S-Equol Ameliorates Menopausal Osteoarthritis in Rats through Reducing Oxidative Stress and Cartilage Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Body Weight
2.4. Histopathologic Analysis of Cartilage
2.5. Serum Sample Collection
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Serum Cholesterol and Triglyceride Analysis
2.8. Serum H2O2 and NO Determination
2.9. Statistical Analysis
3. Results
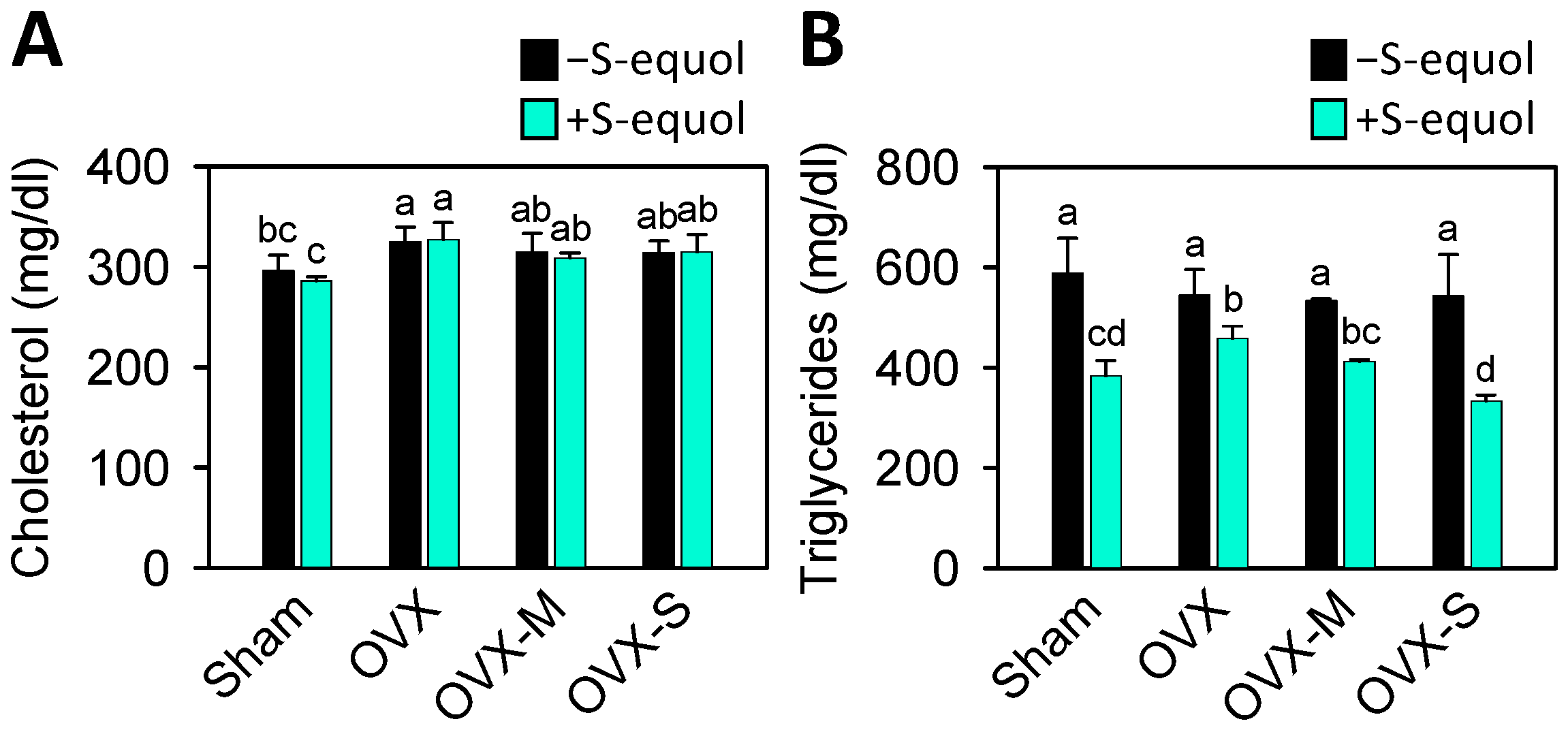
3.1. Body Weight and Physiologic Parameters
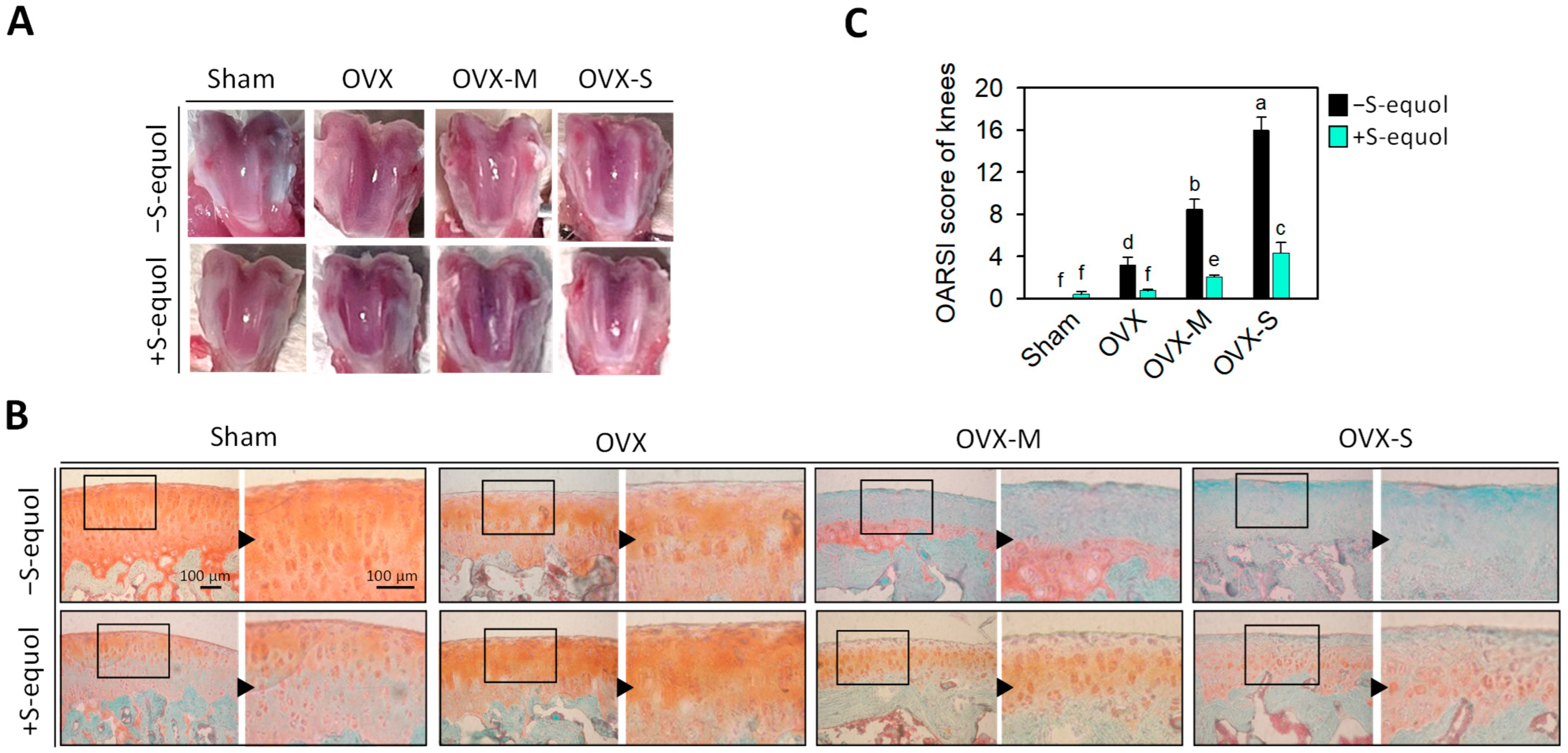
3.2. Macroscopic Observation and Knee-Joint Histopathologic Changes
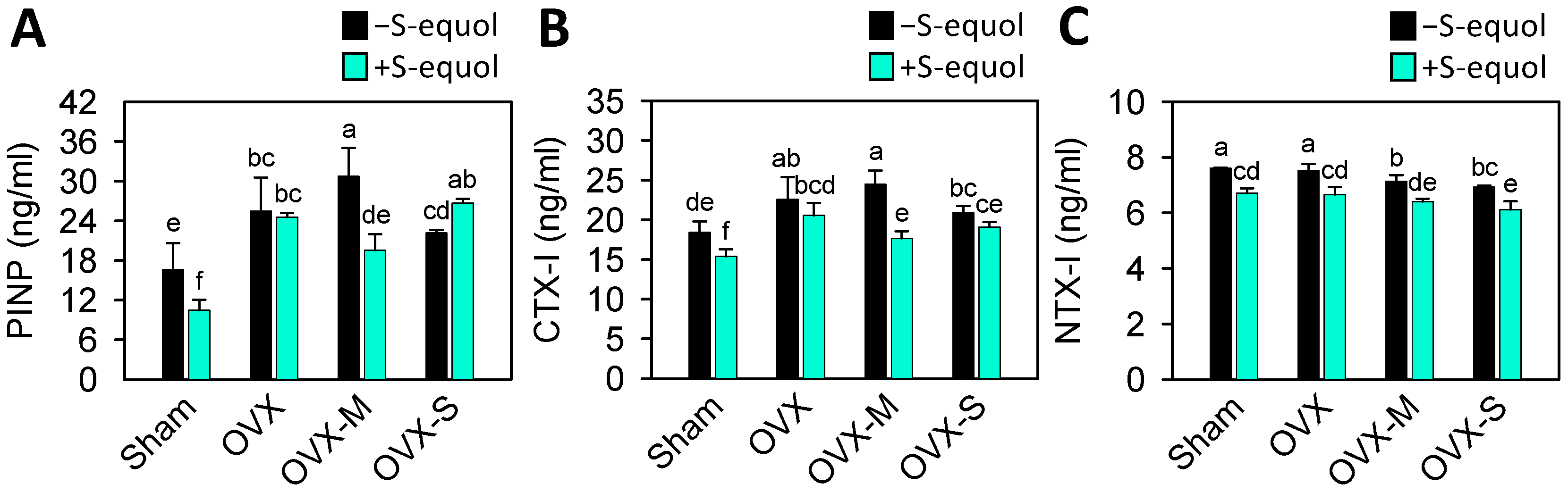
3.3. Serum Biomarkers of Bone Turnover
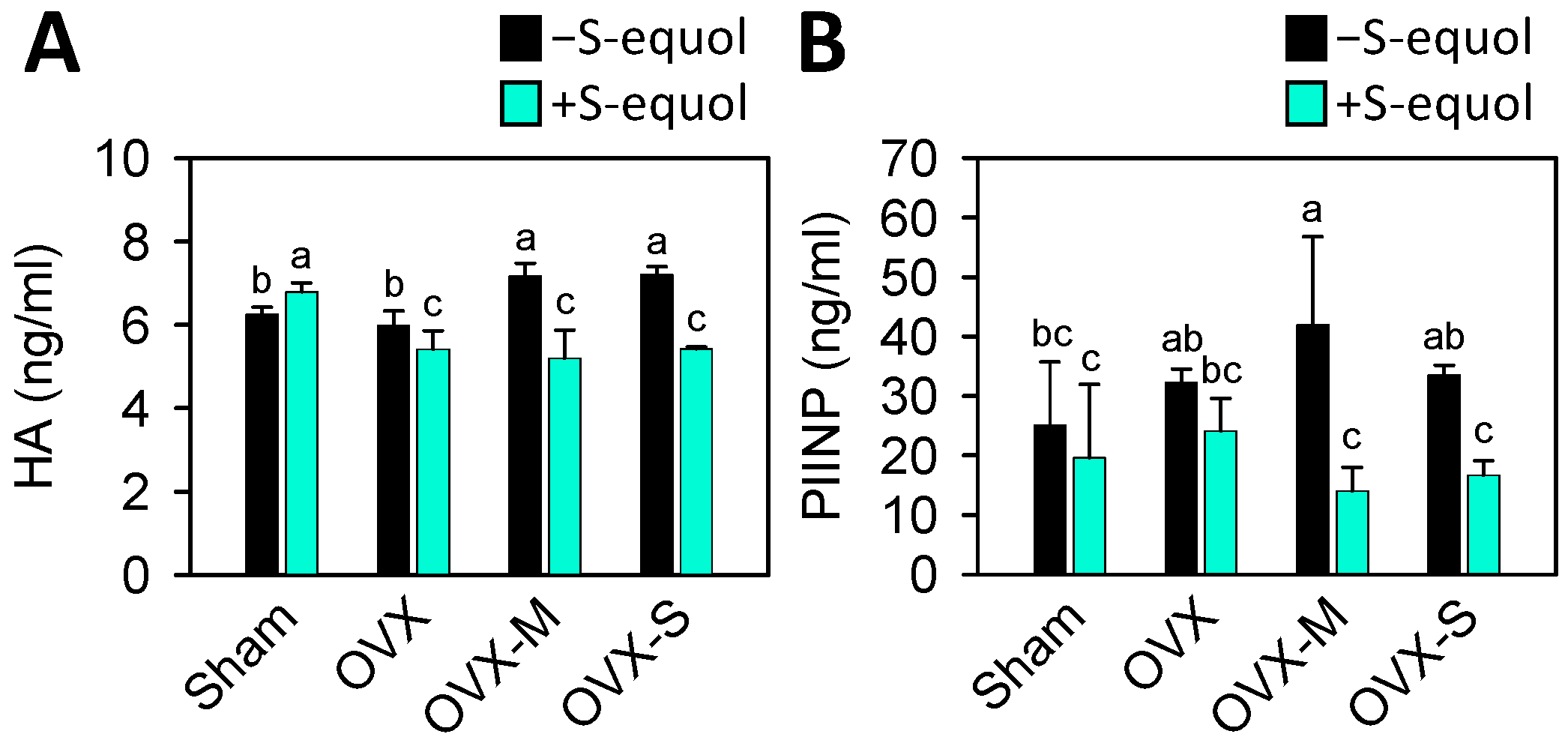
3.4. Serum Biomarkers of Cartilage Degradation
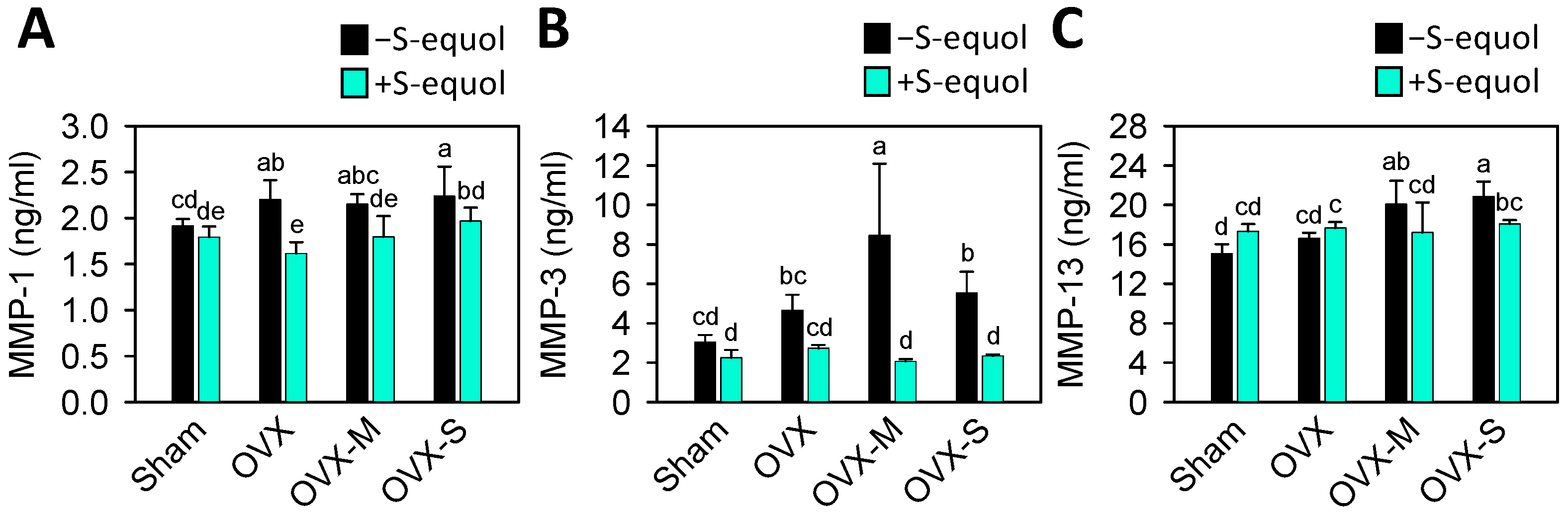
3.5. Serum Biomarkers of Matrix-Degrading Enzymes
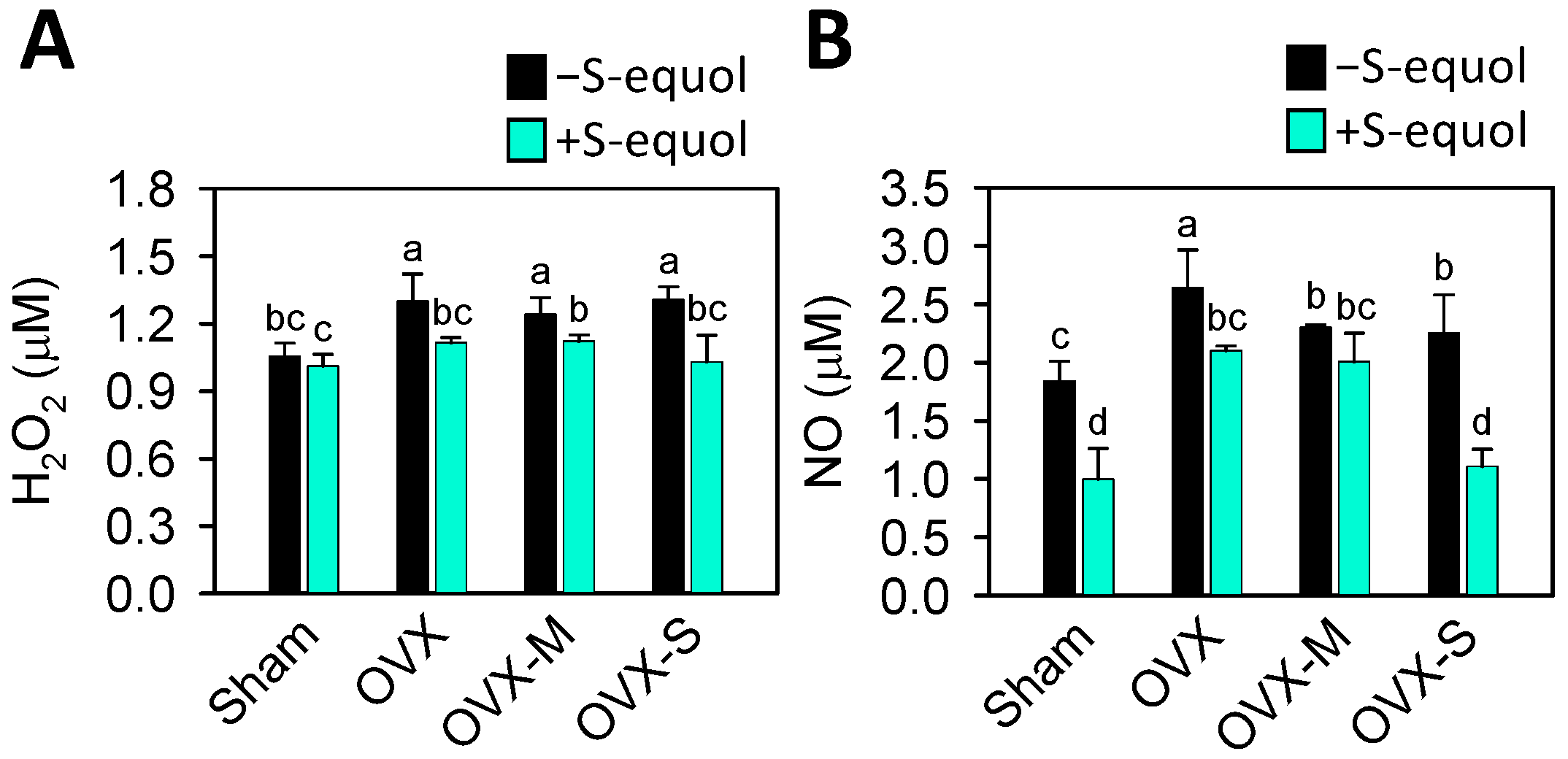
3.6. Oxidative Stress in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.L.; Esa, M.S.; Li, K.H.C.; Krishnan, S.R.G.; Elgallab, G.M.; Pearce, M.S.; Young, D.A.; Birrell, F.N. Osteoporosis, fracture, osteoarthritis & sarcopenia: A systematic review of circulating microRNA association. Bone 2021, 152, 116068. [Google Scholar]
- Mahajan, A.; Patni, R. Menopause and Osteoarthritis: Any Association ? J. Midlife Health 2018, 9, 171–172. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, S.; Han, M.; Gu, Z.; Zhang, Y.; Fan, T.; Zeng, M.; Ruan, G.; Cao, P.; Yang, Q.; et al. Osteoporosis and osteoarthritis: A bi-directional Mendelian randomization study. Arthritis Res. Ther. 2023, 25, 242. [Google Scholar] [CrossRef]
