Abstract
Colostrum is a nutritious milk synthesized by mammals during the postpartum period, and its rich bioactive components has led to a global increase in the consumption of bovine colostrum as a supplement. Bovine colostrum contains key components such as immunoglobulins, oligosaccharides, lactoferrin and lysozyme. It is a special supplement source due to its natural, high bioavailability and high concentrations of growth factors. Growth factors are critical to many physiological functions, and considering its presence in the colostrum, further research must be conducted on its safe application in many bodily disorders. Growth factors contribute to wound healing, muscle and bone development, and supporting growth in children. Additionally, the molecular mechanisms have been explored, highlighting the growth factors roles in cell proliferation, tissue regeneration, and the regulation of immune responses. These findings are crucial for understanding the potential health effects of bovine colostrum, ensuring its safe use, and forming a basis for future clinical applications. This review article examines the growth factors concentration in bovine colostrum, their benefits, clinical studies, and molecular mechanisms.
1. Introduction
Colostrum is a pre-milk substance that is essential for the health and development of newborn mammals. This special pre-milk offers various positive effects thanks to its bioactive components. For example, it enhances athletic performance, aids in the healing of muscle injuries, helps with the development of immunity in newborns, and promotes muscle mass gain [1]. Colostrum’s composition closely resembles that of blood and differs markedly from milk. It contains a range of nutrients, including biologically active compounds and proteins, lipids, lactose, and vital fatty acids [2].
The various bioactive components of colostrum positively affect the body in different ways. For example, growth factors play a role in important metabolic activities such as wound healing, bone and muscle development, and cell proliferation [3,4,5].
Growth factors can vary in concentration depending on their source. Both regular milk and colostrum contain many peptide growth factors that help mammalian cells grow and differentiate. A study reported that within the first 10 h after birth, colostrum contains the optimal concentration of growth factors, shown in Table 1, although this timeframe may vary depending on the source and environmental factors [6]. The two main growth factors, insulin-like growth factors 1 and 2 (IGF-1 and 2) and transforming growth factors alpha and beta (TGF-A and B), are only found in colostrum (Figure 1). These growth factors exhibit remarkable biochemical attributes that contribute to muscle repair and wound healing [7]. Consuming bovine colostrum (BC) may be more effective compared to taking growth factor supplements orally, because BC contains a variety of bioactive components that work synergistically to promote health Also, applying growth factor supplementation topically and systemically rather than orally increases its effectiveness; a study reported that growth factors are degraded by digestive enzymes [8]. In this review article, various types of growth factors found in BC and their health benefits were examined.
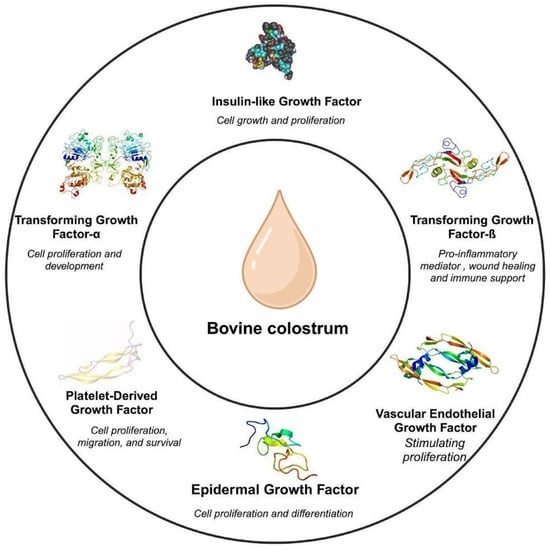
Figure 1.
Growth factors in bovine colostrum [2,9].
Figure 1.
Growth factors in bovine colostrum [2,9].
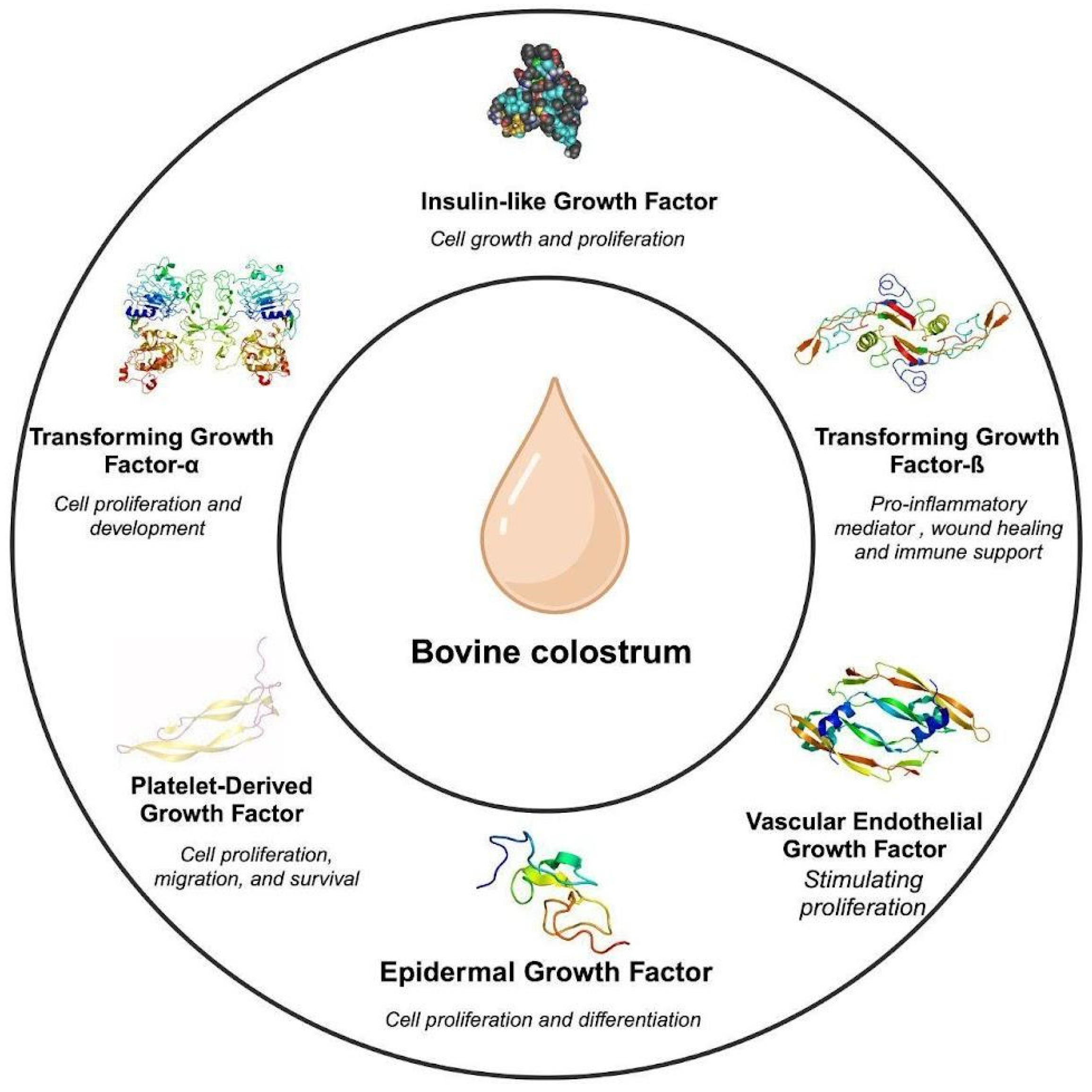

Table 1.
