Effect of a Triterpenoid-Rich Olive Oil on Chronic Kidney Disease in an Experimental Model of Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Reagents
2.2. Olive Fruit Derived Products
- -
- Destoned olive oil (DOO)
- -
- Destoned and dehydrated olive oil (DDOO)
2.3. Experimental Design
2.3.1. Study Design, Care of the Animals, and Sample Size
- -
- Replacement alternatives. The development of an experimental model of type 1 diabetes allows the reproduction of the macro- and microangiopathic complications that develop in patients with diabetes. This allows the evaluation of several biomarkers of vascular, cerebral, renal, and retinal damage, which enables the identification of potential therapeutic targets. This requires a living organism in which all the organ systems affected by chronic hyperglycemia are related, and this objective cannot be achieved in in vitro models.
- -
- Reduction alternatives. The sample size was calculated based on a percentage difference in the main parameters that define diabetic nephropathy (proteinuria and glomerular volume) in treated diabetic animals with respect to untreated animals, calculating a percentage reduction of 30% for both parameters. The number of animals per group was 10, including a replacement rate of 10%.
- -
- Refinement alternatives. All animals were handled by people with extensive experience in animal treatment and with official accreditation for handling experimental animals, and the suffering that could be generated by the procedures used was always reduced, minimizing the number of animal manipulations. Another way to minimize animal stress was to favor the environment in which the procedures were carried out. The animals were treated on a thermal blanket to maintain optimal body temperature, and noise sources were minimized. At the end of the treatment period, the animals were anesthetized with sodium pentobarbital, and after ensuring that the animal did not react to external stimuli, we proceeded to exsanguination and extraction of the organs under study. Subsequently, as this was a non-recovery procedure, the animals were decapitated with a guillotine, as established in the protocols for this type of experiment.
- -
- Presence of dyspnea or hemorrhage or stupor or cachexia was considered endpoint criteria.
- -
- Presence of abnormal or increased secretions (no = 0 points; yes = 1 point); isolation or aggressive attitude towards conspecifics and/or investigator (no = 0 points; yes = 1 point); diarrhea (no = 0 points; yes = 1 point). In case of reaching 2 points, the endpoint criterion would have been applied.
2.3.2. Groups of Study, Inclusion and Exclusion Criteria, and Randomization
- -
- Group of normoglycemic control rats (NCR). In these animals, isotonic saline was administered in each of the procedures while the rest of the animals required the administration of some reagent or the study compounds.
- -
- Group of diabetic control animals (DCR). These animals were induced with diabetes mellitus as explained in the following section, receiving saline instead of the study oils and 3–4 units of semi-slow-acting insulin (Levemir®, Novo Nordisk A/S, Bagsværd, Denmark) subcutaneously, to reduce the possibility of mortality due to excessive hyperglycemia.
- -
- Group of diabetic animals treated with destoned olive oil (DOO) at a dose of 0.5 mL/kg/day orally.
- -
- Group of diabetic animals treated with destoned and dehydrated olive oil (DDOO) at a dose of 0.5 mL/kg/day orally.
2.3.3. Analytical Techniques
Samples
- -
- Non-anticoagulated blood was centrifuged at 3500× g to obtain serum, obtaining aliquots, which were frozen at −80 °C.
- -
- Renal tissue. The right kidney was processed for subsequent histological analysis, and the left kidney for determination of biochemical variables. From the left kidney, the renal cortex was homogenized in 50 mM phosphate-buffered saline, pH 7.0 (1/15 w/v), and then centrifuged at 13,000× g for 15 min at 4 °C. The supernatant was divided into aliquots and frozen at −80 °C.
- -
- 24 h urine as described above.