- Rizou, S.; Chronopoulos, E.; Ballas, M.; Lyritis, G.P. Clinical manifestations of osteoarthritis in osteoporotic and osteopenic postmenopausal women. J. Musculoskelet. Neuronal. Interact. 2018, 18, 208–214. [Google Scholar]
- Ho, W.C.; Chang, C.C.; Wu, W.T.; Lee, R.P.; Yao, T.K.; Peng, C.H.; Yeh, K.T. Effect of Osteoporosis Treatments on Osteoarthritis Progression in Postmenopausal Women: A Review of the Literature. Curr. Rheumatol. Rep. 2024, 26, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Akune, T. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: A 3-year follow-up of the ROAD study. Osteoarthr. Cartil. 2012, 20, 1217–1226. [Google Scholar] [CrossRef]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Yang, X.; Bie, J.; Li, D.; Sun, C.; Zhang, J.; Meng, Y.; Lin, J. Menopausal Hormone Replacement Therapy and the Risk of Ovarian Cancer: A Meta-Analysis. Front. Endocrinol. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ 2020, 371, m3873. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. The antioxidant activity of daidzein metabolites, Odesmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.J.; Wu, J.; Ni, Y.D.; Lu, L.Z.; Zhao, R.Q. Antioxidant effect of a phytoestrogen equol on cultured muscle cells of embryonic broilers. In Vitro Cell Dev. Biol. Anim. 2011, 47, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef]
- Yokoyama, S.; Suzuki, T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Uehara, M.; Sato, Y.; Kimira, M.; Eboshida, A.; Adlercreutz, H.; Watanabe, S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J. Epidemiol. 2000, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Miyanaga, N.; Takashima, N.; Naito, S.; Hirao, Y.; Tsukamoto, T.; Fujioka, T.; Mori, M.; Kim, W.J.; Song, J.M.; et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004, 34, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wahala, K.; Thomas, W.K.; Lampe, J.W. