Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Randomization Procedures
2.3. Intervention Protocol
2.4. Preparation of Curcuminoid Capsules
2.5. Study Outcomes
2.6. Data Collection and Measurement Methods
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Participant Enrollment and Baseline Characteristics
3.2. Curcumin Treatment and Depression Severity
3.3. Glycemic Control Outcomes
3.4. Insulin Resistance, Anti-Inflammatory, and Antioxidative Stress Outcomes
3.5. Weight Measurement Results
3.6. The Effect of Curcumin between Genders
3.7. Adverse Effects
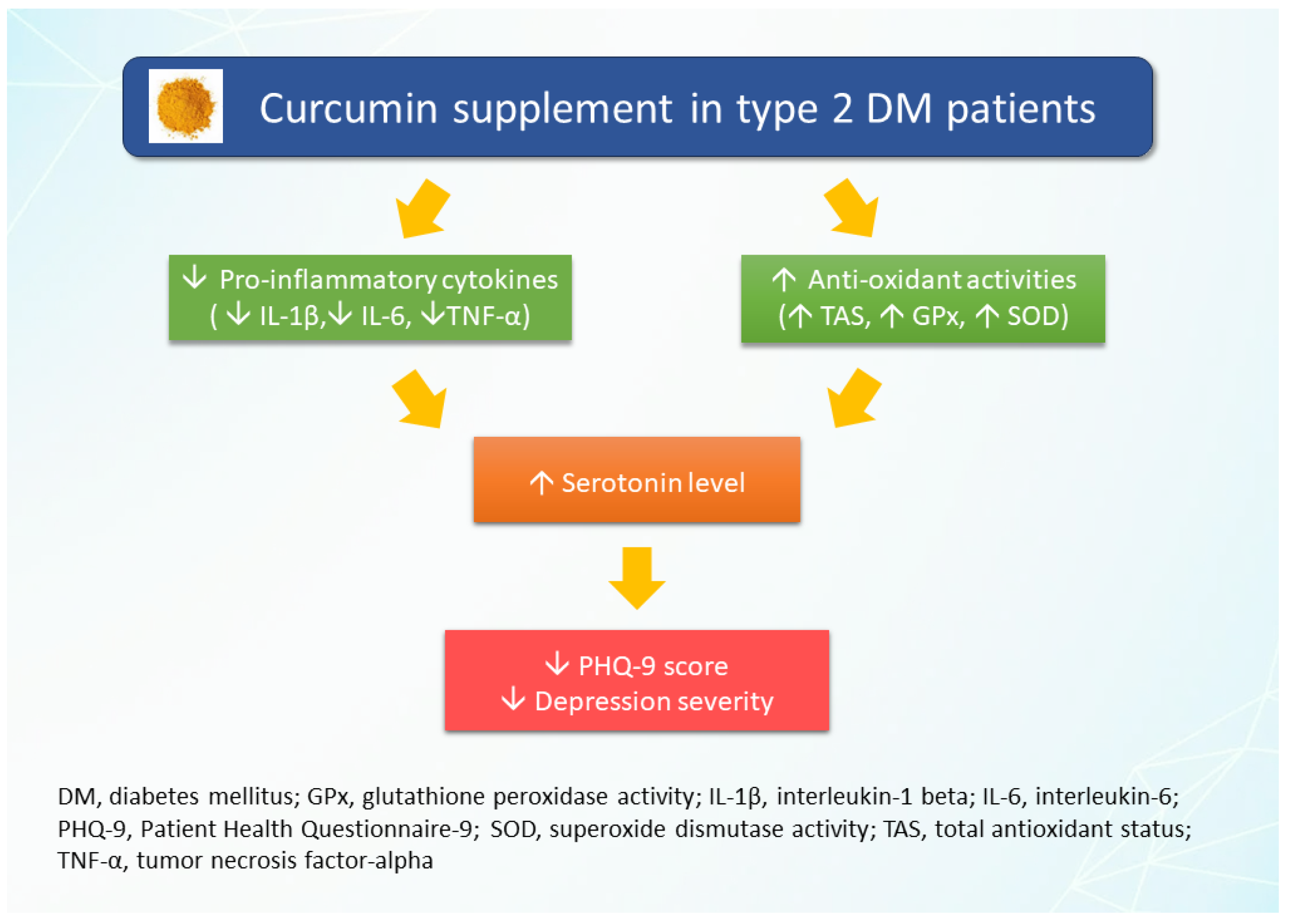
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.C.; Lim, L.-L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.; Eliasson, B.; Kong, A.P.; Ezzati, M. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2020, 396, 2019–2082. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Okoro, C.A.; Denny, C.H.; Greenlund, K.J.; Benjamin, S.M.; Strine, T.W.; Balluz, L.S.; Mokdad, A.H. Risk factors for heart disease and stroke among diabetic persons, by disability status. J. Diabetes Its Complicat. 2005, 19, 201–206. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Forth Edition Text Revision (DSM-IV-TR); American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Word Health Organization. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 11 May 2024).