Serum and Urinary Determination of Biochemical Variables
Oxidative and Nitrosative Stress
Urinary Prostanoids
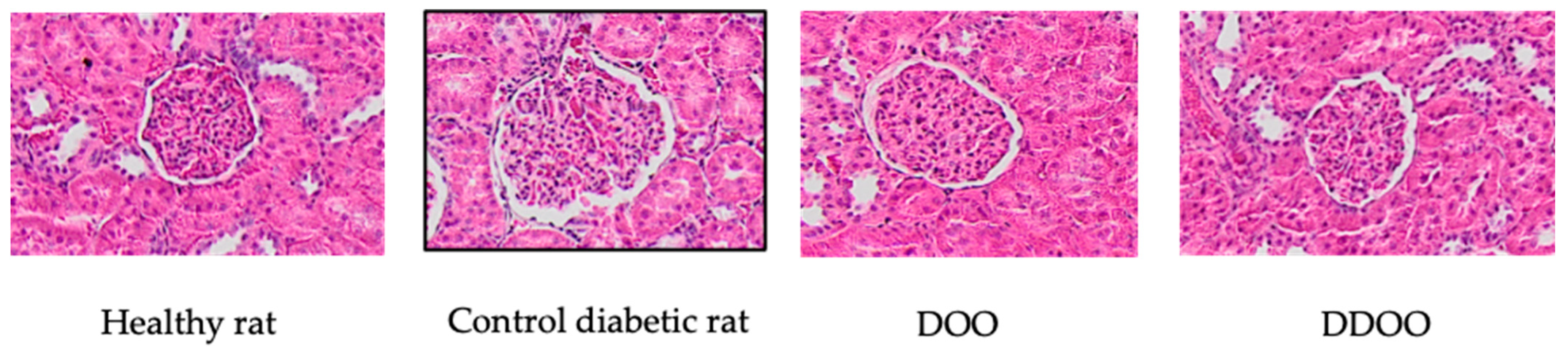
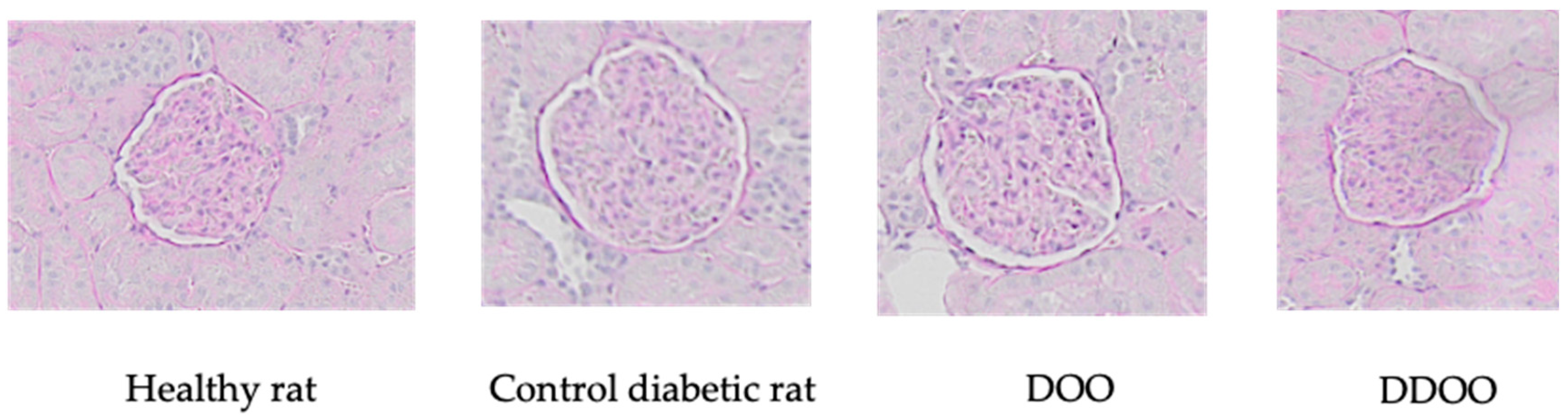
Morphometric Analysis
2.4. Statistical Analysis
3. Results
3.1. Zoometric Variables
3.2. Serum and Urinary Biochemistry
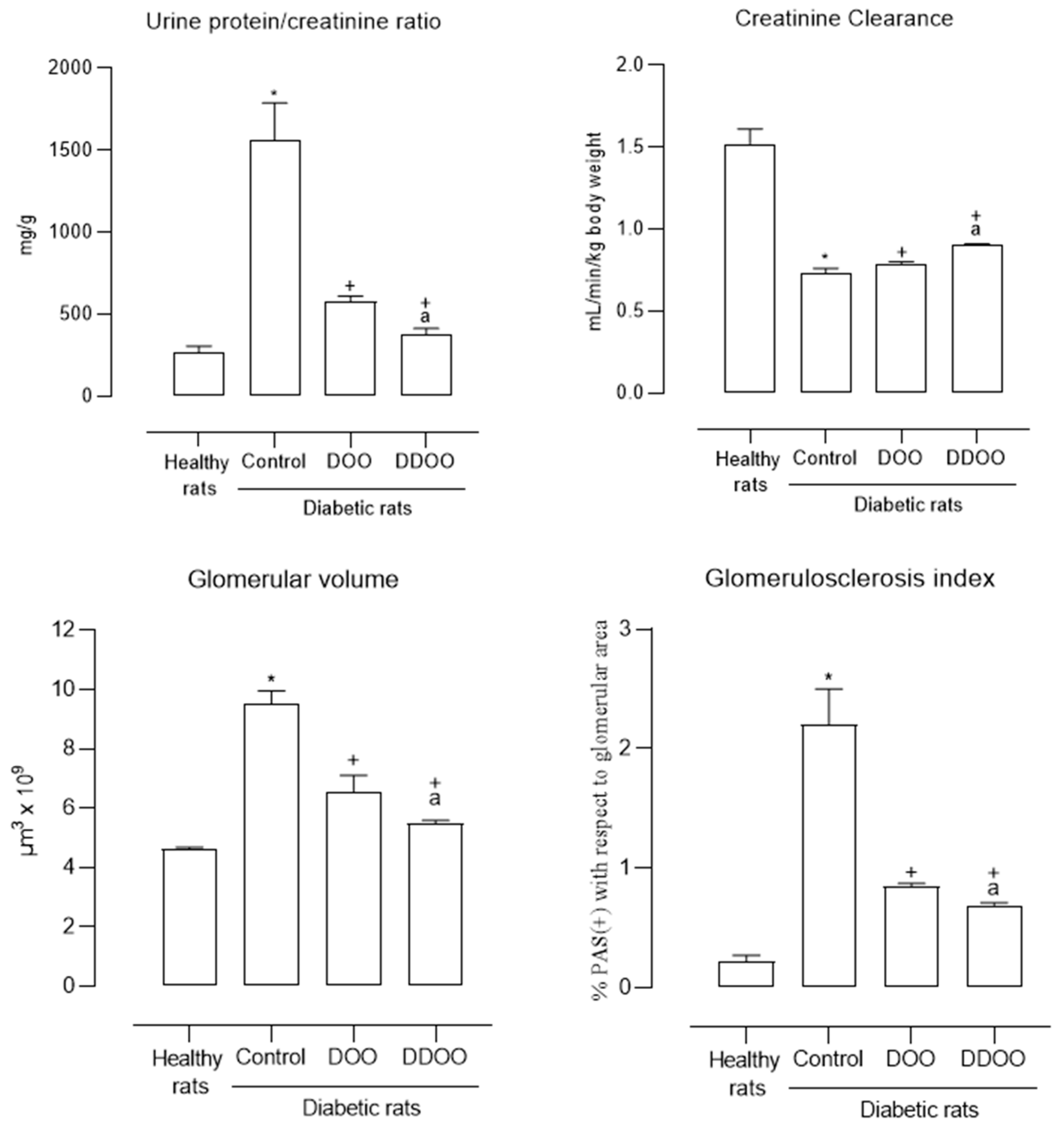
3.3. Kidney Variables
3.4. Oxidative and Nitrosative Stress
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S10–S18. [Google Scholar] [CrossRef]
- Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; Kahan, S.; Khunti, K.; Leon, J.; Lyons, S.K.; Perry, M.L.; Prahalad, P.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar]
- Dagar, N.; Das, P.; Bisht, P.; Taraphdar, A.K.; Velayutham, R.; Arumugam, S. Diabetic nephropathy: A twisted thread to unravel. Life Sci. 2021, 278, 119635. [Google Scholar] [CrossRef]
- Magee, C.; Grieve, D.J.; Watson, C.J.; Brazil, D.P. Diabetic Nephropathy: A Tangled Web to Unweave. Cardiovasc. Drugs Ther. 2017, 31, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Riser, B.L.; Denichilo, M.; Cortes, P.; Baker, C.; Grondin, J.M.; Yee, J.; Narins, R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 25–38. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z. Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy. Front. Endocrinol. 2022, 13, 816400. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Matoba, K.; Utsunomiya, K. Signaling pathways in diabetic nephropathy. Histol. Histopathol. 2016, 31, 1059–1067. [Google Scholar]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 8, 1497449. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 20, 50–63. [Google Scholar] [CrossRef]
- Balakumar, P.; Chakkarwar, V.A.; Krishan, P.; Singh, M. Vascular endothelial dysfunction: A tug of war in diabetic nephropathy? Biomed. Pharmacother. 2009, 63, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 1042. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural antioxidants in the treatment and prevention of diabetic nephropathy; a potential approach that warrants clinical trials. Redox Rep. 2017, 22, 99–118. [Google Scholar] [CrossRef]