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Uehara, M.; Wu, J.; Adlercreutz, H.; Suzuki, K.; Kanazawa, K.; Takeda, K.; Yamada, K.; Ishimi, Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr. 2004, 134, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Tadaishi, M.; Kobori, M.; Tousen, Y.; Kato, M.; Inada, M.; Miyaura, C.; Ishimi, Y. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomized mice. J. Clin. Biochem. Nutr. 2013, 53, 41–48. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Huang, L.W.; Huang, T.C.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Chiu, P.R.; Chang, K.L. S-Equol Protects Chondrocytes against Sodium Nitroprusside-Caused Matrix Loss and Apoptosis through Activating PI3K/Akt Pathway. Int. J. Mol. Sci. 2021, 22, 7054. [Google Scholar] [CrossRef]
- Lin, I.C.; Yamashita, S.; Murata, M.; Kumazoe, M.; Tachibana, H. Equol suppresses inflammatory response and bone erosion due to rheumatoid arthritis in mice. J. Nutr. Biochem. 2016, 32, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hirase, Y.; Okubo, A. Equol production capability and family history as risk factors for hand osteoarthritis in menopausal and postmenopausal women. Cross-sectional study. J. Orthop. Sci. 2024; in press. [Google Scholar]
- Sniekers, Y.H.; Weinans, H.; Bierma-Zeinstra, S.M.; van Leeuwen, J.P.; van Osch, G.J. Animal models for osteoarthritis: The effect of ovariectomy and estrogen treatment—A systematic approach. Osteoarthr. Cartil. 2008, 16, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaisse, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [PubMed]