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar] [PubMed]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and Type 2 Diabetes Over the Lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Gill, B.; El-Halabi, S.; Chen-Li, D.; Lipsitz, O.; Rosenblat, J.D.; Van Rheenen, T.E.; Rodrigues, N.B.; Mansur, R.B.; Majeed, A.; et al. Antidepressant Medications and Weight Change: A Narrative Review. Obesity 2020, 28, 2064–2072. [Google Scholar] [CrossRef]
- Salvi, V.; Mencacci, C.; Barone-Adesi, F. Antidepressant induced weight gain associated with anti-histaminergic activity. BMJ 2018, 362, k3222. [Google Scholar] [CrossRef]
- Nutt, D.J.; Forshall, S.; Bell, C.; Rich, A.; Sandford, J.; Nash, J.; Argyropoulos, S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur. Neuropsychopharmacol. 1999, 9, S81–S86. [Google Scholar] [CrossRef]
- Lee, S.; Paz-Filho, G.; Mastronardi, C.; Licinio, J.; Wong, M.-L. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl. Psychiatry 2016, 6, e759. [Google Scholar] [CrossRef]
- Schwartz, T.L.; Meszaros, Z.S.; Khan, R.; Nihalani, N. How to control weight gain when prescribing antidepressants. Curr. Psychiatry 2007, 6, 43. [Google Scholar]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Julie, S.; Jurenka, M. Anti-inflammatory properties of curcumin, a major constituent. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer US: Boston, MA, USA, 2007; pp. 105–125. [Google Scholar]
- Xu, Y.; Ku, B.-S.; Yao, H.-Y.; Lin, Y.-H.; Ma, X.; Zhang, Y.-H.; Li, X.-J. The effects of curcumin on depressive-like behaviors in mice. Eur. J. Pharmacol. 2005, 518, 40–46. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Wu, H.-L.; Li, Y.-B.; Li, Y.-H.; Guo, J.-B.; Li, X.-J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Xia, X.; Cheng, G.; Pan, Y.; Xia, Z.; Kong, L. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J. Ethnopharmacol. 2007, 110, 356–363. [Google Scholar] [CrossRef]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Sotoudeh, G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2020, 34, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2017 abridged for primary care providers. Clin. Diabetes 2017, 35, 5–26. [Google Scholar] [CrossRef]
- Lotrakul, M.; Sumrithe, S.; Saipanish, R. Reliability and validity of the Thai version of the PHQ-9. BMC Psychiatry 2008, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A New Depression Diagnostic and Severity Measure; Slack Incorporated: Thorofare, NJ, USA, 2002; Volume 32, pp. 509–515. [Google Scholar]
- Saldanha, D.; Kumar, N.; Ryali, V.; Srivastava, K.; Pawar, A.A. Serum Serotonin Abnormality in Depression. Med. J. Armed Forces India 2009, 65, 108–112. [Google Scholar] [CrossRef]
- Trujillo-Hernández, P.E.; Sáenz-Galindo, A.; Saucedo-Cárdenas, O.; Villarreal-Reyna, M.d.l.Á.; Salinas-Santander, M.A.; Carrillo-Cervantes, A.L.; Torres-Obregón, R.; Esparza-González, S.C. Depressive Symptoms are Associated with low Serotonin Levels in Plasma but are not 5–HTTLPR Genotype Dependent in Older Adults. Span. J. Psychol. 2021, 24, e28. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Jirawatnotai, S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J. Diabetes Its Complicat. 2014, 28, 124–129. [Google Scholar] [CrossRef]