- Hernandez, L.F.; Eguchi, N.; Whaley, D.; Alexander, M.; Tantisattamo, E.; Ichii, H. Anti-Oxidative Therapy in Diabetic Nephropathy. Front. Biosci. (Schol. Ed.) 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Tavafi, M. Diabetic nephropathy and antioxidants. J. Nephropathol. 2013, 2, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Urciuoli, S.; Di Lauro, M.; Ruzzolini, J.; Ieri, F.; Vignolini, P.; Di Daniele, F.; Guerriero, C.; Nediani, C.; Di Daniele, N.; et al. Extra Virgin Olive Oil and Cardiovascular Protection in Chronic Kidney Disease. Nutrients 2022, 14, 4265. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.D.; Santiago-Corral, L.; Ortega-Hombrados, L.; Verdugo, C.; Arrebola, M.M.; Martín-Aurioles, E.; Fernández-Prior, M.Á.; Bermúdez-Oria, A.; De La Cruz, J.P.; González-Correa, J.A. The Effect of the Extra Virgin Olive Oil Minor Phenolic Compound 3′,4′-Dihydroxyphenylglycol in Experimental Diabetic Kidney Disease. Nutrients 2023, 15, 377. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.D.; López-Villodres, J.A.; Arrebola, M.M.; Martín-Aurioles, E.; Fernández-Prior, Á.; Bermúdez-Oria, A.; Ríos, M.C.; De La Cruz, J.P.; González-Correa, J.A. Nephroprotective Effect of the Virgin Olive Oil Polyphenol Hydroxytyrosol in Type 1-like Experimental Diabetes Mellitus: Relationships with Its Antioxidant Effect. Antioxidants 2021, 10, 1783. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; De Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, A.R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Domínguez-López, I.; Siscart-Serra, A.; Fraga, N.; Sámano, S.; López-Sabater, C.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Pérez, M. Extra virgin olive oil: A comprehensive review of efforts to ensure its authenticity, traceability, and safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2639–2664. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Monasterio, R.P.; Sánchez-Arévalo, C.M.; Fernández-Gutiérrez, A.; Olmo-Peinado, J.M.; Carrasco-Pancorbo, A. Characterization of New Olive Fruit Derived Products Obtained by Means of a Novel Processing Method Involving Stone Removal and Dehydration with Zero Waste Generation. J. Agric. Food Chem. 2019, 67, 9295–9306. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis, and apoptosis, and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Costabile, G.; Della Pepa, G.; Bozzetto, L.; Annuzzi, G.; Vetrani, C.; Giacco, R.; Della Corte, G.; Conte, F.S.; Di Marino, L.; Rivellese, A.A. Urine 8-isoprostane in relation to adiposity and insulin resistance in individuals at high cardiometabolic risk. Metab. Syndr. Relat. Disord. 2015, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lane, P.H.; Steffes, M.W.; Mauer, S.M. Estimation of glomerular volume: A comparison of four methods. Kidney Int. 1992, 41, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Testa, R.; Genovese, S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 285–292. [Google Scholar] [CrossRef]