- Christgau, S.; Tanko, L.B.; Cloos, P.A.; Mouritzen, U.; Christiansen, C.; Delaisse, J.M.; Hoegh-Andersen, P. Suppression of elevated cartilage turnover in postmenopausal women and in ovariectomized rats by estrogen and a selective estrogen-receptor modulator (SERM). Menopause 2004, 11, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Oestergaard, S.; Sondergaard, B.C.; Hoegh-Andersen, P.; Henriksen, K.; Qvist, P.; Christiansen, C.; Tanko, L.B.; Karsdal, M.A. Effects of ovariectomy and estrogen therapy on type II collagen degradation and structural integrity of articular cartilage in rats: Implications of the time of initiation. Arthritis Rheum. 2006, 54, 2441–2451. [Google Scholar] [CrossRef]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.V.; Czeczko, N.G.; Malafaia, O.; Ribas, J.M.F.; Garcia, J.B.; Miguel, M.T.; Zini, C.; Massignan, A.G. Osteoarthritis model induced by intra-articular monosodium iodoacetate in rats knee. Acta Cir. Bras. 2016, 31, 765–773. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Tian, F.M.; Dai, M.W.; Wang, W.Y.; Shao, L.T.; Zhang, L. Are estrogen-related drugs new alternatives for the management of osteoarthritis? Arthritis Res. Ther. 2016, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, B.M.; Schiphof, D.; Groeneveld, F.P.; Koes, B.W.; van Osch, G.J.; van Meurs, J.B.; Bierma-Zeinstra, S.M. Limited evidence for a protective effect of unopposed oestrogen therapy for osteoarthritis of the hip: A systematic review. Rheumatology 2009, 48, 104–112. [Google Scholar] [CrossRef]
- Watt, F.E. Hand osteoarthritis, menopause and menopausal hormone therapy. Maturitas 2016, 83, 13–18. [Google Scholar] [CrossRef]