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef]
- Semenkovich, K.; Brown, M.E.; Svrakic, D.M.; Lustman, P.J. Depression in Type 2 Diabetes Mellitus: Prevalence, Impact, and Treatment. Drugs 2015, 75, 577–587. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Wang, Y.; Xie, K.; Zhang, Q.; Luan, Q.; Chen, W.; Liu, D. Antidepressant-like effects of curcumin in chronic mild stress of rats: Involvement of its anti-inflammatory action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 47, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective effects of curcumin on IL-1β-induced neuronal apoptosis and depression-like behaviors caused by chronic stress in rats. Front. Cell. Neurosci. 2019, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Afrin, R.; Arumugam, S.; Rahman, A.; Wahed, M.I.I.; Karuppagounder, V.; Harima, M.; Suzuki, H.; Miyashita, S.; Suzuki, K.; Yoneyama, H.; et al. Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-κB translocation. Int. Immunopharmacol. 2017, 44, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ghandadi, M.; Sahebkar, A. Curcumin: An effective inhibitor of interleukin-6. Curr. Pharm. Des. 2017, 23, 921–931. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Rawdin, B.; Mellon, S.; Dhabhar, F.; Epel, E.; Puterman, E.; Su, Y.; Burke, H.; Reus, V.; Rosser, R.; Hamilton, S. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid–biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Panaro, M.A.; La Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as prospective anti-aging natural compound: Focus on brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative stress and major depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Fava, M. Weight gain and antidepressants. J. Clin. Psychiatry 2000, 61, 37–41. [Google Scholar] [PubMed]
- Serretti, A.; Mandelli, L.; Laura, M. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 979. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Powrie, J.K.; Anderson, A.S.; Garthwaite, P.H. Obesity, Weight Loss and Prognosis in Type 2 Diabetes. Diabet. Med. 1990, 7, 228–233. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Chaves Filho, A.J.M.; Lima, C.N.C.; Vasconcelos, S.M.M.; de Lucena, D.F.; Maes, M.; Macedo, D. IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 234–249. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative stress in depression: The link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
| Variable | Placebo | Curcumin | p Value * |
|---|---|---|---|
| Mean (SEM) (n = 114) | Mean (SEM) (n = 113) | ||
| Sex, M:F ratio | 54/80 (0.67) | 62/73 (0.85) | 0.87 † |
| Age, y | 62.26 (0.81) | 60.27 (0.83) | 0.13 |
| BMI, kg/m2 | 26.76 (0.38) | 27.21 (0.37) | 0.41 |
| Weight, kg | 69.50 (1.32) | 69.92 (1.24) | 0.58 |
| Systolic blood pressure | 129.25 (1.28) | 129.76 (1.30) | 0.95 |
| Diastolic blood pressure | 75.84 (1.05) | 75.14 (1.15) | 0.95 |
| PHQ-9 | NA | NA | NA |
| Serotonin, ng/mL | NA | NA | NA |
| FBG, mg/dL | 125.80 (2.22) | 123.65 (1.73) | 0.401 |
| HbA1c, % | 6.26 (0.06) | 6.28 (0.07) | 0.69 |
| HOMA-IR, units | 5.24 (0.24) | 5.38 (0.23) | 0.72 |
| IL-1β, pg/mL | 0.44 (0.02) | 0.42 (0.02) | 0.46 |
