Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- (a)
- Studies that combined eggshell membrane with other nutraceuticals;
- (b)
- Studies lacking necessary quantitative data for the meta-analysis, such as means and standard deviations of the measured outcomes;
- (c)
- Studies without a control group;
- (d)
- Studies where patients did not have knee joint pain or were not diagnosed with osteoarthritis;
- (e)
- Non-original articles such as case reports, editorials, opinion pieces, and reviews; and
- (f)
- Duplicate studies, referring to multiple publications reporting on the same study or patient cohort.
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Items and Quality Assessment
2.5. Synthesis Methods
3. Results
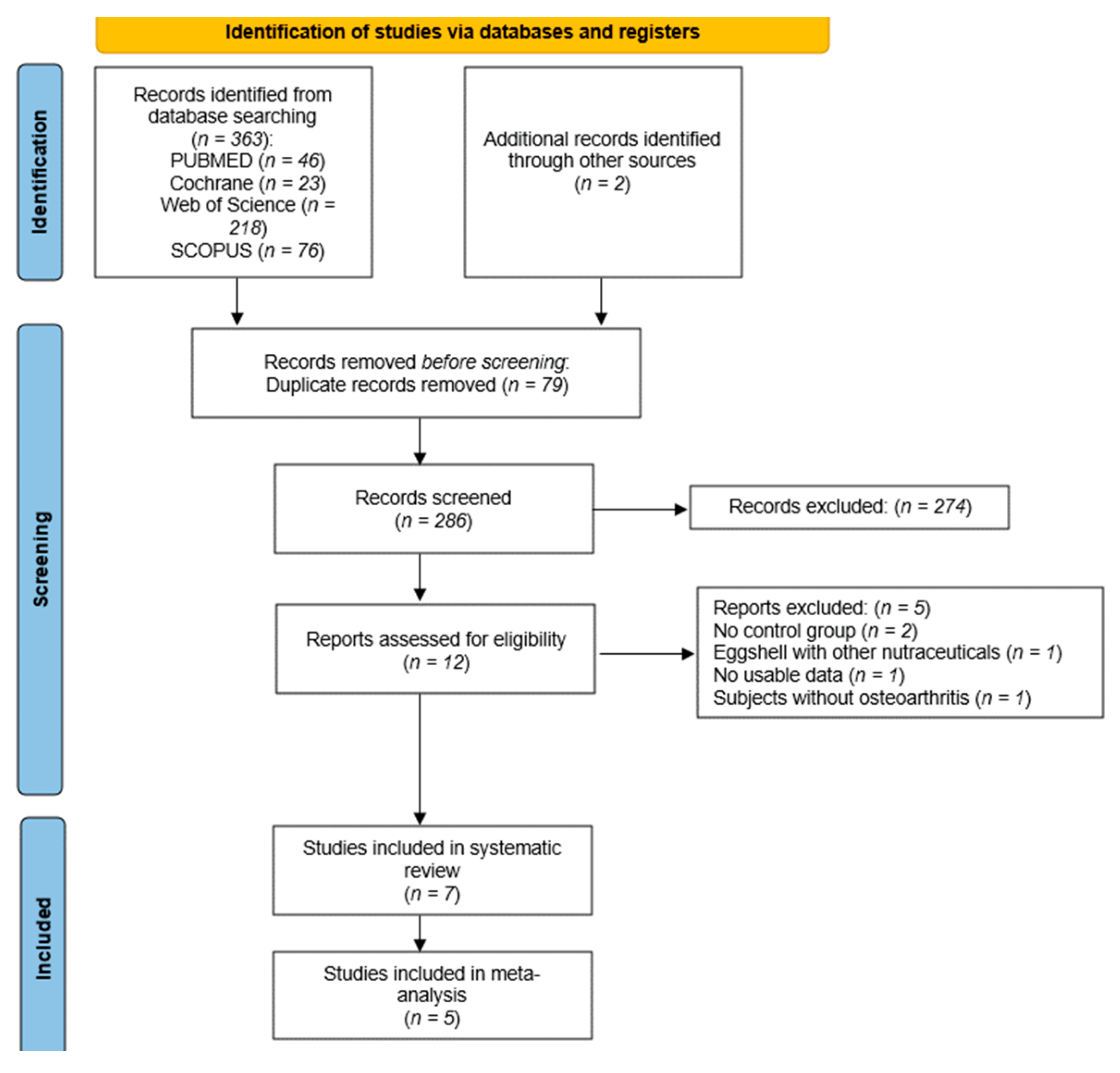
3.1. Study Selection
3.2. Study Characteristics
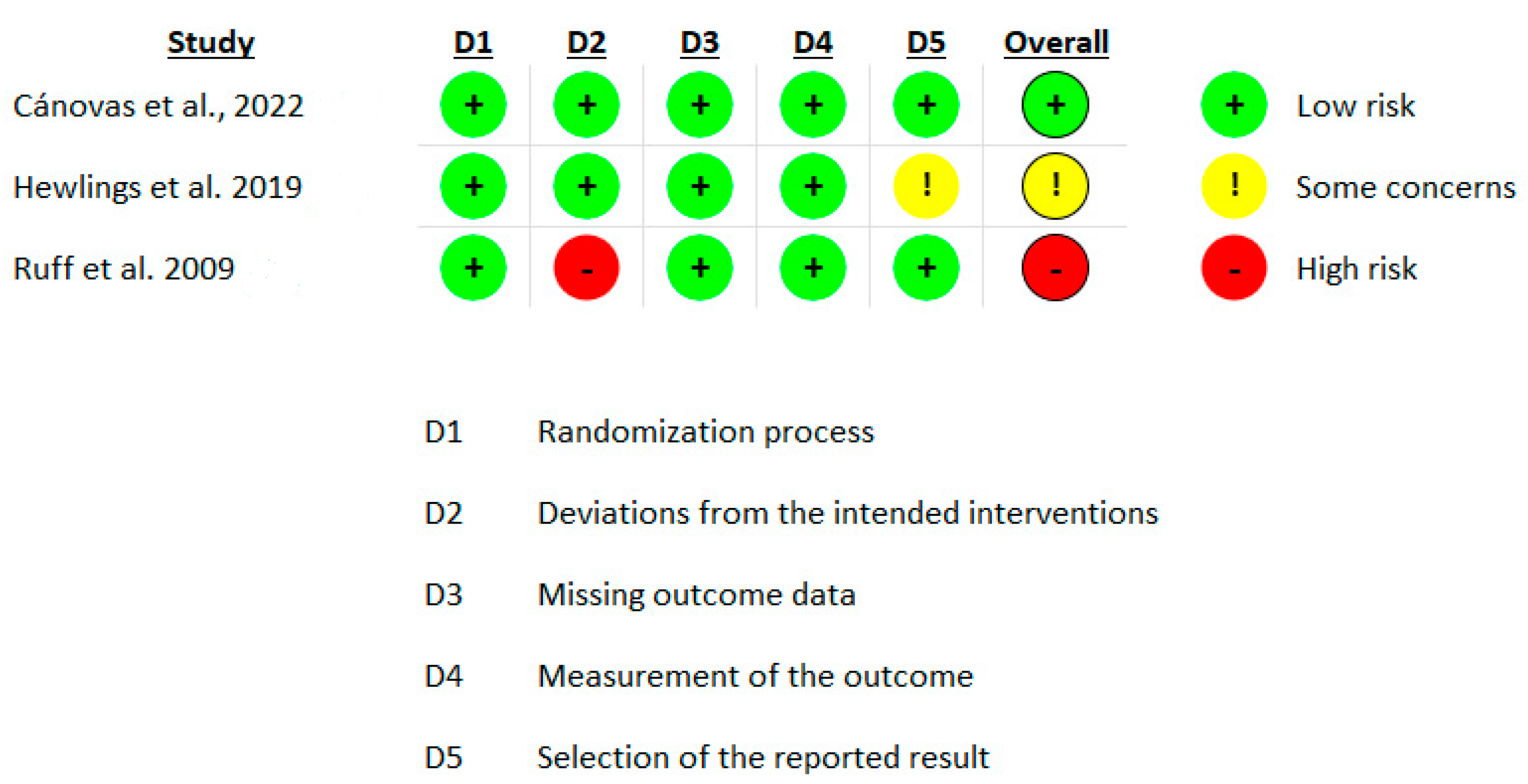
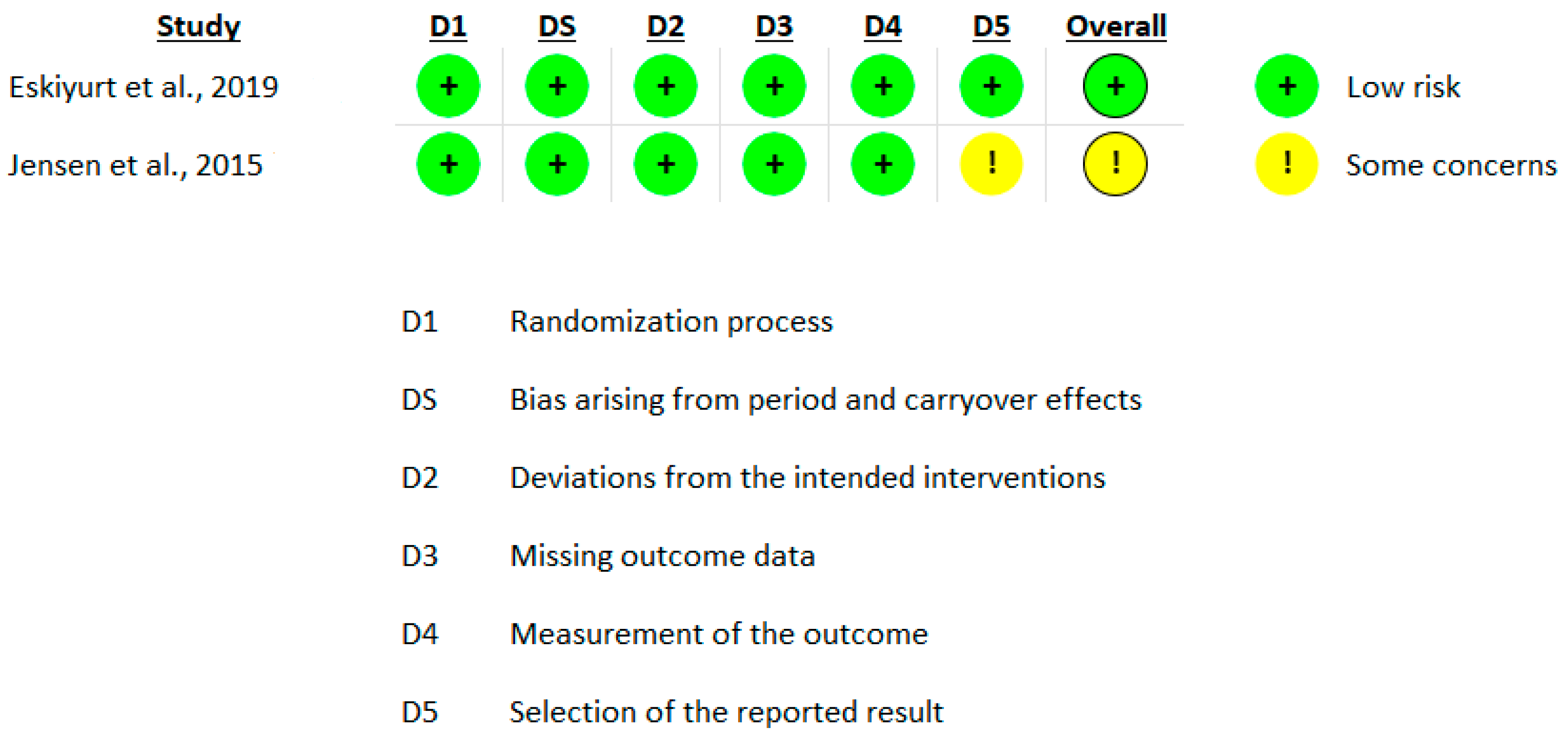
3.3. Risk of Bias in Included Studies
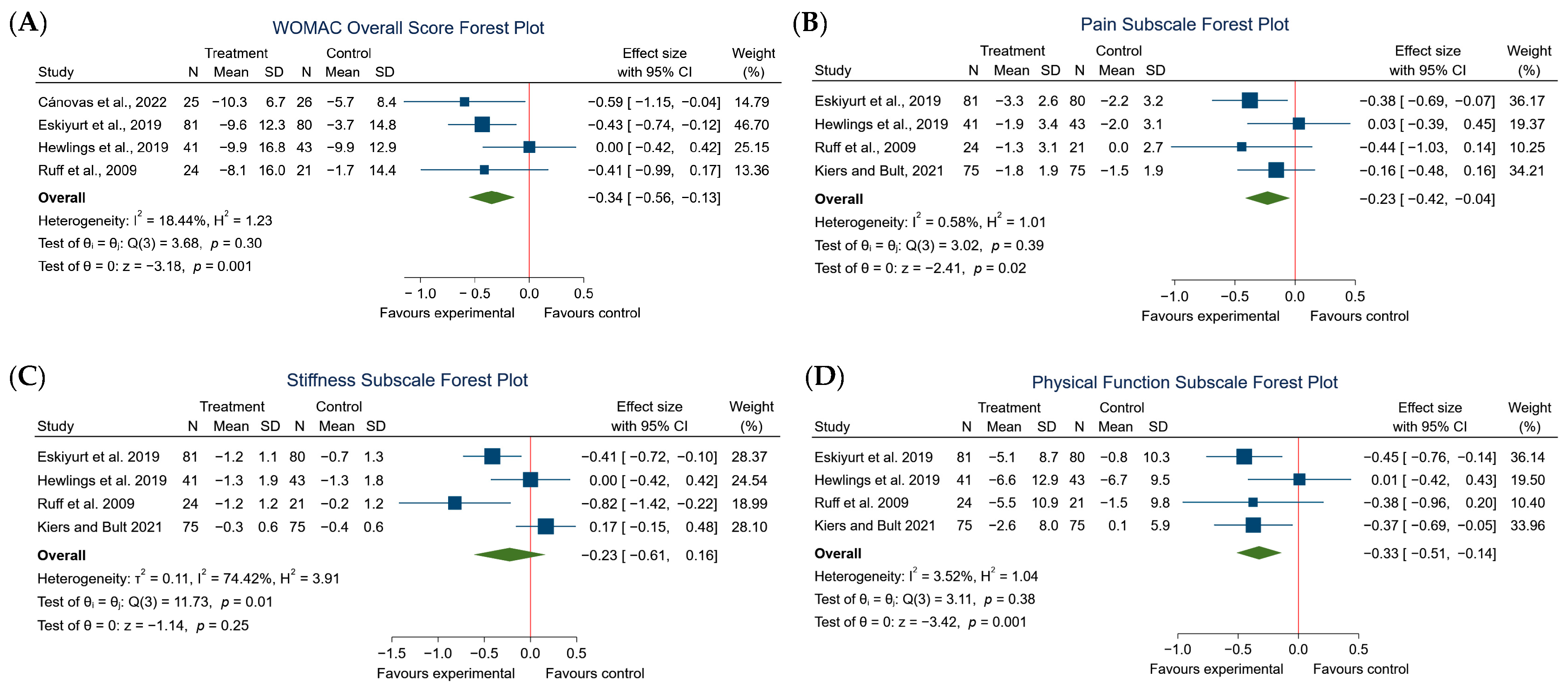
3.4. Effects of the Intervention
3.5. Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee Osteoarthritis Has Doubled in Prevalence since the Mid-20th Century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Rheumatoid Arthritis—Level 3 Cause|Institute for Health Metrics and Evaluation. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-rheumatoid-arthritis-level-3-disease (accessed on 23 July 2024).
