Abstract
Plant Extracts (PE) are natural substances extracted from plants, rich in various bioactive components. Exploring the molecular mechanisms and interactions involved in the vascular protective effects of PE is beneficial for the development of further strategies to protect aging blood vessels. For this review, the content was obtained from scientific databases such as PubMed, China National Knowledge Infrastructure (CNKI), and Google Scholar up to July 2024, using the search terms “Plant extracts”, “oxidative stress”, “vascular aging”, “endothelial dysfunction”, “ROS”, and “inflammation”. This review highlighted the effects of PE in protecting aging blood vessels. Through pathways such as scavenging reactive oxygen species, activating antioxidant signaling pathways, enhancing respiratory chain complex activity, inhibiting mitochondrial-reactive oxygen species generation, improving nitric oxide bioavailability, downregulating the secretion of inflammatory factors, and activating sirtuins 1 and Nrf2 signaling pathways, it can improve vascular structural and functional changes caused by age-related oxidative stress, mitochondrial dysfunction, and inflammation due to aging, thereby reducing the incidence of age-related cardiovascular diseases.
1. Introduction
Vascular senescence refers to a series of degenerative changes in vascular structure and function that occur with age, including the thickening and hardening of the vascular wall, increased arterial stiffness, loss of endothelium-dependent vasodilator function, and endothelial dysfunction [1,2], leading to vascular senescence, the pathology of which is mainly due to oxidative stress, mitochondrial dysfunction, and chronic low-grade inflammation. Aging is linked to a higher occurrence of cardiovascular and cerebrovascular diseases, including hypertension, stroke, and coronary artery disease. These conditions worsen due to alterations in blood vessel structure and function. As the population ages, the incidence of these diseases is rising rapidly, and they have become one of the major causes of death among China’s elderly population [2,3]
PE is extracted from plants using physical or chemical methods, and single or mixed biological active ingredients [4], including mainly polyphenols, polysaccharides, flavonoids, amino acids, saponins and organic acids, etc. These bioactive components are secondary metabolites of plants and have biological functions such as anti-inflammatory, antioxidant and anti-aging [5,6,7], which can improve oxidative stress, mitochondrial function, and can alleviate inflammatory response through single or synergistic effects among their active components [8,9]. In recent years, PE has been shown to extend life expectancy, promote healthy aging, and mitigate the effects of aging on the body. Research in this area has been steadily increasing, with many drugs and healthcare products now being developed to prevent aging or promote healthy aging, and these products aim to exert their antioxidant and anti-inflammatory properties to regulate cellular and biochemical processes in the body [10,11], providing protection against cardiovascular disease [12,13].
2. PE Mitigates Oxidative Stress in Aging Vessels
PE has antioxidant properties that modulate cellular responses, signaling pathways, and chemical mediators associated with oxidative stress processes. Since the free radical theory of aging was first proposed in the 1950s [14], a large number of studies have shown that oxidative stress is an important process in vascular aging [15,16,17]. An excessive or sustained production of reactive oxygen species (ROS), surpassing the buffering capacity of antioxidant defenses or inadequacies in antioxidant enzymes, can result in oxidative stress, which in turn affects the course of age-related cardiovascular disease [16]. ROS are partially reduced metabolites of oxygen that are generated during cellular homeostasis [18], and in the vasculature they are mainly generated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), xanthine oxidase (XO), cyclooxygenase (COX) uncoupling, the electron transport chain (ETC)and nitric oxide synthase NOS [19]. An important source of ROS production in cardiovascular systems is NOX, and its seven isozymes are transmembrane proteins with multiple cytoplasmic and transmembrane subunits that generate superoxide anions during electron transport across membranes [20]. XO is present in endothelial cells (EC) and plasma and generates superoxide anions and other reactive oxygen products by accepting electrons from molecules of oxygen, stimulating lipoprotein receptor-1 (LOX-1) expression on macrophages and vascular smooth muscle cells and increasing ROS production [21,22]. It is the respiratory chain complexes I and III that produce ROS as superoxide anions in mitochondria, and mitochondrial dysfunction or failure of its antioxidant mechanism leads to the leakage of electrons from the ETC to react with O2 to form a superoxide anion. At the same time, mitochondrial-reactive oxygen species (mtROS) interfere with the function of the ETC complex through the iron–sulfur oxide center, thereby exacerbating ROS production [16,23]. NOS is a homodimeric enzyme that is uncoupled in the absence of the cofactor tetrahydrobiopterin or the substrate L-arginine, resulting in impaired production and the release of NO and an increase in highly pro-oxidative superoxide anions [24]. Oxidative stress affects vascular function through the oxidation of critical proteins or the induction of redox-sensitive transcription factors. In the event that NO is inactivated, clearance increases, production decreases, bioavailability declines, and endothelial dysfunction ensues, ultimately resulting in vascular aging [25]. NO reacts with superoxide to produce highly reactive peroxynitrite, which in excess leads to protein nitration, resulting in mitochondrial and endothelial dysfunction [26,27,28,29].