Growth factor concentrations and types in BC.
Table 1.
Growth factor concentrations and types in BC.
| Growth Factor | Concentration in BC (ng/mL) | Concentration in Bovine Milk(ng/mL) | Properties | Reference |
|---|---|---|---|---|
| EGF | 324.2 | 155 (pasteurized milk) | Cell proliferation, survival, and differentiation | [10,11] |
| IGF-1 | 870 | 150 | Cell growth and proliferation | [6,12] |
| IGF-2 | 206 | 2–6 | Regulates cell proliferation and survival | [12,13,14] |
| TGF-β | 100.7 | 4.3 (pasteurized milk) | Pro-inflammatory mediator, stimulating the activation and migration of immune cells and wound healing | [15,16] |
| TGF-α | 200 | - | Cell proliferation, differentiation, and development | [17,18] |
| PDGF | - | - | Cell migration, proliferation, and survival | [19] |
2. Exploring the Growth Factors: Types and Molecular Mechanisms
Bovine colostrum is a rich supplement in terms of growth factor concentrations and the types it contains. Clinical studies have demonstrated the effects of administering growth factors derived from bovine milk or bovine colostrum in various conditions such as diseases and injuries [1,9].
Growth factors have an important role in fundamental cellular processes, including growth, proliferation, differentiation, and survival. They achieve this intricate control by binding to specific receptors on the cell surface, initiating a cascade of intracellular signaling events. This intricate signaling network stimulates the regulation of gene expression and cellular functions, directing the cell’s behavior. Epidermal Growth Factor (EGF), TGF-β, platelet-derived growth factor, and IGFs are the main types of growth factors.
2.1. Epidermal Growth Factor (EGF)
EGF is a compact protein consisting of approximately 53 amino acids categorized within the EGF ligand family [20]. Members of this ligand family, including EGF, exhibit structural resemblances and interact with a group of receptors known as ErbB receptors, among which EGFR stands out as extensively studied [21]. Numerous cell types, including fibroblasts, endothelial cells, and epithelial cells, produce EGF [22]. Typically, it is generated as a transmembrane precursor and subsequently undergoes cleavage by specific proteases. This process liberates the mature, active EGF ligand into the extracellular environment [23]. EGF exhibits high-affinity binding to the extracellular domain of EGFR. This binding pocket within EGFR is formed by loops and β-sheets, creating a specific docking site for EGF [24]. When EGF binds to EGFR, the receptor’s conformation changes significantly, exposing previously hidden areas and facilitating the initiation of further signaling pathways [24]. This binding triggers the receptor to form dimers and phosphorylate tyrosine residues in its cytoplasmic domain [10]. This phosphorylation activates pathways including Ras/MAPK and PI3K/Akt, which control cell proliferation, survival, and differentiation [25,26]. It is worth noting that EGF is just one member of a larger EGF ligand family. Each ligand may have slightly different binding affinities and can activate distinct signaling pathways within the cell depending on the specific ErbB receptor it interacts with [27]. This adds complexity and potential for tailored cellular responses. The existence of EGF-like domains in other proteins suggests possible communication between various signaling pathways that involve the ErbB receptor family [28].
2.2. Transforming Growth Factor Beta (TGF-β)
The TGF-β superfamily, of which there are three members (TGF-β1, TGF-β2, and TGF-β3), includes the cytokine TGF-β. Within cells, TGF-β molecules are first generated as precursor proteins [29]. These precursors undergo cleavage to form mature TGF-β ligands, which remain bound to a latency-associated peptide (LAP) in a non-covalent manner [30,31]. This association prevents the premature activation of TGF-β signaling [31]. The release and activation of the TGF-β-LAP complex require additional processing, which can happen through different mechanisms such as proteolytic cleavage or interaction with integrins (cell surface receptors) or thrombospondin-1 (a matrix protein) [32]. After activation, the mature TGF-β ligand exhibits strong binding to specific transmembrane TGF-β receptors (TGFBRs) located on the cell surface [33]. The TGFBRs undergo a conformational shift brought on by ligand interaction, which causes them to dimerize. Signal transduction requires this dimerization, and the dimerized TGFBRs phosphorylate particular Smad proteins (Smad2 and Smad3) inside the cell [33]. Subsequently, these phosphorylated Smads form heterodimers with Smad4, a shared partner [33]. Once within the nucleus, the Smad complexes interact with co-activators or DNA to regulate gene expression and trigger biological reactions [34].
2.3. Platelet-Derived Growth Factor (PDGF)
PDGF is present in four isoforms, namely, PDGF-AA, PDGF-AB, PDGF-BB, and PDGF-DD, each composed of homodimers or heterodimers of A and B chains [19]. These isoforms attach to particular PDGF receptors (PDGFRs) on the cell surface. PDGFRα and PDGFRβ are two types of PDGFRs that are members of the receptor tyrosine kinase (RTK) family [19]. PDGF-AA predominantly interacts with PDGFRα, whereas PDGF-BB binds to both PDGFRα and PDGFRβ. PDGF-AB can bind to either receptor. Upon the binding of the ligand, the PDGFR undergoes a conformational change [19]. Ligand binding triggers the activation and dimerization of PDGFRs, involving homodimers (α-α or β-β) or heterodimers (α-β) [19]. This dimerization process provides a binding site for intracellular signaling molecules, initiating downstream signaling pathways. PDGFR activation triggers multiple downstream signaling pathways, such as the Ras-MAPK (Figure 2), PI3K-Akt (Figure 2), and PLCγ pathways, depending on the specific receptor and cellular context [35]. Eventually, these pathways result in a variety of biological reactions, such as cell migration, proliferation, and survival.