- Tanamas, S.K.; Wijethilake, P.; Wluka, A.E.; Davies-Tuck, M.L.; Urquhart, D.M.; Wang, Y.; Cicuttini, F.M. Sex hormones and structural changes in osteoarthritis: A systematic review. Maturitas 2011, 69, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, G.; Bean, A.C.; Iijima, H.; Jackson, N.; Thurston, R.C.; Ambrosio, F. Uncovering the “riddle of femininity” in osteoarthritis: A systematic review and meta-analysis of menopausal animal models and mathematical modeling of estrogen treatment. Osteoarthr. Cartil. 2023, 31, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.R.; Hu, Y.C.; Huang, T.C.; Hsieh, B.S.; Yeh, J.P.; Cheng, H.L.; Huang, L.W.; Chang, K.L. Vitamin C Protects Chondrocytes against Monosodium Iodoacetate-Induced Osteoarthritis by Multiple Pathways. Int. J. Mol. Sci. 2016, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S24–S34. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Leeming, D.J.; Dam, E.B.; Henriksen, K.; Alexandersen, P.; Pastoureau, P.; Altman, R.D.; Christiansen, C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. 2008, 16, 638–646. [Google Scholar] [CrossRef]
- Kumm, J.; Tamm, A.; Lintrop, M.; Tamm, A. Diagnostic and prognostic value of bone biomarkers in progressive knee osteoarthritis: A 6-year follow-up study in middle-aged subjects. Osteoarthr. Cartil. 2013, 21, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO); the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif. Tissue Int. 2019, 104, 235–238. [Google Scholar] [CrossRef]
- Gillett, M.J.; Vasikaran, S.D.; Inderjeeth, C.A. The Role of PINP in Diagnosis and Management of Metabolic Bone Disease. Clin. Biochem. Rev. 2021, 42, 3–10. [Google Scholar] [CrossRef]