PE can scavenge ROS to alleviate oxidative stress in four ways: (1) the presence of phenolic hydroxyl groups on polyphenol molecules in PE directly scavenges ROS [30]. (2) PE activates antioxidant signaling pathways, balances cellular reactive oxygen species, improves ROS overproduction due to vascular aging, mitigates the glutathione (GSH) depletion resulting from antioxidant system impairment, and concurrently reinstates the functionality of antioxidant enzymes like superoxide dismutase (SOD) [31,32,33]; GSH functions as a nucleophilic metabolite that directly reduces ROS levels, whereas SOD inhibits peroxide anion generation, neutralizes superoxides, and hinders peroxynitrite formation and the reduction in transition metal ions [34,35]. (3) PE mediates the clearance of ROS through the regulation of nuclear factor erythroid 2-related factor-2 (Nrf2). Nrf2 is an evolutionarily conserved redox-sensitive transcription factor, demonstrating significant vasoprotective properties. Aging promotes the downregulation and dysfunction of Nrf2 levels in the vascular system, and its downregulation exacerbates oxidative stress by exacerbating the susceptibility of senescent vascular cells to ROS-mediated cellular and molecular damage [10,36,37,38]. PE enhances the DNA-binding activity of Nrf2 or upregulates its protein expression, and Nrf2 induces the expression of antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), and GSH. PE mediates glutathione S-transferases (GST) and heme oxygenase-1 (HO-1) so that they reduce ROS production by being expressed in conjunction with an antioxidant response element (ARE) in the promoter region of the gene, relieving the oxidative stress. Moreover, PE increased GPx expression via Nrf2, converting peroxides into their corresponding alcohols, and H2O2 mediated the monocyte chemoattractant protein-1 (MCP-1)-induced inflammatory factor expression; furthermore, the expression of vascular cellular adhesion molecule-1 (VCAM-1) was inhibited. This process helps to scavenge ROS and mitigate oxidative damage to blood vessels [32,39,40]. (4) PE has been shown to decrease the expression levels of COX-1 and COX-2 in aging blood vessels, and COX-1 and COX-2 are involved in ROS production, causing oxidative damage. Concurrently, the production of oxidized low-density lipoproteins (Ox-LDL) occurs, with the oxidation of lipoproteins serving as the primary stage in the pathogenesis of atherosclerosis, resulting in injury to EC. Both have increased expression in the vascular senescent state [41,42]. In addition to Ox-LDL, oxidative stress and inflammation are major factors in the development of atherosclerosis [43]. Furthermore, PE also resists oxidative stress by decreasing Nox1 expression [44], as well as inducing AMPK phosphorylation to significantly upregulate eNOS expression to generate NO, preventing eNOS uncoupling to generate a superoxide anion [45]. In conclusion, PE has the ability to scavenge ROS and mitigate oxidative stress by enhancing the activity of antioxidant enzymes such as GSH and SOD, modulating the Nrf2 pathway, and downregulating COX and Nox1 expression levels and other pathways.
3. PE Reducing Mitochondrial Dysfunction in Blood Vessels
Mitochondria are double-membrane-enveloped organelles that control energy production and apoptosis, among other cellular functions, and play a pivotal role in modulating the aging process. With age, the efficacy of the respiratory chain diminishes, leading to increased electron leakage and ROS production, and reduced cellular ATP synthesis; mtROS production is associated with age-related vascular dysfunction [46]. In aging vessels, increased mtROS has been linked to dysfunction in the electron transport chain, exacerbated by a decreased cellular GSH content and impaired Nrf2-mediated antioxidant defense responses [47,48,49,50]. Concurrently, impaired mitochondrial biogenesis in EC and smooth muscle cells in the vasculature negatively affects cellular energy, and the increased induction of mtROS production via defective electron flow through the electron transport chain leads to vascular injury [25]. It was shown that PE improved the mitochondrial respiratory chain complex’s activity, upregulated SOD, CAT, and peroxiredoxin 3 (PRDX3) expression, increased ATP generation, decreased mtROS release, and maintained mitochondrial redox homeostasis; moreover, it inhibited cytochrome c release due to mitochondrial permeabilization, thereby preventing mitochondrial-mediated apoptosis and vascular senescence [51,52,53,54]. At the same time, PE promotes the restoration of mitochondrial ATP production and inhibits the release of cytochrome c through the activation of (mitogen-activated protein kinase kinase) MEK signaling and the upregulation of the anti-apoptotic protein B cell lymphoma-2 (Bcl-2) [51]. In addition, PE stimulates peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), regulates mitochondrial biogenesis, and inhibits mtROS generation [55]; PGC-1α is a transcription co-activator that regulates mitochondrial biogenesis and the expression of antioxidant enzymes, and the decreased expression or impaired function of PGC-1α can result in mitochondrial dysfunction and the induction of senescence [56].