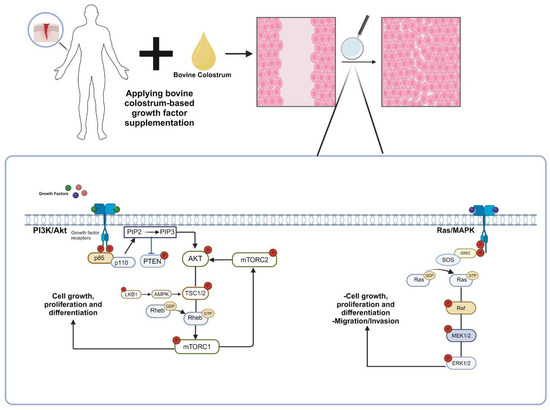
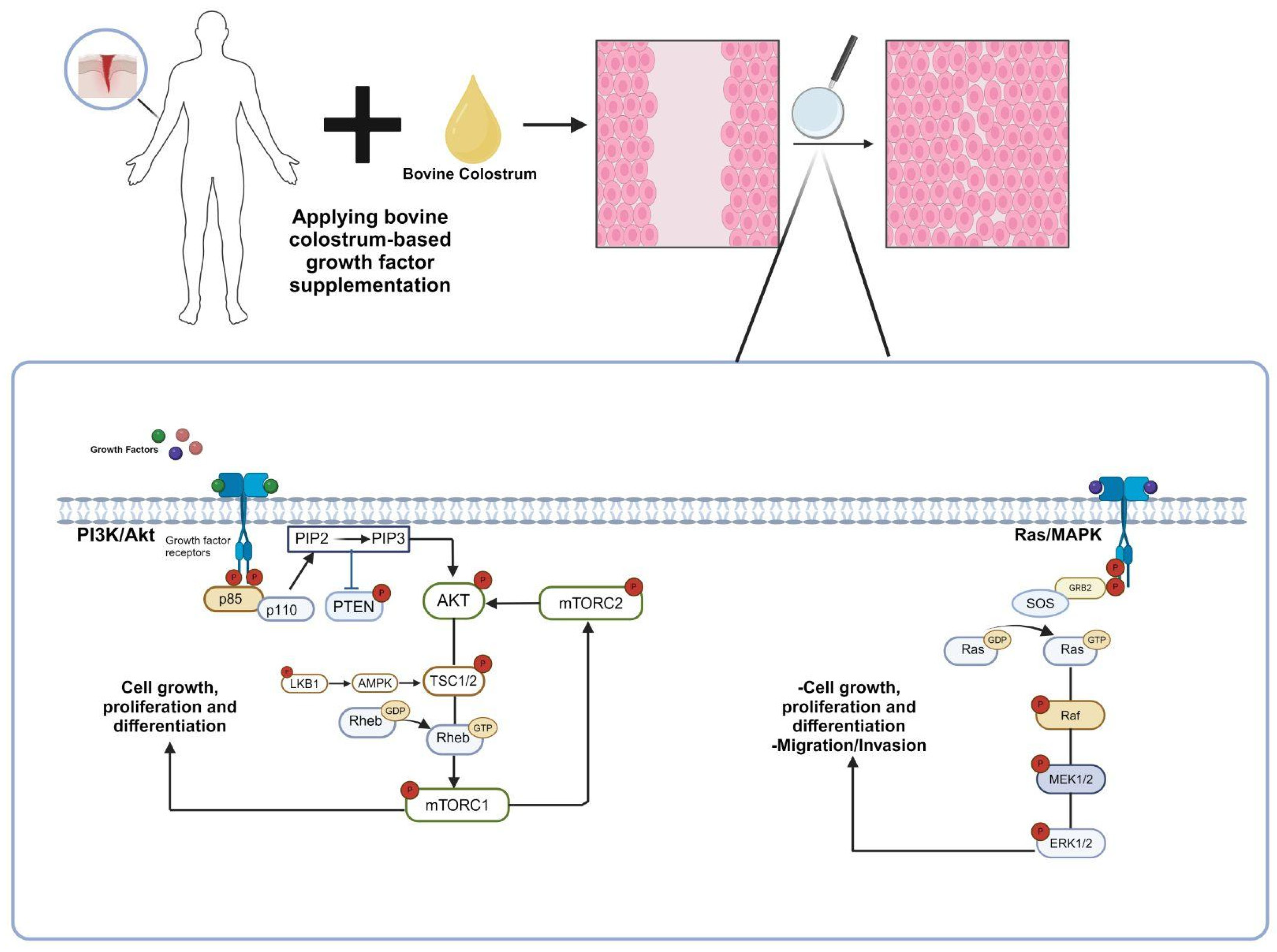
Figure 2.
The molecular mechanism of the contribution of growth factors found in bovine colostrum to wound healing. Growth factors found in bovine colostrum stimulate the Ras/MAPK and PI3K/Akt signaling pathways through receptor binding, promoting the proliferation and differentiation of cells in the affected area. Consequently, the wound-healing process is accelerated [25,26]. (p85: regulatory subunit of phosphatidylinositol 3-kinase, p110: catalytic subunit of phosphatidylinositol 3-kinase, PTEN: phosphatase and tensin homolog, Akt: protein kinase B, PIP2: phosphatidylinositol (4,5)-bisphosphate, PIP3: phosphatidylinositol (3,4,5)-trisphosphate, TSC: tuberous sclerosis complex, Rheb: Ras homolog enriched in brain, mTORC1: mechanistic target of rapamycin complex, LKB1: liver kinase B1, AMPK1: AMP-activated protein kinase, GRB2: growth factor receptor-bound protein 2, SOS: Son of Sevenless, Ras: rat sarcoma, Raf: rapidly accelerated fibrosarcoma, MEK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinase).
2.4. Insulin-Like Growth Factors (IGFs)
IGFs bind to specific cell surface receptors, primarily the IGF-1 receptor (IGF1R), and to a lesser extent, the insulin receptor (IR) [36]. IGF1R is a transmembrane receptor tyrosine kinase (RTK) and associated with several intracellular pathways, including the RAS-MAPK and PI3K-AKT (Figure 2) [37]. Certain tyrosine residues in the receptor’s cytoplasmic domain become autophosphorylated when IGF binds, causing the receptor to undergo a conformational shift [38]. The phosphorylated tyrosine residues on IGF1R serve as docking sites for adaptor proteins containing Src homology 2 (SH2) domains [39]. Upon binding to these sites, adaptor proteins, including Grb2 and Shc, become activated, starting downstream signaling pathways [40]. This leads to the activation of major signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, which regulates cell proliferation and survival, and the PI3K/Akt/mTOR pathway (Figure 2), which is involved in cell growth and metabolism [12].
It should be noted that the molecular mechanisms of growth factors, as indicated in Figure 2 and discussed in the text, can lead to side effects if used improperly. For instance, uncontrolled cell proliferation may contribute to tumor formation. Therefore, consulting experts in the field of growth factor supplementation is crucial.
3. Clinical Trials and Effects of Growth Factors on Various Diseases
Growth factors are essential for many physiological functions, including accelerating the healing of wounds by stimulating tissue repair and cell proliferation. Additionally, the reason for using BC as a source of growth factors is its high bioavailability and synergistic activity. Research studies have reported results supporting this phenomenon [41,42]. Their ability to promote the regeneration of injured tissues makes them promise for the treatment of musculoskeletal injuries and disorders like osteoarthritis. Furthermore, growth factors strengthen the body’s defenses against infections and illnesses by increasing the generation and function of immune cells. Studies indicate that their advantages surpass immune support, pointing to wider uses in maintaining health and managing illnesses. In this part, molecular mechanisms of various types of GF and their applications are discussed.