- Martínez-Rojas, M.Á.; Balcázar, H.; Ponce-Nava, M.S.; González-Soria, I.; Marquina-Castillo, B.; Pérez-Villalva, R.; Bobadilla, N.A. A short treatment with resveratrol after a renal ischemia-reperfusion injury prevents maladaptive repair and long-term chronic kidney disease in rats. J. Physiol. 2024, 602, 1835–1852. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Kanda, H.; Yamawaki, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 10, 857–864. [Google Scholar] [CrossRef]
- De La Cruz, J.P.; Pérez de Algaba, I.; Martín-Aurioles, E.; Arrebola, M.M.; Ortega-Hombrados, L.; Rodríguez-Pérez, M.D.; Fernández-Prior, M.A.; Bermúdez-Oria, A.; Verdugo, C.; González-Correa, J.A. Extra Virgin Oil Polyphenols Improve the Protective Effects of Hydroxytyrosol in an In Vitro Model of Hypoxia-Reoxygenation of Rat Brain. Brain Sci. 2021, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Vallejo-Carmona, L.; Arrebola, M.M.; Martín-Aurioles, E.; Rodriguez-Pérez, M.D.; Ortega-Hombrados, L.; Verdugo, C.; Fernández-Prior, M.A.; Bermúdez-Oria, A.; González-Correa, J.A. Synergistic Effect of 3′,4′-Dihidroxifenilglicol and Hydroxytyrosol on Oxidative and Nitrosative Stress and Some Cardiovascular Biomarkers in an Experimental Model of Type 1 Diabetes Mellitus. Antioxidants 2021, 10, 1983. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Roos, B.; Duthie, G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015, 54, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Binou, P.; Stergiou, A.; Kosta, O.; Tentolouris, N.; Karathanos, V.T. Positive contribution of hydroxytyrosol-enriched wheat bread to HbA1c levels, lipid profile, markers of inflammation and body weight in subjects with overweight/obesity and type 2 diabetes mellitus. Eur. J. Nutr. 2023, 62, 2165–2176. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P. Hypolipidemic and hypoglycemic activities of a oleanolic acid derivative from Malva parviflora on streptozotocin-induced diabetic mice. Arch. Pharm. Res. 2017, 40, 550–562. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.I.; Vilchez, P.; et al. Effects of virgin olive oils differing in their bioactive compound contents on biomarkers of oxidative stress and inflammation in healthy adults: A randomized double-blind controlled trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef]