- Eastell, R.; Pigott, T.; Gossiel, F.; Naylor, K.E.; Walsh, J.S.; Peel, N.F.A. Diagnosis of Endocrine Disease: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyere, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Milazzo, R.; Savasi, V.M.; Cetin, I. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. Int. J. Mol. Sci. 2021, 22, 1732. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Markers 2016, 2016, 4895050. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, Q.; Guo, P.; Huang, Y.; Ye, Z.; Hu, J. Antichondrocyte apoptosis effect of genistein in treating inflammationinduced osteoarthritis. Mol. Med. Rep. 2020, 22, 2032–2042. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, L. The protective activity of genistein against bone and cartilage diseases. Front. Pharmacol. 2022, 13, 1016981. [Google Scholar] [CrossRef]
- Gundogdu, G.; Demirkaya Miloglu, F.; Gundogdu, K.; Tasci, S.Y.; Albayrak, M.; Demirci, T.; Cetin, M. Investigation of the efficacy of daidzein in experimental knee osteoarthritis-induced with monosodium iodoacetate in rats. Clin. Rheumatol. 2020, 39, 2399–2408. [Google Scholar] [CrossRef]
- Toda, T.; Sugioka, Y.; Koike, T. Soybean isoflavone can protect against osteoarthritis in ovariectomized rats. J. Food Sci. Technol. 2020, 57, 3409–3414. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; Perez-Lopez, F.R.; Escobar, G.S.; Palla, G.; Montt-Guevara, M.; Cecchi, E.; Genazzani, A.R.; Simoncini, T.; Research Group for the Omega Women’s Health Project. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas 2014, 79, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Baudart, P.; Louati, K.; Marcelli, C.; Berenbaum, F.; Sellam, J. Association between osteoarthritis and dyslipidaemia: A systematic literature review and meta-analysis. RMD Open 2017, 3, e000442. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Anzai, Y.; Tanji, N.; Imaizumi, H.; Fujita, M.; Hayashi, M.; Abe, K.; Ohira, H. Association of equol with obesity in postmenopausal women. Menopause 2021, 28, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat. Clin. Pract. Rheumatol. 2006, 2, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five-year prospective cohort study. Arthritis Res. Ther. 2015, 17, 283. [Google Scholar] [CrossRef]
- Filkova, M.; Senolt, L.; Braun, M.; Hulejova, H.; Pavelkova, A.; Sleglova, O.; Kupka, K.; Gatterova, J.; Pavelka, K. Serum hyaluronic acid as a potential marker with a predictive value for further radiographic progression of hand osteoarthritis. Osteoarthr. Cartil. 2009, 17, 1615–1619. [Google Scholar] [CrossRef]
- Aigner, T.; Zhu, Y.; Chansky, H.H.; Matsen, F.A., 3rd; Maloney, W.J.; Sandell, L.J. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999, 42, 1443–1450. [Google Scholar] [CrossRef]
- Sharif, M.; Kirwan, J.; Charni, N.; Sandell, L.J.; Whittles, C.; Garnero, P. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis--association with disease progression. Rheumatology 2007, 46, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of Matrix Metalloproteinase (MMP) Gene Polymorphisms With Knee Osteoarthritis: A Review of the Literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed]
- Ping, S.H.; Tian, F.M.; Zhao, Z.M.; Liang, C.Y.; Liu, F.; Wang, Y.D.; Zhang, L. Protective effects of equol on the cartilage and subchondral bone in ovariectomized rats with osteoarthritis. Iran. J. Basic. Med. Sci. 2022, 25, 1228–1233. [Google Scholar]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 492–496. [Google Scholar] [CrossRef]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.L.; Pearsall, A.W.; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Remst, D.F.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