- Chen, X.; Tang, H.; Lin, J.; Zeng, R. Temporal Trends in the Disease Burden of Osteoarthritis from 1990 to 2019, and Projections until 2030. PLoS ONE 2023, 18, e0288561. [Google Scholar] [CrossRef]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain. Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Chaplin, S. NICE on the Diagnosis and Management of Osteoarthritis. Prescriber 2023, 34, 15–16. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis. Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Kiers, J.L.; Bult, J.H.F. Mildly Processed Natural Eggshell Membrane Alleviates Joint Pain Associated with Osteoarthritis of the Knee: A Randomized Double-Blind Placebo-Controlled Study. J. Med. Food 2021, 24, 292–298. [Google Scholar] [CrossRef] [PubMed]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of Collagen Supplementation on Osteoarthritis Symptoms: A Meta-Analysis of Randomized Placebo-Controlled Trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, Y.; Shi, L.; Chen, H.; Li, L.; Ning, Z.; Zeng, D.; Wang, D. Advances in Eggshell Membrane Separation and Solubilization Technologies. Front. Vet. Sci. 2023, 10, 1116126. [Google Scholar] [CrossRef]
- Nakano, T.; Ikawa, N.; Ozimek, L. Chemical Composition of Chicken Eggshell and Shell Membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, F.; Abellán-Ruíz, M.S.; García-Muñoz, A.M.; Luque-Rubia, A.J.; Victoria-Montesinos, D.; Pérez-Piñero, S.; Sánchez-Macarro, M.; López-Román, F.J. Randomised Clinical Trial to Analyse the Efficacy of Eggshell Membrane to Improve Joint Functionality in Knee Osteoarthritis. Nutrients 2022, 14, 2340. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D.; Schneider, L.V. A Randomized, Double-Blind, Placebo-Controlled, Prospective Clinical Trial Evaluating Water-Soluble Chicken Eggshell Membrane for Improvement in Joint Health in Adults with Knee Osteoarthritis. J. Med. Food 2019, 22, 875–884. [Google Scholar] [CrossRef]
- Ruff, K.J.; Winkler, A.; Jackson, R.W.; DeVore, D.P.; Ritz, B.W. Eggshell Membrane in the Treatment of Pain and Stiffness from Osteoarthritis of the Knee: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Clinical Study. Clin. Rheumatol. 2009, 28, 907–914. [Google Scholar] [CrossRef]
- Kannan, R.; Bakthavatchalam, S.; Murugesan, S.; Kumar, B.; Deb, B.; Marimuthu, C.; Rajendran, P. A Randomized, Open-Label, Multicentered Parallel-Group Clinical Study to Evaluate the Efficacy and Safety of Joint CoreTM Compared to Jointace DNTM in Osteoarthritis Patients. J. Curr. Res. Sci. Med. 2022, 8, 44–51. [Google Scholar]
- Kulshreshtha, G.; Diep, T.; Hudson, H.-A.; Hincke, M.T. High Value Applications and Current Commercial Market for Eggshell Membranes and Derived Bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-C.; Zhao, J.-Y.; Ahn, D.U.; Jin, Y.-G.; Huang, X. Separation and Identification of Highly Efficient Antioxidant Peptides from Eggshell Membrane. Antioxidants 2019, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 13 February 2023).
- Brunello, E.; Masini, A. NEM Brand Eggshell Membrane Effective in the Treatment of Pain and Stiffness Associated with Osteoarthritis of the Knee in an Italian Study Population. Int. J. Clin. Med. 2016, 7, 169–175. [Google Scholar] [CrossRef]
- Danesch, U. NEM Brand Eggshell Membrane Effective in the Treatment of Pain Associated with Knee and Hip Osteoarthritis: Results from a Six Center, Open Label German Clinical Study. J. Arthritis 2014, 3, 136. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell Membrane: A Possible New Natural Therapeutic for Joint and Connective Tissue Disorders. Results from Two Open-Label Human Clinical Studies. Clin. Interv. Aging, 2009; 4, 235–240. [Google Scholar] [CrossRef]
- Ruff, K.J.; Morrison, D.; Duncan, S.A.; Back, M.; Aydogan, C.; Theodosakis, J. Beneficial Effects of Natural Eggshell Membrane versus Placebo in Exercise-Induced Joint Pain, Stiffness, and Cartilage Turnover in Healthy, Postmenopausal Women. Clin. Interv. Aging 2018, 13, 285–295. [Google Scholar] [CrossRef]