- Qin, X.; Qiu, C.; Zhao, L. Maslinic acid protects vascular smooth muscle cells from oxidative stress through Akt/Nrf2/HO-1 pathway. Mol. Cell. Biochem. 2014, 390, 61–67. [Google Scholar] [CrossRef]
- Márquez, A.; de la Puerta, R.A.; Fernández-Arche, A.; Ruiz-Gutiérrez, V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Rodríguez-Rodríguez, R.; González-Díez, M.; Rodríguez, C.; Herrera, M.D.; Ruiz-Gutierrez, V.; Badimon, L. Oleanolic acid induces prostacyclin release in human vascular smooth muscle cells through a cyclooxygenase-2-dependent mechanism. J. Nutr. 2008, 138, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, J.; Baek, M.C.; Bae, J.S. Novel factor Xa inhibitor, maslinic acid, with antiplatelet aggregation activity. J. Cell. Physiol. 2020, 235, 9445–9456. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Destoned and Dehydrated Olive Oil (DDOO) | Destoned Olive Oil (DOO) |
|---|---|---|
| Acidity (%) | 0.11 ± 0.01 | 0.11 ± 0.01 |
| Peroxide value (meqO2/kg) | 10.22 ± 1.12 | 9.05 ± 0.0 |
| K270 | 0.15 ± 0.01 | 0.16 ± 0.01 |
| K232 | 1.55 ± 0.03 | 1.74 ± 0.03 |
| Delta K | <0.01 | <0.01 |
| Ethyl esters (mg/kg) | 12 ± 2 | 14 ± 2 |
| Ethyl palmitate (mg/kg) | 4 ± 0 | 6 ± 1 |
| Ethyl oleate (mg/kg) | 6 ± 1 | 7 ± 1 |
| Waxes (mg/kg) | 45 ± 2 | 32 ± 1 |
| Fatty acid composition | ||
| Myristic acid (%) | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Palmitic acid (%) | 12.92 ± 1.11 | 11.58 ± 0.79 |
| Palmitoleic acid (%) | 1.22 ± 0.04 | 1.1 ± 0.05 |
| Margaric acid (%) | 0.05 ± 0.00 | 0.05 ± 0.00 |
| Margaroleic acid (%) | 0.08 ± 0.00 | 0.07 ± 0.00 |
| Stearic Acid (%) | 2.48 ± 0.71 | 2.95 ± 0.55 |
| Oleic Acid (%) | 78.05 ± 3.54 | 77.59 ± 2.98 |
| Linoleic Acid (%) | 4.01 ± 0.55 | 4.52 ± 0.72 |
| Linolenic Acid (%) | 1.02 ± 0.04 | 0.91 ± 0.03 |
| Arachidic Acid (%) | 0.35 ± 0.01 | 0.32 ± 0.01 |
| Eicosanoic Acid (%) | 0.15 ± 0.01 | 0.21 ± 0.01 |
| Bhenenic Acid (%) | 0.12 ± 0.01 | 0.15 ± 0.01 |
| Lignoceric acid (%) | 0.09 ± 0.01 | 0.07 ± 0.00 |
| Total sterols (mg/kg) | 1428 ± 18 | 1227 ± 12 |
| Cholesterol (%) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Brassicasterol (%) | <0.1 | <0.1 |