3.1. Wound Healing
EGF and TGF-β1 are critical factors in wound healing, each playing distinct but complementary roles. EGF stimulates granulation tissue development, decreases inflammation, and encourages re-epithelialization to speed up wound closure [43]. In a clinical study, it was reported that EGF derived from bovine colostrum plays a role in the healing process of intestinal epithelial wounds [44]. On the other hand, TGF-β1 initially acts as a pro-inflammatory mediator, stimulating immune cell activation and migration. As the healing process progresses, TGF-β1 transitions to an anti-inflammatory role crucial for tissue repair and inflammation resolution [15].
Adding to these critical growth factors, PDGF orchestrates essential processes in wound healing. PDGF functions as a powerful chemoattractant, drawing fibroblasts, neutrophils, monocytes, and smooth muscle cells to the injury site [3,45]. This recruitment is vital for initiating the repair process and laying the foundation for tissue regeneration. Additionally, PDGF activates macrophages, triggering them to release growth factors that further stimulate healing mechanisms [3,45]. Furthermore, PDGF promotes the proliferation of fibroblasts, increasing their numbers within the wound area. Fibroblasts are crucial for producing the extracellular matrix (ECM), which provides structural support and scaffolding for tissue repair [46]. By enhancing ECM production, PDGF significantly contributes to the formation of new tissue and the restoration of tissue integrity.
The IGF family, in addition to EGF, TGF-β, and PDGF, plays a major role in the intricate series of processes involved in wound healing. IGFs stimulate epithelial cell migration and proliferation, which improves re-epithelialization, an essential stage of the healing process that replaces the skins or mucosal protective layer over the wound site [47,48]. Furthermore, IGFs stimulate the proliferation of fibroblasts, which are pivotal in producing collagen and other components of the ECM [48]. This activity is crucial for providing structural support to the healing tissue and promoting wound contraction. Understanding the collaborative roles of EGF, TGF-β1, IGF, and PDGF underscores their potential as targeted therapies to optimize wound healing outcomes. Using a mouse excisional wound model, it was shown that treatment with bovine milk extracellular vesicles (EVs) promoted re-epithelialization, activated angiogenesis, and enhanced extracellular matrix maturation [49]. Together, they effectively manage inflammatory responses, promote tissue regeneration, and accelerate the overall healing process.
3.2. Gastrointestinal Health
IGF-1 plays a critical role in enhancing intestinal cell growth and supporting overall gut health. Research has demonstrated its ability to increase nutrient absorption, promote mucosal growth, and boost intestinal weight in studies involving piglets [50,51]. These findings suggest that IGF-1 could be a beneficial therapeutic option for improving intestinal structure and function. IGF-1 also shows promise in protecting against cytokine-induced apoptosis, which helps to maintain the integrity of intestinal epithelial cells and reduces damage during inflammation [52]. This dual action of promoting growth and protecting against inflammation underscores IGF-1’s potential in enhancing overall gut health. In summary, IGF-1 emerges as a valuable candidate for future research and potential therapies aimed at enhancing intestinal health through its role in cell growth stimulation and protective effects against inflammation. Several reports have evaluated the delivery of TGF-β in foods, in enteral formulas, or directly by gavage in animal models of mucosal inflammation [53]. The preclinical and clinical findings show that TGF-β supplementation decreases mucosal and systemic inflammation in Crohn’s disease and ulcerative colitis [53].
3.3. Cancer
In oncology, while EGF is essential for normal cellular functions, its overexpression or dysregulation is associated with various cancers. As a result, EGF and its receptor (EGFR) are targets for cancer therapies, with several EGFR inhibitors having been developed to block the proliferative signals in cancer cells [54]. In the context of cancer, IGF-2 is often overexpressed in various tumors, contributing to tumor growth and progression [55]. It plays a crucial role in oncogenesis due to its capacity to stimulate cell division and prevent apoptosis. Thus, targeting the IGF-2 signaling pathway is being investigated as a possible therapeutic approach for the treatment of cancer [56,57]. However, the adaptability of TGF-β2 goes far beyond bone production, involving a variety of biological processes such as immunological regulation, wound healing, cell development, inflammation management, and even cancer metastasis [58,59,60].
3.4. Bone and Muscle Health
IGF-1 is a crucial peptide hormone involved in the growth and developmental processes of mammals [61]. This growth factor system influences the vasculature through a variety of physiological effects, operating via both endocrine and autocrine/paracrine mechanisms [62]. IGF-1 is a hormone that supports and stimulates growth, and studies have shown that it increases osteoblastic activity [63]. Additionally, from a different perspective, exercise or training activates the GH and IGF-1 axis. Conversely, exercise also increases catabolic pro-inflammatory cytokines, such as interleukin-6 (IL-6) [64]. The anabolic GH and IGF-1 axis are crucial because its activation after exercise enhances muscle growth and repair, increases protein synthesis, and promotes fat metabolism, leading to improved muscle strength, reduced body fat, and overall better physical performance [64].
Also, one study aimed to improve muscle injury recovery in elderly people [65] (Table 2). Muscle stem cells become less common as people age, but the ones that remain can still regenerate in a manner similar to those of youth [65]. The research showed that changes in the muscle environment with age reduce the regenerative capacity of these cells. IGF-2 levels were observed to be lower in the regenerated muscles of older mice based on protein analysis. By promoting stem cell proliferation and blood vessel creation while decreasing the formation of fat cells, pro-IGF-2 supplementation of elderly mice resulted in enhanced muscle regeneration [65].

Table 2.
Clinical trials on growth factor supplementation.
3.5. Neurological and Mental Health
Growth factors play a crucial role in regulating cellular activities during both the embryonic and postnatal stages. Minor alterations in the expression patterns of these factors can significantly impact brain development. These initial changes can lead to various neuroanatomical and biochemical variations observed in the later stages of brain maturation [89]. Abnormal levels of growth factors have been implicated in the development and clinical presentation of multiple psychiatric disorders [89].
IGF-2 is a critical regulator of cellular processes such as proliferation, migration, differentiation, and apoptosis (programmed cell death) [90]. IGF-2 exerts its cellular effects through three distinct receptors: IGF-1 receptor (IGF-1R), insulin receptor (IR), and IGF-2 receptor (IGF-2R) [91]. Binding to these receptors triggers the activation of critical intracellular signaling pathways, including PI3K/Akt and MAPK, and these pathways serve as molecular keys regulating cellular growth, survival, and metabolism (Table 2) (Figure 2) [91]. While IGF-2 was traditionally seen as a fetal growth driver, new research has revealed its continued significance in adulthood [92]. Scientists have found high levels of IGF-2 gene expression in the central nervous system, where it appears to play a critical role in memory consolidation, especially involving the hippocampus [93]. Intriguingly, IGF-2 may be linked to memory-related disorders like schizophrenia, depression, etc. While its exact role remains unclear, further research holds promise for new treatments and diagnoses in these critical conditions [92].