| IL-6, pg/mL | 8.71 (0.11) | 8.96 (0.12) | 0.34 |
| TNF-α, pg/mL | 5.01 (0.14) | 4.78 (0.13) | 0.24 |
| TAS, μmol/trolox eq/L | 1.60 (0.01) | 1.58 (0.01) | 0.30 |
| Glutathione peroxidase activity, U/mL | 6583.78 (218.65) | 6693.82 (206.44) | 0.90 |
| Superoxide dismutase activity, U/mL | 241.09 (4.60) | 237.59 (4.38) | 0.77 |
| Malondialdehyde, μmol/L | 2.01 (0.04) | 1.95 (0.04) | 0.28 |
| Creatinine, mg/dL | 0.87 (0.02) | 0.86 (0.02) | 0.77 |
| AST, U/L | 25.01 (0.87) | 25.34 (0.80) | 0.58 |
| ALT, U/L | 27.58 (1.56) | 30.09 (1.50) | 0.08 |
| History of cerebrovascular disease | 7 (6.1%) | 5 (4.4%) | 0.78 † |
| History of coronary artery disease | 9 (7.8%) | 8 (7.1%) | 1.00 † |
| History of hypertension | 82 (71.9%) | 76 (67.2%) | 0.53 † |
| History of diabetic nephropathy | 18 (15.8%) | 28 (24.8%) | 0.13 † |
| History of dyslipidemia | 104 (77.6%) | 101 (74.8%) | 0.84 † |
| Outcomes | Follow-Up Period (mo) | Placebo | Curcumin | p Value | ||
|---|---|---|---|---|---|---|
| Mean | Minimum–Maximum | Mean | Minimum–Maximum | |||
| PHQ-9 | 0 | 11.22 | 3–15 | 11.59 | 3.00–15.00 | NS |
| 3 | 11.81 | 5–15 | 9.97 | 3.00–14.00 | <0.0001 | |
| 6 | 12.23 | 5–15 | 8.91 | 3.00–14.00 | <0.0001 | |
| 9 | 12.48 | 4–15 | 8.26 | 3.00–13.00 | <0.0001 | |
| 12 | 12.84 | 6–16 | 7.66 | 2.00–13.00 | <0.0001 | |
| Serotonin, ng/mL | 0 | 99.51 | 70.40–132.00 | 100.39 | 71.28–132.00 | NS |
| 3 | 103.26 | 70.40–132.00 | 104.23 | 70.40–132.00 | NS | |
| 6 | 102.44 | 48.14–154.35 | 136.50 | 87.35–198.76 | 0.0001 | |
| 9 | 101.23 | 48.19–153.56 | 143.35 | 94.35–193.33 | <0.0001 | |
| 12 | 100.60 | 46.59–150.23 | 151.03 | 99.87–199.87 | <0.0001 | |
| HbA1c, % | 0 | 6.26 | 4.80–8.90 | 6.28 | 4.40–9.50 | NS |
| 3 | 6.44 | 5.00–8.90 | 6.26 | 4.70–9.20 | <0.01 | |
| 6 | 6.46 | 5.10–9.00 | 6.25 | 4.50–8.30 | <0.01 | |
| 9 | 6.47 | 5.00–10.40 | 6.19 | 4.10–8.20 | <0.05 | |
| 12 | 6.47 | 5.00–10.50 | 6.12 | 4.20–8.40 | <0.05 | |
| Glucose | 0 | 125.08 | 91–285 | 123.65 | 79–178 | NS |
| 3 | 128.93 | 100–195 | 124.40 | 80–171 | NS | |
| 6 | 130.34 | 77–231 | 122.82 | 79–204 | <0.01 | |
| 9 | 130.93 | 97–201 | 118.67 | 75–165 | <0.01 | |
| 12 | 130.71 | 98–194 | 115.49 | 70–160 | <0.05 | |
| HOMA-IR | 0 | 5.24 | 1.70–21.80 | 5.38 | 1.20–14.20 | NS |
| 3 | 5.88 | 2.00–17.00 | 5.25 | 1.70–12.80 | <0.05 | |
| 6 | 5.93 | 1.80–17.90 | 5.17 | 1.60–16.50 | <0.05 | |
| 9 | 6.02 | 2.20–19.80 | 5.02 | 1.30–11.50 | <0.05 | |
| 12 | 6.04 | 2.30–18.00 | 4.86 | 1.20–11.00 | <0.05 | |
| IL-1β, pg/mL | 0 | 0.44 | 0.01–0.86 | 0.46 | 0.01–0.88 | NS |
| 3 | 0.46 | 0.02–0.87 | 0.45 | 0.01–0.87 | NS | |
| 6 | 0.71 | 0.20–1.74 | 0.43 | 0.15–1.54 | <0.001 | |
| 9 | 0.72 | 0.20–1.65 | 0.41 | 0.12–0.99 | <0.001 | |
| 12 | 074 | 0.32–1.86 | 0.31 | 0.10–1.39 | <0.001 | |
| IL-6, pg/mL | 0 | 8.71 | 7.04–10.56 | 8.96 | 7.04–10.56 | NS |
| 3 | 8.89 | 7.04–10.56 | 8.72 | 7.04–10.56 | NS | |
| 6 | 12.84 | 5.21–17.99 | 7.54 | 3.11–14.99 | <0.001 | |
| 9 | 14.30 | 7.65–19.66 | 6.82 | 3.2–13.24 | <0.001 | |
| 12 | 15.84 | 4.33–19.66 | 6.12 | 3.09–12.40 | <0.001 | |
| TNF-α, pg/mL | 0 | 5.01 | 2.64–7.04 | 4.77 | 2.64–7.04 | NS |
| 3 | 5.16 | 2.64–7.04 | 4.84 | 2.64–7.04 | NS | |