| Cholesterol (%) | 2.4 ± 0.1 | 2.9 ± 0.0 |
| Stigmasterol (%) | 0.9 ± 0.1 | 0.5 ± 0.0 |
| B-Sitosterol (%) | 96.4 ± 3.2 | 93.2 ± 3.0 |
| D7-Stigmastenol (%) | 0.3 ± 0.0 | 0 |
| Erythrodiol + Uvaol (%) | 3.6 ± 0.1 | 0.2 ± 0.0 |
| Total triterpenic acids (mg/kg) | 845.21 ± 16.80 | 122.1 ± 5.07 |
| Oleanolic acid (mg/kg) | 261.04 ± 10.84 | 37.59 ± 2.12 |
| Maslinic acid (mg/Kg) | 574.72 ± 14.01 | 80.53 ± 2.30 |
| Ursolic acid (mg/kg) | 9.45 ± 0.10 | 4.02 ± 0.09 |
| Chlorophyll Pigments (mg/kg) | 32.84 ± 1.02 | 31.66 ± 1.58 |
| Carotenoid Pigments (mg/kg) | 7.44 ± 0.23 | 8.95 ± 0.17 |
| Squalane (mg/100 g) | 594.32 ± 24.51 | 388.05 ± 32.87 |
| Tocoferoles (mg/kg) | 421.16 ± 12.75 | 388.04 ± 14.60 |
| Total phenols (ppm) | 752.15 ± 23.15 | 722.61 ± 20.07 |
| 3,4-dihydroxyphenylglycol | 1.32 ± 0.02 | 1.17 ± 0.02 |
| Hydroxytyrosol | 12.14 ± 0.44 | 8.01 ± 0.22 |
| Tyrosol | 12.07 ± 1.33 | 4.52 ± 1.74 |
| Vanillic acid | 0 | 0 |
| HT acetate | 14.1 5 ± 0.55 | 0 |
| Nuzhenide | 7.12 ± 0.75 | 0 |
| Oleuropein derivative 1 | 0 | 0 |
| Oleuropein derivative 2 | 28.74 ± 2.21 | 52.94 ± 4.50 |
| Ligustroside derivative | 57.09 ± 3.18 | 62.15 ± 2.33 |
| Sum | 132.63 ± 7.52 | 128.91 ± 8.45 |
| Hydroxytyrosol (HT) potential (ppm) | 33.05 | 27.60 |
| % of potential HT approx. | 0.0033 | 0.0028 |
| Control Healthy Rats | Control Diabetic Rats (DRs) | DRs Treated with Destoned Olive Oil | DRs Treated with Destoned and Dehydrated Olive Oil | |
|---|---|---|---|---|
| Body weight (g) | ||||
| Day 1 | 298 ± 9.2 | 286 ± 10.1 | 290 ± 8.3 | 293 ± 9.0 |
| Day 60 | 505 ± 8.3 | 380 ± 19.1 * | 419 ± 35.0 + | 397 ± 33.1 + |
| % increase | 157 ± 10.6 | 134 ± 17.5 * | 149 ± 21.3 + | 141 ± 29.9 + |
| Kidney weight (% respect to body weight) | 0.6 ± 0.05 | 1.0 ± 0.07 * | 0.8 ± 0.06 + | 0.8 ± 0.05 + |
| Chow consumption (g/day) | 32.4 ± 5.1 | 21.4 ± 2.3 * | 23.8 ± 3.3 + | 27.2 ± 5.2 + |
| Drinking water (mL/day) | 43.3 ± 16.5 | 145 ± 27.5 * | 90.5 ± 17.0 + | 90.6 ± 15.5 + |
| Control Healthy Rats | Control Diabetic Rats (DRs) | DRs Treated with Destoned Olive Oil | DRs Treated with Destoned and Dehydrated Olive Oil | |
|---|---|---|---|---|
| Serum | ||||
| Glucose (mg/dL) | 98.8 ± 6.0 | 531 ± 10.1 * | 508 ± 13.1 + | 435 ± 9.9 +a |
| Creatinine (mg/dL) | 0.6 ± 0.05 | 1.0 ± 0.07 * | 0.8 ± 0.06 + | 0.8 ± 0.05 + |
| Total proteins (g/dL) | 6.3 ± 0.1 | 6.1 ± 0.3 | 6.2 ± 0.3 | 5.8 ± 0.2 |