3.6. Eye Health
The fluid inside the human eye, called aqueous humor, helps nourish the parts like the cornea and lens. This fluid contains special signaling molecules, and TGF-β2 is the most common one [94]. The concentration of a signaling molecule known as TGF-β2 increases in the fluid of the eye during certain eye conditions. This occurs in conditions such as proliferative vitreoretinopathy (PVR), diabetic retinopathy, and glaucoma [95]. Studies in mice lacking specific TGF-β isoforms have revealed their distinct roles in eye development [95,96]. While deficiencies in TGF-β1 or TGF-β3 do not cause eye problems, mice lacking TGF-β2 exhibit several malformations. These include a thinner cornea missing its inner cell layer, an undeveloped front chamber, an immature retina, and blood vessels persisting in the normally clear vitreous gel. All isoforms of TGF-β, including TGF-β3, are demonstrably expressed during fetal eye development. The specific pattern of TGF-β3 expression observed in the ganglion cell layer, photoreceptor layer, and choriocapillaris during the second trimester suggests its potential involvement in several critical processes. These processes likely include morphogenesis, development, and/or differentiation of the fovea, a specialized region responsible for central vision [97].
4. Growth Factors as Health Products
Given their therapeutic promise in wound healing, tissue regeneration, and disease management, growth factors—naturally occurring proteins—have attracted significant attention in the production of health products. These proteins can stimulate cellular growth, proliferation, and differentiation.
Growth factors can be presented in various forms in the medical and dietary supplementation fields. As shown in Table 3, different types of growth factors can be incorporated as the main active ingredients in these products. These products can be used as supportive treatments for conditions such as wound formation due to chronic diseases, tissue damage, growth disorders, hormonal imbalances, and skin aging. Healthcare professionals may use growth factors not only as supportive treatments, but also as therapeutic drugs. The wide variety of growth factors and their unique properties allow for the discovery of new applications beyond the current usage areas. In particular, the ability of growth factors to promote tissue regeneration holds the potential for broader therapeutic applications through advanced studies. In addition to medical treatments, these products can also be used to enhance performance and overall well-being in healthy individuals [98].

Table 3.
Growth factor-based products.
Growth factor therapy has several limitations. A key issue is the short half-life of growth factors in tissues, requiring a continuous angiogenic stimulus to maintain new vessel growth [105]. Systemic expression can lead to harmful side effects in distant organs, such as promoting tumor growth or arthritis [105]. For instance, VEGFs can increase vascular permeability and edema, with pericardial effusion being a dose-limiting side effect [106]. Therefore, consulting medical experts is crucial for growth factor supplementation. The source, whether bovine or not, is less important due to the fundamental biological actions of these proteins. The effects of the fundamental mechanisms, which are also seen in treatments using bovine colostrum-derived growth factors (listed in Table 2), can potentially be replicated by the patented products shown in Table 3.
Colostrum-derived growth factors are currently under research for their effectiveness and safety in treating various health conditions, focusing on their potential benefits in immune support, gastrointestinal health, and wound healing. Ongoing studies aim to clarify their role in improving gut integrity, enhancing immune response, and promoting tissue repair without significant adverse effects. In summary, the use of growth factors in the medical and nutritional supplementation fields represents a significant innovation that can support existing treatments and enable new therapeutic approaches. It is expected that this field will continue to expand, with growth factors being used in a wider range of applications in the future.
5. Conclusions and Future Outlook
BC is gaining increasing attention due to its various bioactive components. Among these components, growth factors stand out for their significant role in physiological processes. BC is a unique and powerful source of these development factors since it naturally contains them, unlike other supplements or synthetic substitutes.
It has been shown that these growth factors have many positive effects on the body. For instance, they can promote wound healing by encouraging tissue repair processes and cell proliferation. Growth factors may also be useful as therapeutic agents for diseases such as osteoarthritis or musculoskeletal injuries because they encourage the regeneration of deteriorating tissues. Unlike other sources, BC’s related growth factors are highly bioavailable and work together to improve these healing processes. Growth factors also play a critical role in strengthening the immune system by increasing the generation and activity of immune cells, which improves defense against infections and illnesses. The naturally occurring growth factors in BC promote a powerful immunological response, making it a better choice than manufactured immune boosters. Moreover, studies indicate that growth factors might be involved in more than just immune support. Some research suggests that they may serve as biomarkers for the diagnosis of diseases, demonstrating their involvement in pathological processes. Through comprehending the complex mechanisms by which growth factor’s function, researchers hope to open up new approaches to the diagnosis and treatment of disease.
The potential of BC as a helpful supplement for improving general health and well-being is highlighted by the complex impacts of growth factors. However, more research is needed to understand the effects of BC on health. Future studies can help us better understand the detailed mechanisms of action of growth factors and their effects on different health conditions. Furthermore, future research could investigate the best dosages and administration methods for enhancing the effects of colostrum-derived growth factors. Furthermore, we may uncover additional features of growth factors that could improve or broaden the benefits of colostrum ingestion. In this way, we can more comprehensively evaluate the role of BC in health and better understand its potential health benefits.
