08 |
| Body mass index (kg/m2) | 23.24 ± 2.78 | 24.45 ± 1.19 | 0.11 | 0.06 |
| Fat mass (%) | 12.13 ± 3.89 | 12.85 ± 4.42 | 0.36 | 0.03 |
| Baseline serum ferritin (ng/mL) | 144.20 ± 43.29 | 149.50 ± 68.95 | 0.44 | 0.02 |
| Baseline serum iron (µmol/L) | 24.53 ± 13.16 | 28.27 ± 19.01 | 0.50 | 0.01 |
| Number of Training Units per Week | CR 1 (km) | CR 2 (km) | CROSS 1 (km) | CROSS 2 (km) | Speed (km) | ||
|---|---|---|---|---|---|---|---|
| General preparation period | Mean | 5.00 | 60.94 | 11.58 | 7.58 | 3.91 | 0.88 |
| SD | 0.83 | 16.29 | 4.21 | 2.78 | 2.88 | 0.60 | |
| Pre-start period | Mean | 5.70 | 67.39 | 13.52 | 14.38 | 5.7 | 1.57 |
| SD | 0.85 | 11.96 | 2.60 | 4.35 | 2.65 | 0.59 |
| Variable | Supplemented Group (n = 16) | Placebo (Control) Group (n = 19) | p | Effect Size (η2) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| VO2 max (mL/kg/min) | 53.73 ± 6.04 | 54.40 ± 5.68 | 0.74 | <0.01 |
| Distance (km) | 2.908 ± 0.263 | 2.939 ± 0.254 | 0.75 | <0.01 |
| Variable | Effect | F | df | p | Effect Size (η2) | Post hoc Outcome |
|---|---|---|---|---|---|---|
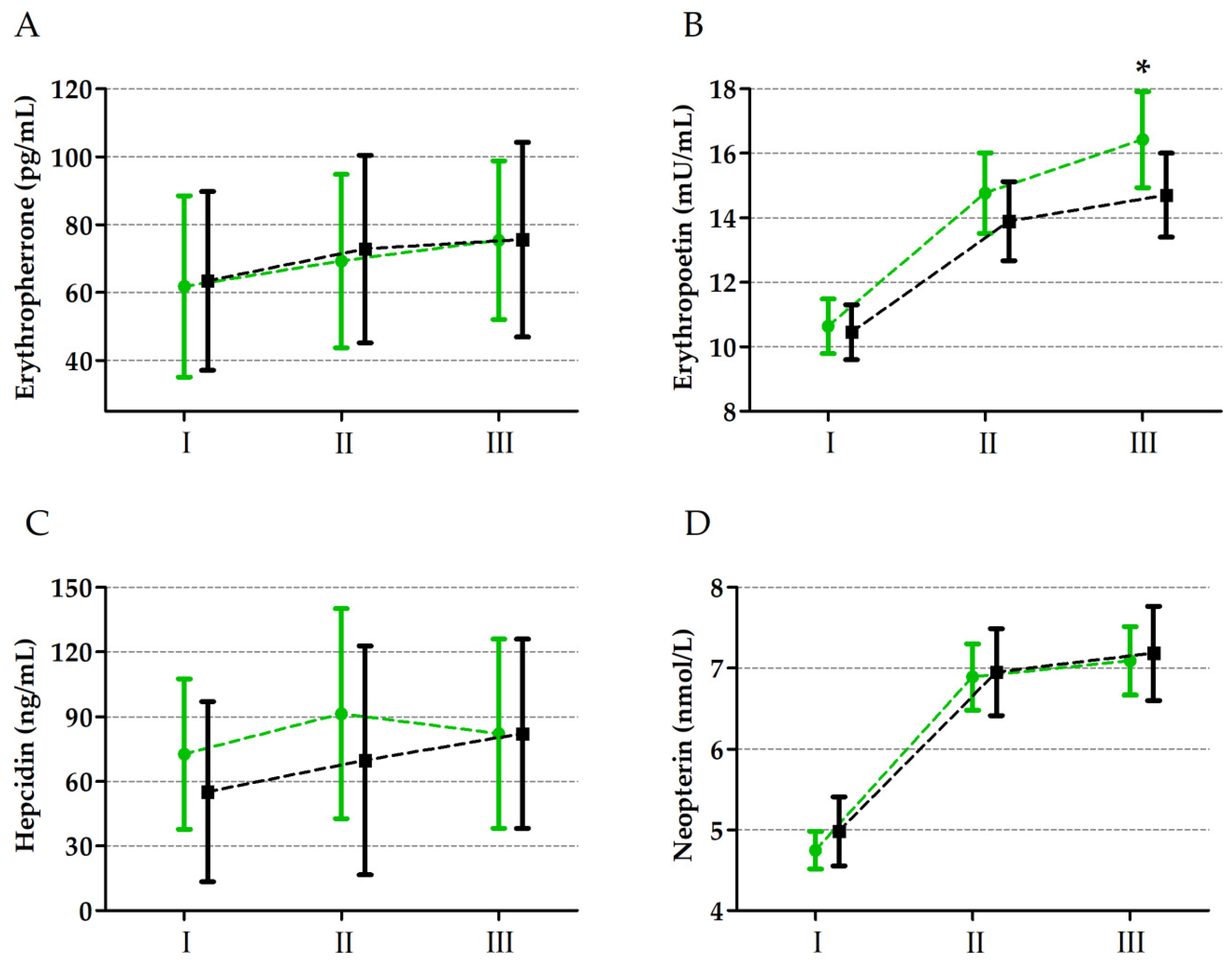
| Erythroferrone | GR | 0.04 | 1, 33 | 0.83 | <0.01 | I < II < III |
| UM | 33.6 | 2, 66 | 0.01 * | 0.49 | ||
| GR × UM | 0.55 | 2, 66 | 0.57 | 0.01 | ||
| Erythropoietin | GR | 3.39 | 1, 33 | 0.07 | 0.09 | |
| UM | 743.92 | 2, 66 | 0.01 * | 0.95 | I < II < III | |
| GR × UM | 4.04 | 2, 66 | 0.01 * | 0.16 | C-III < S-III | |
| Hepcidin | GR | 0.94 | 1, 33 | 0.33 | 0.02 | |
| UM | 12.51 | 2, 66 | 0.01 * | 0.27 | I < II, III | |
| GR × UM | 0.74 | 2, 66 | 0.47 | 0.02 | ||
| Neopterin | GR | 0.79 | 1, 33 | 0.37 | 0.02 | |
| UM | 996.39 | 2, 66 | 0.01 * | 0.97 | I < II < III | |
| GR × UM | 2.07 | 2, 66 | 0.13 | 0.05 |
| Variable | Change | Supplemented Group | Placebo (Control) Group | ||
|---|---|---|---|---|---|
| Iron | Ferritin | Iron | Ferritin | ||
| Erythroferrone | Δ II–I | 0.41 | 0.27 | 0.07 | 0.48 * |
| Δ III–I | 0.34 | 0.28 | 0.23 | 0.45 * | |
| Erythropoietin | Δ II–I | 0.13 | −0.07 | 0.09 | 0.28 |
| Δ III–I | 0.24 | 0.01 | −0.08 | 0.12 | |
| Hepcidin | Δ II–I | −0.31 | −0.51 * | −0.14 | 0.46 * |
| Δ III–I | −0.04 | −0.30 | −0.08 | 0.30 | |
| Neopterin | Δ II–I | −0.09 | 0.31 | 0.13 | 0.42 |
| Δ III–I | −0.06 | 0.36 | 0.52 * | 0.31 | |
| Variable | Effect | F | df | p | Effect Size (η2) | Post hoc Outcome |