| Total cholesterol (mg/dL) | 53.4 ± 3.5 | 86.4 ± 4.0 * | 70.6 ± 2.1 + | 63.2 ± 3.7 +a |
| LDL cholesterol (mg/dL) | 21.4 ± 1.4 | 44.8 ± 4.7 * | 26.7 ± 5.1 + | 17.3 ± 2.7 +a |
| HDL cholesterol (mg/dL) | 18.6 ± 1.5 | 15.3 ± 0.8 * | 27.9 ± 3.4 + | 32.1 ± 1.4 + |
| Triglycerides (mg/dL) | 56.7 ± 7.7 | 149 ± 8.0 * | 106 ± 3.5 + | 85.5 ± 5.1 +a |
| Urine Creatinine (mg/dL) | 114 ± 8.3 | 66.6 ± 3.5 * | 78.1 ± 5.6 + | 85.1 ± 4.2 + |
| Proteinuria (mg/L) | 14.8 ± 1.2 | 100 ± 10.3 * | 56.5 ± 5.7 + | 18.5 ± 1.7 +a |
| Glucosuria (g/L) | 0.0 ± 0.0 | 7.3 ± 0.7 * | 6.2 ± 0.6 + | 4.8 ± 0.4 +a |
| 11-dH-TxB2 (ng/mg creatinine) | 4.7 ± 0.6 | 13.2 ± 0.6 * | 8.8 ± 0.6 + | 5.1 ± 0.4 +a |
| 6-keto-PGF1α (pg/mg creatinine) | 19.5 ± 0.3 | 7.4 ± 0.6 * | 13.2 ± 0.4 + | 17.7 ± 0.7 +a |
| Control Healthy Rats | Control Diabetic Rats (DRs) | DRs Treated with Destoned Olive Oil | DRs Treated with Destoned and Dehydrated Olive Oil | |
|---|---|---|---|---|
| Serum | ||||
| TBARS (nmol/mL) | 4.6 ± 0.9 | 10.1 ± 0.6 * | 4.2 ± 1.1 + | 1.7 ± 0.2 +a |
| 8-OhdG (ng/mL) | 17.7 ± 0.5 | 29.0 ± 1.8 * | 4.5 ± 0.2 + | 1.5 ± 0.2 +a |
| GSH (nmol/mL) | 139 ± 8.6 | 90.2 ± 0.9 * | 106 ± 3.5 + | 139 ± 1.0 +a |
| GSHpx (nmol/min/mL) | 30.7 ± 1.2 | 8.0 ± 0.7 * | 18.5 ± 0.9 + | 27.7 ± 2.4 +a |
| TAC (IU/mL) | 19.6 ± 0.6 | 14.6 ± 0.8 * | 17.3 ± 1.0 + | 18.7 ± 0.6 +a |
| 3-nitrotyrosine (pg/mL) | 16.2 ± 1.1 | 73.3 ± 2.1 * | 49.1 ± 1.4 + | 42.9 ± 1.4 +a |
| Kidney | ||||
| TBARS (nmol/mg protein) | 3.8 ± 0.5 | 24.0 ± 0.8 * | 15.8 ± 0.2 + | 14.2 ± 0.1 +a |
| 8-isoprostane (ng/mg creatinine) | 7.4 ± 0.5 | 53.9 ± 0.7 * | 16.4 ± 0.6 + | 14.6 ± 0.5 +a |
| 8-OHdG (ng/0.1 g tissue) | 7.2 ± 0.8 | 13.8 ± 0.7 * | 9.7 ± 0.1 + | 8.3 ± 0.3 +a |
| GSH (µmol/0.1 g tissue) | 521 ± 25.0 | 129 ± 13.6 * | 187 ± 3.1 + | 291 ± 7.1 +a |
| GSHpx (nmol/min/0.1 g tissue) | 105 ± 5.3 | 59.0 ± 2.1 * | 89.8 ± 3.3 + | 98.2 ± 1.5 +a |
| TAC (IU/0.1 g tissue) | 104 ± 2.9 | 35.7 ± 3.2 * | 83.5 ± 1.5 + | 97.7 ± 3.1 + |
| 3-nitrotyrosine (pg/0.1 g tissue) | 22.7 ± 1.5 | 131 ± 6.9 * | 74.1 ± 1.6 + | 59.7 ± 4.1 +a |
| Prot/Creat | CrCl | GV | GlS | |
|---|---|---|---|---|
| Prot/Creat | ---- | −0.946 * | 0.898 * | 0.968 * |
| CrCl | −0.946 * | ---- | −0.798 ** | −0.942 * |
| GV | 0.898 * | −0.798 ** | ---- | 0.879 * |
| GlS | 0.968 * | −0.942 * | 0.879 * | ---- |
| TBARS (s) | 0.932 * | −0.881 * | 0.903 * | 0.918 * |
| 8-OH-dG (s) | 0.973 * | −0.958 * | 0.855 * | 0.984 * |