Author Contributions
Conceptualization, S.K.; writing—original draft preparation, Y.M.Y. and H.D. writing—review and editing, S.K., J.M.M.L., A.C.M.P., M.L., F.K., W.K., M.B., H.E.-S., A.R. and J.L.d.B.A.; visualization, Y.M.Y. and H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This paper does not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yalçıntaş, Y.M.; Baydemir, B.; Duman, H.; Eker, F.; Bayraktar Biçen, A.; Ertürk, M.; Karav, S. Exploring the Impact of Colostrum Supplementation on Athletes: A Comprehensive Analysis of Clinical Trials and Diverse Properties. Front. Immunol. 2024, 15, 1395437. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 Prevention, Treatment, and Recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Ginjala, V.; Pakkanen, R. Determination of Transforming Growth Factor-Β1 (TGF-Β1) and Insulin-Like Growth Factor 1 (IGF-1) in Bovine Colostrum Samples. J. Immunoass. 1998, 19, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.; Gauthier, S.F.; Drouin, R.; Poubelle, P.E.; Pouliot, Y. In Vitro Digestion of Proteins and Growth Factors in a Bovine Whey Protein Extract as Determined Using a Computer-Controlled Dynamic Gastrointestinal System (TIM-1). Food Dig. 2011, 2, 13–22. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. Epidermal Growth Factor. Annu. Rev. Biochem. 1979, 48, 193–216. [Google Scholar] [CrossRef]
- Yagi, H.; Suzuki, S.; Noji, T.; Nagashima, K.; Kuroume, T. Epidermal Growth Factor in Cow’s Milk and Milk Formulas. Acta Paediatr. 1986, 75, 233–235. [Google Scholar] [CrossRef] [PubMed]
- White, M.F.; Kahn, C.R. Insulin Action at a Molecular Level—100 Years of Progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef] [PubMed]
- Skaar, T.C.; Vega, J.R.; Pyke, S.N.; Baumrucker, C.R. Changes in Insulin-like Growth Factor-Binding Proteins in Bovine Mammary Secretions Associated with Pregnancy and Parturition. J. Endocrinol. 1991, 131, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.R.; Gibson, C.A.; Skaar, T.C.; Hadsell, D.L.; Baumrucker, C.R. Insulin-like Growth Factor (IGF)-I and -II and IGF Binding Proteins in Serum and Mammary Secretions during the Dry Period and Early Lactation in Dairy Cows. J. Anim. Sci. 1991, 69, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.A.; Borchers, A.T.; Li, M.; Flavell, R.A.; Bowlus, C.L.; Ansari, A.A.; Gershwin, M.E. Transforming Growth Factor β (TGF-β) and Autoimmunity. Autoimmun. Rev. 2005, 4, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.-L.; Goddard, C.; Regester, G.O.; Ballard, F.J.; Belford, D.A. Transforming Growth Factor β in Bovine Milk: Concentration, Stability and Molecular Mass Forms. J. Endocrinol. 1996, 151, 77–86. [Google Scholar] [CrossRef]
- Okada, M.; Ohmura, E.; Kamiya, Y.; Murakami, H.; Onoda, N.; Iwashita, M.; Wakai, K.; Tsushima, T.; Shizume, K. Transforming Growth Factor (TGF)—α in Human Milk. Life Sci. 1991, 48, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, A. Transforming Growth Factor α and β (TGF-α and TGF-β). In Angiogenesis in Health, Disease and Malignancy; Salajegheh, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 331–337. ISBN 978-3-319-28140-7. [Google Scholar]
- Heldin, C.-H. Targeting the PDGF Signaling Pathway in Tumor Treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef]
- Boonstra, J.; Rijken, P.; Humbel, B.; Cremers, F.; Verkleij, A.; en Henegouwen, P.v.B. The Epidermal Growth Factor. Cell Biol. Int. 1995, 19, 413–430. [Google Scholar] [CrossRef]
- Jacobi, N.; Seeboeck, R.; Hofmann, E.; Eger, A. ErbB Family Signalling: A Paradigm for Oncogene Addiction and Personalized Oncology. Cancers 2017, 9, 33. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; López-Saura, P.; González-López, T.; Castro-Santana, M.D.; López-Mola, E.; Guillén-Nieto, G.; Herrera-Martinez, L. Epidermal Growth Factor in Clinical Practice—A Review of Its Biological Actions, Clinical Indications and Safety Implications. Int. Wound J. 2009, 6, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Adrain, C.; Freeman, M. Regulation of Receptor Tyrosine Kinase Ligand Processing. Cold Spring Harb. Perspect. Biol. 2014, 6, a008995. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.P.J.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Zhu, H.-J.; Walker, F.; Frenkel, M.J.; Hoyne, P.A.; et al. Crystal Structure of a Truncated Epidermal Growth Factor Receptor Extracellular Domain Bound to Transforming Growth Factor Alpha. Cell 2002, 110, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F. PI3K/Akt: Getting It Right Matters. Oncogene 2008, 27, 6473–6488. [Google Scholar] [CrossRef]
- Waterman, H.; Sabanai, I.; Geiger, B.; Yarden, Y. Alternative Intracellular Routing of ErbB Receptors May Determine Signaling Potency. J. Biol. Chem. 1998, 273, 13819–13827. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β Signaling in Fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Zilberberg, L.; Todorovic, V.; Dabovic, B.; Horiguchi, M.; Couroussé, T.; Sakai, L.Y.; Rifkin, D.B. Specificity of Latent TGF-β Binding Protein (LTBP) Incorporation into Matrix: Role of Fibrillins and Fibronectin. J. Cell. Physiol. 2012, 227, 3828–3836. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β Signaling and the Fibrotic Response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ Signalling in Context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Z.; Chen, Q.; Zhang, W.-Y.; Zhang, H.-H.; Ma, Y.; Zhang, S.-Z.; Fang, J.; Yu, C.-H. PDGF Signaling Pathway in Hepatic Fibrosis Pathogenesis and Therapeutics (Review). Mol. Med. Rep. 2017, 16, 7879–7889. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Bentov, I.; Werner, H. CHAPTER 193—Insulin-Like Growth Factor. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Burlington, ON, Canada, 2006; pp. 1385–1392. ISBN 978-0-12-369442-3. [Google Scholar]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 Activates Its Receptor. eLife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed]
- Jadwin, J.A.; Curran, T.G.; Lafontaine, A.T.; White, F.M.; Mayer, B.J. Src Homology 2 Domains Enhance Tyrosine Phosphorylation in Vivo by Protecting Binding Sites in Their Target Proteins from Dephosphorylation. J. Biol. Chem. 2018, 293, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.A.; Engelmann, B.W.; Jablonowski, K.; Higginbotham, K.; Stergachis, A.B.; Nash, P.D. SRC Homology 2 Domain Binding Sites in Insulin, IGF-1 and FGF Receptor Mediated Signaling Networks Reveal an Extensive Potential Interactome. Cell Commun. Signal. 2012, 10, 27. [Google Scholar] [CrossRef]
- Shing, C.M.; Hunter, D.C.; Stevenson, L.M. Bovine Colostrum Supplementation and Exercise Performance. Sports Med. 2009, 39, 1033–1054. [Google Scholar] [CrossRef]