| 6 | 5.91 | 2.18–14.88 | 4.23 | 1.46–10.5 | <0.001 | |
| 9 | 6.37 | 2.24–14.98 | 3.81 | 1.43–9.44 | <0.001 | |
| 12 | 6.77 | 2.14–15.37 | 3.46 | 1.33–8.59 | <0.001 | |
| TAS, μmol trolox eq/L | 0 | 1.60 | 1.20–1.98 | 1.59 | 1.25–1.89 | NS |
| 3 | 1.61 | 1.26–2.20 | 1.70 | 1.35–2.14 | <0.05 | |
| 6 | 1.63 | 1.20–1.98 | 1.73 | 1.32–2.01 | <0.05 | |
| 9 | 1.63 | 1.09–2.94 | 1.82 | 1.42–2.94 | <0.05 | |
| 12 | 1.63 | 1.21–2.45 | 1.86 | 1.49–2.58 | <0.05 | |
| RANSEL, U/mL | 0 | 6583.78 | 1083–13,199 | 6693.82 | 1124–13,012 | NS |
| 3 | 6537.38 | 3540–19,484 | 7380.96 | 3497–15,378 | <0.05 | |
| 6 | 6501.03 | 2500–18,386 | 8273.31 | 4376–17,679 | <0.05 | |
| 9 | 5675.5 | 3090–10,834 | 11,048.48 | 6089–16,548 | <0.001 | |
| 12 | 5153.41 | 3489–10,234 | 13,143.50 | 5787–20,987 | <0.001 | |
| RANSOD, U/mL | 0 | 241.09 | 133.00–379.00 | 241.59 | 144.00–362.00 | NS |
| 3 | 237.19 | 133.00–420.00 | 244.93 | 151.00–362.00 | <0.05 | |
| 6 | 224.35 | 120.00–420.00 | 265.74 | 144.00–450.00 | <0.001 | |
| 9 | 202.45 | 120.00–280.00 | 280.38 | 189.00–450.00 | <0.001 | |
| 12 | 176.34 | 112.00–218.00 | 328.90 | 215.00–468.00 | <0.001 | |
| MDA, μmol/L | 0 | 2.01 | 1.20–3.30 | 2.00 | 1.20–3.23 | NS |
| 3 | 2.03 | 1.08–3.42 | 2.01 | 1.03–4.22 | NS | |
| 6 | 2.32 | 1.15–3.90 | 1.90 | 0.99–3.51 | <0.001 | |
| 9 | 2.34 | 1.22–5.59 | 1.66 | 0.84–3.28 | <0.001 | |
| 12 | 2.40 | 1.20–5.59 | 1.45 | 0.93–2.60 | <0.001 | |
| BMI, kg/m2 | 0 | 26.94 | 16.45–35.18 | 27.35 | 20.40–36.58 | NS |
| 3 | 26.98 | 16.88–40.37 | 26.56 | 19.15–44.81 | <0.05 | |
| 6 | 26.96 | 17.31–40.79 | 25.90 | 18.31–43.71 | <0.01 | |
| 9 | 28.00 | 16.88–40.79 | 26.02 | 19.14–42.61 | <0.001 | |
| 12 | 29.34 | 17.72–42.11 | 25.94 | 17.90–42.24 | <0.001 | |
| Body weight, kg | 0 | 69.50 | 61–112 | 69.92 | 71–120 | NS |
| 3 | 69.53 | 62–113 | 67.72 | 70–117 | <0.05 | |
| 6 | 69.47 | 63–119 | 66.06 | 69–135 | <0.01 | |
| 9 | 72.01 | 63–117 | 66.08 | 68–117 | <0.01 | |
| 12 | 75.30 | 63–140 | 63.85 | 68–114 | <0.001 | |
| Severity of Depression | Placebo (n = 114) | Curcumin (n = 113) | p Value * | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | Improved ⵜ | Baseline | 12 Months | Improved ⵜ | |||
| PHQ-9 | Minimal, (0–4) | 3 (2.63%) | 1 (0.88%) | 3/114 (2.6%) | NA | 9 (7.96%) | 23/113 (20.4%) | <0.000001 |
| Mild, (5–9) | 15 (13.16%) | 8 (7.02%) | 17 (15.05%) | 91 (80.53%) | ||||
| Moderate, (10–14) | 93 (81.58%) | 94 (82.45%) | 89 (78.76%) | 13 (11.51%) | ||||
| Moderately Severe (15–19) | 3 (2.63%) | 11 (9.65%) | 7 (6.19%) | NA | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2414. https://doi.org/10.3390/nu16152414
Yaikwawong M, Jansarikit L, Jirawatnotai S, Chuengsamarn S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients. 2024; 16(15):2414. https://doi.org/10.3390/nu16152414
Chicago/Turabian StyleYaikwawong, Metha, Laddawan Jansarikit, Siwanon Jirawatnotai, and Somlak Chuengsamarn. 2024. "Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial" Nutrients 16, no. 15: 2414. https://doi.org/10.3390/nu16152414
APA StyleYaikwawong, M., Jansarikit, L., Jirawatnotai, S., & Chuengsamarn, S. (2024). Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients, 16(15), 2414. https://doi.org/10.3390/nu16152414