- Eskiyurt, N.; Sarıdoğan, M.; Şenel, K.; Günaydın, R.; Erdal, A.; Özyiğit, E.; Akarırmak, Ü.; Şendur, Ö.; Barut, K.; Akyuz, G.; et al. Efficacy and Safety of Natural Eggshell Membrane (NEM®) in Patients with Grade 2/3 Knee Osteoarthritis: A Multi-Center, Randomized, Doubleblind, Placebo-Controlled, Single-Crossover Clinical Study. J. Am. Helicopter Soc. 2019, 8, 1000285. [Google Scholar]
- Jensen, G.S.; Lenninger, M.R.; Beaman, J.L.; Taylor, R.; Benson, K.F. Support of Joint Function, Range of Motion, and Physical Activity Levels by Consumption of a Water-Soluble Egg Membrane Hydrolyzate. J. Med. Food 2015, 18, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Casado-Santos, A.; La Nuez-García, M.A.; Álvarez-Rodríguez, P.; González-Cubero, E.; González-Rodríguez, Y.; Luisa González-Fernández, M.; Villar-Suárez, V. Anti-Inflammatory and Regenerative Effects of MKARE® Eggshell Membrane: An in vitro Osteoarthritis Model and Placebo-Controlled Clinical Study. J. Funct. Foods 2024, 116, 106119. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Moseng, T.; Vlieland, T.P.M.V.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Effect of Weight Reduction in Obese Patients Diagnosed with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 2007, 66, 433–439. [Google Scholar] [CrossRef]
- Wang, H. The Potential of Collagen Treatment for Comorbid Diseases. Polymers 2023, 15, 3999. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef]

| Authors | Country | N | Women n (%) | Age (Years) | BMI kg/m2 | Design RCT | Dosage (mg) | Duration | Disease Severity | Outcome | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cánovas et al. (2022) [19] | Spain | HD = 25 LD = 24 CG = 26 | 52.0 | 38.4 | 25.1 | Double blind, parallel | HD = 500 LD = 300 | 8 weeks | ACR functional grades I-III | WOMAC and VAS | For VAS scale, all groups showed a statistically significant decrease in pain perception at the end of the study. In particular, the high-dose group showed a statistically significant reduction in pain compared to the control group. Regarding the WOMAC results, all groups showed a significant decrease in the WOMAC score, indicating an improvement in functional capacity and quality of life. | Eggshell membrane effectively reduced knee pain and stiffness in osteoarthritis patients, with the response being dose dependent. |
| Eskiyurt et al. (2019) [34] | Turkey | IG = 81 CG = 80 | 83.8 | 57.2 | 29.5 | Double blind, crossover | 500 | 90 days | Kellgren-Lawrence grade 2 and 3 | WOMAC | Significant improvements in WOMAC scores (pain, stiffness, function) were observed in the NEM group within 7 to 30 days. The percentage of subjects experiencing greater decreases in WOMAC—pain score was significantly higher in the 90-day NEM group compared to the 60-day group. After 90 days, the original placebo group showed marked clinical improvement upon addition of NEM, resulting in no significant difference in WOMAC scores compared to the original NEM group. | NEM was effective in providing rapid and persistent clinically meaningful improvements in the WOMAC scores for subjects with moderate-to-severe osteoarthritis of the knee. The study also confirmed that NEM was safe and well tolerated, with no occurrence of serious adverse events. |
| Hewlings et al. (2019) [20] | USA | IG = 41 CG = 43 | 72.0 | 53.3 | 28.2 | Double blind, parallel | 450 | 84 days | ACR criteria (minimum 3 criteria) | WOMAC | The change in the composite WOMAC score was statistically different from baseline in the Study Product cohort at all subsequent visits, 3 (day 5), 4 (day 28), 5 (day 57), and 6 (day 86) by t-test. The mean change from baseline in the Placebo cohort was not statistically different from baseline in visit 3 (day 5). It was statistically different from baseline in visits 4 (day 28), 5 (day 57), and 6 (day 86) by t-test. | The study concluded that the consumption of eggshell membrane showed significant improvement in physical performance, mobility, and joint stiffness within 5 days when compared with a placebo. These improvements were maintained over the 12-week study period. The study also confirmed the safety of product, with no observed human safety concerns. |