- Mero, A.; Kähkönen, J.; Nykänen, T.; Parviainen, T.; Jokinen, I.; Takala, T.; Nikula, T.; Rasi, S.; Leppäluoto, J. IGF-I, IgA, and IgG Responses to Bovine Colostrum Supplementation during Training. J. Appl. Physiol. 2002, 93, 732–739. [Google Scholar] [CrossRef]
- Andasari, V.; Lü, D.; Swat, M.; Feng, S.; Spill, F.; Chen, L.; Luo, X.; Zaman, M.; Long, M. Computational Model of Wound Healing: EGF Secreted by Fibroblasts Promotes Delayed Re-Epithelialization of Epithelial Keratinocytes. Integr. Biol. 2018, 10, 605–634. [Google Scholar] [CrossRef] [PubMed]
- Blais, M.; Pouliot, Y.; Gauthier, S.; Boutin, Y.; Lessard, M. A Gene Expression Programme Induced by Bovine Colostrum Whey Promotes Growth and Wound-Healing Processes in Intestinal Epithelial Cells. J. Nutr. Sci. 2014, 3, e57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Gartner, M.H.; Benson, J.D.; Caldwell, M.D. Insulin-like Growth Factors I and II Expression in the Healing Wound. J. Surg. Res. 1992, 52, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Alejandro-Osorio, A.L.; Grorud, K.W.; Martinez, D.A.; Vailas, A.C.; Grindeland, R.E.; Vanderby, R. Systemic Administration of IGF-I Enhances Healing in Collagenous Extracellular Matrices: Evaluation of Loaded and Unloaded Ligaments. BMC Physiol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef]
- Alexander, A.N.; Carey, H.V. Oral IGF-I Enhances Nutrient and Electrolyte Absorption in Neonatal Piglet Intestine. Am. J. Physiol. 1999, 277, G619–G625. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B. Intestinal Hormones and Growth Factors: Effects on the Small Intestine. World J. Gastroenterol. 2009, 15, 385–406. [Google Scholar] [CrossRef]
- Allen, T.R.; Krueger, K.D.; Hunter, W.J.; Agrawal, D.K. Evidence That Insulin-like Growth Factor-1 Requires Protein Kinase C-Epsilon, PI3-Kinase and Mitogen-Activated Protein Kinase Pathways to Protect Human Vascular Smooth Muscle Cells from Apoptosis. Immunol. Cell Biol. 2005, 83, 651–667. [Google Scholar] [CrossRef]
- Hatamzade Esfahani, N.; Day, A.S. The Role of TGF-β, Activin and Follistatin in Inflammatory Bowel Disease. Gastrointest. Disord. 2023, 5, 167–186. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Baselga, J. The EGF Receptor Family as Targets for Cancer Therapy. Oncogene 2000, 19, 6550–6565. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; Gongoll, S.; Langner, C.; Mengel, M.; Piso, P.; Klempnauer, J.; Rüschoff, J.; Kreipe, H.; von Wasielewski, R. IGF-1R, IGF-1 and IGF-2 Expression as Potential Prognostic and Predictive Markers in Colorectal-Cancer. Virchows Arch. 2003, 443, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Liefers-Visser, J.A.L.; Meijering, R.A.M.; Reyners, A.K.L.; van der Zee, A.G.J.; de Jong, S. IGF System Targeted Therapy: Therapeutic Opportunities for Ovarian Cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Y.; Shi, L.Z.; Kanneganti, T.-D.; Chi, H. Signaling by the Phosphatase MKP-1 in Dendritic Cells Imprints Distinct Effector and Regulatory T Cell Fates. Immunity 2011, 35, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Manokawinchoke, J.; Pavasant, P.; Sawangmake, C.; Limjeerajarus, N.; Limjeerajarus, C.N.; Egusa, H.; Osathanon, T. Intermittent Compressive Force Promotes Osteogenic Differentiation in Human Periodontal Ligament Cells by Regulating the Transforming Growth Factor-β Pathway. Cell Death Dis. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 Accelerates Bladder Cancer Progression via miR-1305/Tgf-Β2/Smad3 Pathway. Mol. Cancer 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Le Roith, D. Insulin-Like Growth Factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef]
- Delafontaine, P.; Song, Y.-H.; Li, Y. Expression, Regulation, and Function of IGF-1, IGF-1R, and IGF-1 Binding Proteins in Blood Vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef]
- Laron, Z.; Klinger, B.; Jensen, L.T.; Erster, B. Biochemical and Hormonal Changes Induced by One Week of Administration of rIGF-I to Patients with Laron Type Dwarfism. Clin. Endocrinol. 1991, 35, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Nemet, D. Exercise and the GH-IGF-I Axis. In Endocrinology of Physical Activity and Sport; Hackney, A.C., Constantini, N.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–84. ISBN 978-3-030-33376-8. [Google Scholar]
- Ikemoto-Uezumi, M.; Uezumi, A.; Tsuchida, K.; Fukada, S.; Yamamoto, H.; Yamamoto, N.; Shiomi, K.; Hashimoto, N. Pro-Insulin-Like Growth Factor-II Ameliorates Age-Related Inefficient Regenerative Response by Orchestrating Self-Reinforcement Mechanism of Muscle Regeneration. Stem Cells 2015, 33, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; van Breda, E.; Verlaan, G.; Smeets, R. Effects of Oral Bovine Colostrum Supplementation on Serum Insulin-like Growth Factor-i Levels. Nutrition 2002, 18, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Picardo, M. Bovine Colostrum Promotes Growth and Migration of the Human Keratinocyte HaCaT Cell Line. Growth Factors 2009, 27, 448–455. [Google Scholar] [CrossRef]
- Doillon, C.J.; Lehance, F.; Bordeleau, L.-J.; Laplante-Campbell, M.-P.; Drouin, R. Modulatory Effect of a Complex Fraction Derived from Colostrum on Fibroblast Contractibility and Consequences on Repair Tissue. Int. Wound J. 2011, 8, 280–290. [Google Scholar] [CrossRef]
- Kovacs, D.; Maresca, V.; Flori, E.; Mastrofrancesco, A.; Picardo, M.; Cardinali, G. Bovine Colostrum Induces the Differentiation of Human Primary Keratinocytes. FASEB J. 2020, 34, 6302–6321. [Google Scholar] [CrossRef] [PubMed]
- Jogi, R.; Tager, M.J.; Perez, D.; Tsapekos, M. Bovine Colostrum, Telomeres, and Skin Aging. J. Drugs Dermatol. 2021, 20, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-A.; Park, H.-J.; Han, M.-G.; Lee, R.; Kim, J.-S.; Park, J.-H.; Lee, W.-Y.; Song, H. Fermented Colostrum Whey Upregulates Aquaporin-3 Expression in, and Proliferation of, Keratinocytes via P38/c-Jun N-Terminal Kinase Activation. Food Sci. Anim. Resour. 2021, 41, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Joshi, A.; Singh, N. Natural Cocktail of Bioactive Factors Conjugated on Nanofibrous Dressing for Improved Wound Healing. Biomater. Adv. 2022, 143, 213163. [Google Scholar] [CrossRef]
- Han, G.; Kim, H.; Kim, D.E.; Ahn, Y.; Kim, J.; Jang, Y.J.; Kim, K.; Yang, Y.; Kim, S.H. The Potential of Bovine Colostrum-Derived Exosomes to Repair Aged and Damaged Skin Cells. Pharmaceutics 2022, 14, 307. [Google Scholar] [CrossRef]
- Wu, B.; Gao, M.; Bai, X. Therapeutic Effect of the Combination of Fractional Laser and Recombinant Bovine Basic Fibroblast Growth Factor in Acne Scar Patients. Trop. J. Pharm. Res. 2024, 23, 125–131. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Baumgartner, A.; Gomez, J.E.; Najafzadeh, M. The Efficacy of a Novel Biological Formulation from Bovine Milk Colostrum Exosomes and Its Growth Factors in Enhancing the Process of Wound Healing. J. Biol. 2024, 11. [Google Scholar] [CrossRef]