|---|---|---|---|---|---|---|
| Ferritin | GR | 1.75 | 1, 33 | 0.19 | 0.05 | |
| UM | 1.06 | 2, 66 | 0.34 | 0.03 | ||
| GR × UM | 1.35 | 2, 66 | 0.26 | 0.04 | ||
| Iron | GR | 0.65 | 1, 33 | 0.45 | 0.01 | |
| UM | 13.25 | 2, 66 | 0.01 * | 0.25 | II < I, III | |
| GR × UM | 9.11 | 2, 66 | 0.01 * | 0.19 | CII < CI, CIII | |
| TIBC | GR | 28.16 | 1, 33 | 0.01 * | 0.42 | C < S |
| UM | 109.51 | 2, 66 | 0.01 * | 0.74 | I > II < III | |
| GR × UM | 43.62 | 2, 66 | 0.01 * | 0.53 | CI > CII < CIII; CI, CII < SI, SII | |
| UIBC | GR | 29.97 | 1, 33 | 0.01 * | 0.44 | C < S |
| UM | 6.64 | 2, 66 | 0.01 * | 0.14 | I < II, III | |
| GR × UM | 2.07 | 2, 66 | 0.01 * | 0.16 | CI < CII, CII; CI < SI |
| Variable | Unit | Supplemented Group | Placebo (Control) Group | ||
|---|---|---|---|---|---|
| Baseline | 24 h after the Run | Baseline | 24 h after the Run | ||
| Cardiac Troponin T | pg/mL | 1.70 ± 0.65 | 6.65 ± 2.90 #* | 1.63 ± 0.83 | 10.42 ± 4.13 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankiewicz, B.; Mieszkowski, J.; Kochanowicz, A.; Brzezińska, P.; Niespodziński, B.; Kowalik, T.; Waldziński, T.; Kowalski, K.; Borkowska, A.; Reczkowicz, J.; et al. Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial. Nutrients 2024, 16, 2479. https://doi.org/10.3390/nu16152479
Stankiewicz B, Mieszkowski J, Kochanowicz A, Brzezińska P, Niespodziński B, Kowalik T, Waldziński T, Kowalski K, Borkowska A, Reczkowicz J, et al. Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial. Nutrients. 2024; 16(15):2479. https://doi.org/10.3390/nu16152479
Chicago/Turabian StyleStankiewicz, Błażej, Jan Mieszkowski, Andrzej Kochanowicz, Paulina Brzezińska, Bartłomiej Niespodziński, Tomasz Kowalik, Tomasz Waldziński, Konrad Kowalski, Andżelika Borkowska, Joanna Reczkowicz, and et al. 2024. "Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial" Nutrients 16, no. 15: 2479. https://doi.org/10.3390/nu16152479
APA StyleStankiewicz, B., Mieszkowski, J., Kochanowicz, A., Brzezińska, P., Niespodziński, B., Kowalik, T., Waldziński, T., Kowalski, K., Borkowska, A., Reczkowicz, J., Daniłowicz-Szymanowicz, L., & Antosiewicz, J. (2024). Effect of Single High-Dose Vitamin D3 Supplementation on Post-Ultra Mountain Running Heart Damage and Iron Metabolism Changes: A Double-Blind Randomized Controlled Trial. Nutrients, 16(15), 2479. https://doi.org/10.3390/nu16152479