| 3-Nty (s) | 0.877 * | −0.768 *** | 0.861 * | 0.832 * |
| 8-isoprostanes | 0.929 * | −0.852 * | 0.864 * | 0.924 * |
| TAC (s) | −0.873 * | 0.807 ** | −0.839 * | −0.827 * |
| GSH (s) | −0.891 * | 0.805 ** | −0.785 ** | −0.875 * |
| GSHpx (s) | −0.917 * | 0.840 * | −0.835 * | −0.935 * |
| TBARS (k) | 0.916 * | −0.969 * | 0.815 * | 0.946 * |
| 8-OH-dG (k) | 0.937 * | −0.917 * | 0.769 **** | 0.915 * |
| 3-Nty (k) | 0.952 * | −0.918 * | 0.871 * | 0.948 * |
| TAC (k) | −0.950 * | 0.896 * | −0.862 * | −0.936 * |
| GSH (k) | −0.945 * | 0.872 * | −0.869 * | −0.934 * |
| GSHpx (k) | −0.910 * | 0.947 * | −0.744 ***** | −0.935 * |
| TxB2 | 0.971 * | −0.885 * | 0.881 * | 0.965 * |
| PGI2 | −0.967 * | 0.939 * | −0.850 * | −0.963 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Cruz, J.P.; Osuna-Esteban, L.; Rodríguez-Pérez, M.D.; Ortega-Hombrados, L.; Sánchez-Tévar, A.M.; Martín-Aurioles, E.; Fernández-Prior, M.Á.; Pérez-Burillo, S.; Espejo-Calvo, J.A.; González-Correa, J.A. Effect of a Triterpenoid-Rich Olive Oil on Chronic Kidney Disease in an Experimental Model of Diabetes Mellitus. Nutrients 2024, 16, 2794. https://doi.org/10.3390/nu16162794
De La Cruz JP, Osuna-Esteban L, Rodríguez-Pérez MD, Ortega-Hombrados L, Sánchez-Tévar AM, Martín-Aurioles E, Fernández-Prior MÁ, Pérez-Burillo S, Espejo-Calvo JA, González-Correa JA. Effect of a Triterpenoid-Rich Olive Oil on Chronic Kidney Disease in an Experimental Model of Diabetes Mellitus. Nutrients. 2024; 16(16):2794. https://doi.org/10.3390/nu16162794
Chicago/Turabian StyleDe La Cruz, José Pedro, Laura Osuna-Esteban, María Dolores Rodríguez-Pérez, Laura Ortega-Hombrados, Ana María Sánchez-Tévar, Esther Martín-Aurioles, María África Fernández-Prior, Sergio Pérez-Burillo, Juan Antonio Espejo-Calvo, and José Antonio González-Correa. 2024. "Effect of a Triterpenoid-Rich Olive Oil on Chronic Kidney Disease in an Experimental Model of Diabetes Mellitus" Nutrients 16, no. 16: 2794. https://doi.org/10.3390/nu16162794
APA StyleDe La Cruz, J. P., Osuna-Esteban, L., Rodríguez-Pérez, M. D., Ortega-Hombrados, L., Sánchez-Tévar, A. M., Martín-Aurioles, E., Fernández-Prior, M. Á., Pérez-Burillo, S., Espejo-Calvo, J. A., & González-Correa, J. A. (2024). Effect of a Triterpenoid-Rich Olive Oil on Chronic Kidney Disease in an Experimental Model of Diabetes Mellitus. Nutrients, 16(16), 2794. https://doi.org/10.3390/nu16162794