| Group (n = 9) | Before Treatment (g) | After Treatment (g) | Increase Rate (%) |
|---|---|---|---|
| Sham | 233.7 ± 8.7 | 317.7 ± 17.2 | 135.9 ± 1.3 e |
| Sham + S-equol | 240.3 ± 8.7 | 317.3 ± 13.7 | 132.0 ± 0.5 f |
| OVX | 231.7 ± 17.3 | 383.7 ± 15.8 | 165.9 ± 3.1 c |
| OVX + S-equol | 231.0 ± 10.2 | 376.3 ± 19.6 | 162.9 ± 0.7 c |
| OVX-M | 224.3 ± 6.9 | 400.0 ± 29.1 | 178.2 ± 4.2 ab |
| OVX-M + S-equol | 243.7 ± 6.8 | 380.0 ± 12.8 | 155.9 ± 0.5 d |
| OVX-S | 228.0 ± 5.9 | 400.3 ± 13.5 | 175.5 ± 0.8 b |
| OVX-S+ S-equol | 230.0 ± 5.1 | 405.5 ± 4.5 | 179.0 ± 1.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-C.; Huang, T.-C.; Huang, L.-W.; Cheng, H.-L.; Hsieh, B.-S.; Chang, K.-L. S-Equol Ameliorates Menopausal Osteoarthritis in Rats through Reducing Oxidative Stress and Cartilage Degradation. Nutrients 2024, 16, 2364. https://doi.org/10.3390/nu16142364
Hu Y-C, Huang T-C, Huang L-W, Cheng H-L, Hsieh B-S, Chang K-L. S-Equol Ameliorates Menopausal Osteoarthritis in Rats through Reducing Oxidative Stress and Cartilage Degradation. Nutrients. 2024; 16(14):2364. https://doi.org/10.3390/nu16142364
Chicago/Turabian StyleHu, Yu-Chen, Tzu-Ching Huang, Li-Wen Huang, Hsiao-Ling Cheng, Bau-Shan Hsieh, and Kee-Lung Chang. 2024. "S-Equol Ameliorates Menopausal Osteoarthritis in Rats through Reducing Oxidative Stress and Cartilage Degradation" Nutrients 16, no. 14: 2364. https://doi.org/10.3390/nu16142364
APA StyleHu, Y.-C., Huang, T.-C., Huang, L.-W., Cheng, H.-L., Hsieh, B.-S., & Chang, K.-L. (2024). S-Equol Ameliorates Menopausal Osteoarthritis in Rats through Reducing Oxidative Stress and Cartilage Degradation. Nutrients, 16(14), 2364. https://doi.org/10.3390/nu16142364