- Roohelhami, E.; Vahdat Shariatpanahi, Z.; Ardehali, S.H. Colostrum Supplement, IGF-1, and Diarrhea in Mechanically-Ventilated Patients: A Double-Blind, Randomized, Placebo-Controlled Study. Nutr. Clin. Métabolisme 2023, 37, 227–232. [Google Scholar] [CrossRef]
- Costa, M.A.; Wu, C.; Pham, B.V.; Chong, A.K.S.; Pham, H.M.; Chang, J. Tissue Engineering of Flexor Tendons: Optimization of Tenocyte Proliferation Using Growth Factor Supplementation. Tissue Eng. 2006, 12, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Fu, S.C.; Qin, L.; Rolf, C.; Chan, K.M. Supplementation-Time Dependence of Growth Factors in Promoting Tendon Healing. Clin. Orthop. Relat. Res. 2006, 448, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Belford, D.A.; Rogers, M.-L.; Regester, G.O.; Francis, G.L.; Smithers, G.W.; Liepe, I.J.; Priebe, I.K.; Ballard, F.J. Milk-Derived Growth Factors as Serum Supplements for the Growth of Fibroblast and Epithelial Cells. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Eremia, S.C.; de Boo, H.A.; Bloomfield, F.H.; Oliver, M.H.; Harding, J.E. Fetal and Amniotic Insulin-Like Growth Factor-I Supplements Improve Growth Rate in Intrauterine Growth Restriction Fetal Sheep. Endocrinology 2007, 148, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, L.C.; Lee, G.C.; Huang, K.L.; Mauck, R.L. Growth Factor Supplementation Improves Native and Engineered Meniscus Repair in Vitro. Acta Biomater. 2012, 8, 3687–3694. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The Effect of Anisotropic Collagen-GAG Scaffolds and Growth Factor Supplementation on Tendon Cell Recruitment, Alignment, and Metabolic Activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Brown, G.L.; Nanney, L.B.; Griffen, J.; Cramer, A.B.; Yancey, J.M.; Curtsinger, L.J.; Holtzin, L.; Schultz, G.S.; Jurkiewicz, M.J.; Lynch, J.B. Enhancement of Wound Healing by Topical Treatment with Epidermal Growth Factor. N. Engl. J. Med. 1989, 321, 76–79. [Google Scholar] [CrossRef]
- Brown, G.L.; Curtsinger, L.; Jurkiewicz, M.J.; Nahai, F.; Schultz, G. Stimulation of Healing of Chronic Wounds by Epidermal Growth Factor. Plast. Reconstr. Surg. 1991, 88, 189–194. [Google Scholar] [CrossRef]
- Acosta, J.B.; Savigne, W.; Valdez, C.; Franco, N.; Alba, J.S.; Rio, A.D.; López-Saura, P.; Guillén, G.; Lopez, E.; Herrera, L.; et al. Epidermal Growth Factor Intralesional Infiltrations Can Prevent Amputation in Patients with Advanced Diabetic Foot Wounds. Int. Wound J. 2006, 3, 232–239. [Google Scholar] [CrossRef]
- Tabrizi, M.; Chams-Davatchi, C.; Esmaeeli, N.; Noormohammadpoor, P.; Safar, F.; Etemadzadeh, H.; Ettehadi, H.; Gorouhi, F. Accelerating Effects of Epidermal Growth Factor on Skin Lesions of Pemphigus Vulgaris: A Double-Blind, Randomized, Controlled Trial. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 79–84. [Google Scholar] [CrossRef]
- Fernández-Montequín, J.I.; Betancourt, B.Y.; Leyva-Gonzalez, G.; Mola, E.L.; Galán-Naranjo, K.; Ramírez-Navas, M.; Bermúdez-Rojas, S.; Rosales, F.; García-Iglesias, E.; Berlanga-Acosta, J.; et al. Intralesional Administration of Epidermal Growth Factor-Based Formulation (Heberprot-P) in Chronic Diabetic Foot Ulcer: Treatment up to Complete Wound Closure. Int. Wound J. 2009, 6, 67–72. [Google Scholar] [CrossRef]
- Sigalet, D.L.; Martin, G.R.; Butzner, J.D.; Buret, A.; Meddings, J.B. A Pilot Study of the Use of Epidermal Growth Factor in Pediatric Short Bowel Syndrome. J. Pediatr. Surg. 2005, 40, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth Factors as Clinical Biomarkers of Prognosis and Diagnosis in Psychiatric Disorders. Cytokine Growth Factor Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2012, 59, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2021, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, O.V.; Ordyan, N.E. Insulin-Like Growth Factor 2: New Roles for a Known Molecule. Neurosci. Behav. Physi. 2022, 52, 175–182. [Google Scholar] [CrossRef]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A Critical Role for IGF-II in Memory Consolidation and Enhancement. Nature 2011, 469, 491–497. [Google Scholar] [CrossRef]
- Saika, S. TGFβ Pathobiology in the Eye. Lab. Investig. 2006, 86, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Saika, S.; Liu, C.-Y.; Azhar, M.; Sanford, L.P.; Doetschman, T.; Gendron, R.L.; Kao, C.W.-C.; Kao, W.W.-Y. TGFβ2 in Corneal Morphogenesis during Mouse Embryonic Development. Dev. Biol. 2001, 240, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sanford, L.P.; Ormsby, I.; Groot, A.C.G.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFβ2 Knockout Mice Have Multiple Developmental Defects That Are Non-Overlapping with Other TGFβ Knockout Phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.A. Transforming growth factor—ß in human foetal eye. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3544. [Google Scholar]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Eskens, O.; Amin, S. Challenges and Effective Routes for Formulating and Delivery of Epidermal Growth Factors in Skin Care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Polak, M.; Woelfle, J.; Houchard, A.; EU IGFD Registry Study Group. Effectiveness and Safety of rhIGF-1 Therapy in Children: The European Increlex® Growth Forum Database Experience. Horm. Res. Paediatr. 2015, 83, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Hee, C.K.; Roach, S.; Snel, L.B. Safety of Recombinant Human Platelet-Derived Growth Factor-BB in Augment(®) Bone Graft. J. Tissue Eng. 2012, 3, 2041731412442668. [Google Scholar] [CrossRef] [PubMed]
- Frew, S.E.; Rezaie, R.; Sammut, S.M.; Ray, M.; Daar, A.S.; Singer, P.A. India’s Health Biotech Sector at a Crossroads. Nat. Biotechnol. 2007, 25, 403–417. [Google Scholar] [CrossRef]
- Senet, P. Becaplermin gel (Regranex gel). Ann. Dermatol. Venereol. 2004, 131, 351–358. [Google Scholar] [CrossRef]
- Aldag, C.; Teixeira, D.N.; Leventhal, P.S. Skin Rejuvenation Using Cosmetic Products Containing Growth Factors, Cytokines, and Matrikines: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, J.E.; Rissanen, T.T.; Kivelä, A.; Ylä-Herttuala, S. Growth Factor-Induced Therapeutic Angiogenesis and Arteriogenesis in the Heart–Gene Therapy. Cardiovasc. Res. 2005, 65, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Rutanen, J.; Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Silvennoinen, P.; Kivelä, A.; Hedman, A.; Hedman, M.; Heikura, T.; Ordén, M.-R.; et al. Adenoviral Catheter-Mediated Intramyocardial Gene Transfer Using the Mature Form of Vascular Endothelial Growth Factor-D Induces Transmural Angiogenesis in Porcine Heart. Circulation 2004, 109, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).