| Ruff et al. (2009) [21] | USA | IG = 29 CG = 31 | NR | NR | NR | Double blind, parallel | 500 | 60 days | ACR functional grades I–III | WOMAC | The study found that NEM® supplementation significantly improved pain and stiffness scores compared to placebo at all time points. Rapid improvements were observed within just 10 days of supplementation. While function and overall WOMAC scores also showed improvement, these did not reach statistical significance. The beneficial effects on pain and stiffness were maintained or further improved at the 60-day mark. | The conclusions of the study suggest that eggshell membrane supplementation could be an effective and safe option for the relief of discomfort and inflexibility associated with knee osteoarthritis. The supplement was found to provide rapid relief, with significant improvements observed as early as 10 days after the start of the treatment. |
| Kiers and Bult (2021) [15] | Netherlands | IG = 75 CG = 75 | 53.3 | 63.4 | NR | Double blind, parallel | 300 | 84 days | Positive OA diagnosis with self-reported knee pain score ≥ 3 and <9 | KOOS and NSR-P | KOOS scores were similar for both the eggshell membrane and placebo groups at the start of the study. However, the effect was significant for two of the five KOOS category scores, namely “Pain” and “Daily Life” functioning, showing long-lasting improvement of 5–8 points on a 0–100 scale of complaint categories. The pain relief effects maximized after 3 weeks and decreased only slightly until measurements finished in week 12. NRS-P scores decreased at a similar rate for both groups during the first six weeks of treatment. | The study concludes that the eggshell membrane extract appears to be effective in alleviating pain and improving daily life functioning in individuals with knee osteoarthritis. The beneficial effects were observed across multiple categories of KOOS scale, indicating a potential for broad impact on quality of life. However, these effects were not mirrored in the results from NRS-P, suggesting that the specific benefits of eggshell membrane may be more nuanced than general pain relief. |
| Jensen et al. (2015) [35] | USA | IG = 13 CG = 12 | 63.0 | 52.5 | 30.0 | Double blind, crossover | 450 | 4 weeks | Mild to moderate physical limitations | VAS | Participants who had been experiencing chronic pain in their knees (among other joints) for at least 6 months reported significant improvements in their condition after consuming water-soluble eggshell membrane. This suggests that water-soluble eggshell membrane may be beneficial in reducing knee pain in individuals with chronic joint conditions. | The consumption of water-soluble egg membrane was associated with significant improvements in joint function, comfort during daily activities, and increased physical activity. Notably, significant improvements in the range of motion were observed for the neck, shoulders, back, hips, knees, and ankles during water-soluble egg membrane consumption compared to placebo. |
| Casado-Santos et al. (2024) [36] | Spain | IG = 19 CG = 18 | 56.0 | 51.4 | 29.4 | Double blind, parallel | 300 | 60 days | Positive OA diagnosis with symptoms of pain, stiffness, or functionality problems | WOMAC | The RM-ANOVA analysis of variance showed a significant overall improvement in patients treated with MKARE®, and ESM-RT compared to day 0 in both 30- and 60-days timespans. | MKARE® effectively reduced knee pain and improved physical function in osteoarthritis patients, with significant improvements observed over the 60-day study period. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Muñoz, A.M.; Abellán-Ruiz, M.S.; García-Guillén, A.I.; Victoria-Montesinos, D. Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2640. https://doi.org/10.3390/nu16162640
García-Muñoz AM, Abellán-Ruiz MS, García-Guillén AI, Victoria-Montesinos D. Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(16):2640. https://doi.org/10.3390/nu16162640
Chicago/Turabian StyleGarcía-Muñoz, Ana María, María Salud Abellán-Ruiz, Ana Isabel García-Guillén, and Desirée Victoria-Montesinos. 2024. "Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis" Nutrients 16, no. 16: 2640. https://doi.org/10.3390/nu16162640
APA StyleGarcía-Muñoz, A. M., Abellán-Ruiz, M. S., García-Guillén, A. I., & Victoria-Montesinos, D. (2024). Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients, 16(16), 2640. https://doi.org/10.3390/nu16162640