PE mediates mitochondrial biosynthesis and functional improvement and in-creases expression of antioxidant enzymes through activation of sirtuins 1 (SIRT1), PGC-1α deacetylation [57]. The Sirtuins are NAD+-dependent protein deacetylases whose expression decreases with age, where SIRT1 regulates mitochondrial function in the vascular system, controlling mitochondrial biology and mtROS production, cellular energy metabolism, and the removal of damaged mitochondria through autophagy [47,58,59]. Mitophagy is a mechanism of the selective autophagy process that refers to the autophagic removal of damaged mitochondria, and plays an important role in regulating their function [60]. Whereas abnormal mitochondrial autophagy leads to mitochondrial dysfunction, the frequency of abnormalities in this autophagic process increases with age, which in turn leads to vascular dysfunction; concurrently, PE attenuates mitochondrial dysfunction by impairing ROCK1-mediated mitochondria-specific autophagy [61]. Rho-associated protein kinase (ROCK) is an effector participating in multiple cellular processes; among them, ROCK1 is a protein Ser/Thr kinase involved in the regulation of actin-myosin contraction and stabilization, apoptosis, and gene expression [62]. Moreover, Xiang et al. [61] found that PE downregulated the expression levels of Bcl-2 interacting protein 1 (Beclin1) and Ser/Thr kinase PINK1 and E3 ubiquitin ligase Parkin (PINK1/Parkin). Beclin1 and PINK1/Parkin signaling activate autophagy, and Beclin1 is a key regulator of autophagy, interacting with apoptosis pathway regulatory proteins and playing an important role in apoptosis [63]. Whereas PINK1/Parkin synergistically senses mitochondrial functional status, PINK1 accumulates on the surface of dysfunctional mitochondria while recruiting and activating the E3 ubiquitin ligase activity of Parkin, which labels damaged mitochondria for degradation via the autophagy pathway [60]. In summary, PE attenuates mitochondrial dysfunction and further delays EC senescence through the modulation of SIRT1, increasing respiratory chain complex activity, activating of PGC-1α, and downregulating Beclin1 expression.
4. PE Delays Endothelial Senescence
A hierarchical branching network of arteries, capillaries, and veins arrange the ECs that are functionally integrated into organs to support growth, function, and repair. The vascular endothelium is located between the blood and the vessel wall, and is a continuous single-layer screen that controls the exchange of substances between the lumen, the vessel wall, and thin-walled tissues, with the main function of producing factors such as NO, CO, prostacyclin, endothelin, and others to regulate vascular tone and vascular function, and to maintain vascular homeostasis [64,65]. In contrast, the aging of the vascular system is a pathophysiological process in which ECs undergo characteristic morphological and molecular changes. During this process, increased ROS, mitochondrial dysfunction, or DNA damage promote cellular senescence and the senescence-associated secretory phenotype (SASP) in the vascular system, promote an increased production of inflammatory cytokines and chemokines as well as alterations in the synthesis of lipid mediators, and lead to impaired vasodilatation, increased arterial stiffness, and an increased inflammatory state [66].
PE reduces the proportion of senescence-associated β-galactosidase (Sa-β-gal)-positive cells; Sa-β-gal acts as a biomarker in response to cellular senescence in vivo, and PE downregulates the expression of p16/p21/p53, all of which are established markers of senescence. A prolonged and high expression of p16 will accelerate cell cycle arrest and induce cellular senescence. While p53/p21 is a key pathway in cellular senescence, p21 is located downstream of p53, which binds to and activates the promoters of downstream target genes and participates in proliferation, apoptosis, and other processes. While p53/p21 is a key pathway in cellular senescence, p21 is located downstream of p53, which binds to and activates the promoters of downstream target genes and participates in proliferation, apoptosis and other processes. As a target gene, p21 upregulates the suppression of the activity of cell cycle protein kinases, thereby impeding gene expression and preventing cells from progressing into the S-phase of DNA replication. This ultimately leads to the inhibition of DNA synthesis and the arrest of the cell cycle [67,68]. PE also significantly increased the number of cells in the S-phase of the cell cycle and inhibited cell cycle protein D1, thereby attenuating vascular endothelial senescence [69]. Furthermore, Donato et al. [70] discovered that senescent cells will accumulate in normal arterial tissue as age progresses, and their research confirmed an increase in senescence markers in the EC extracted from the arteries and veins of healthy older adults, whose expression was negatively correlated with endothelial function. Roos et al. [71] showed that vascular endothelial function could be improved by eliminating senescent cells and p16. In addition, PE reduces senescent cell viability and decreases SASP pro-inflammatory cytokine production [6]. The expression of many SASP components is regulated by the activity of nuclear factor kappa B (NF-κB), and PE reduces the SASP expression by inhibiting the activity of NF-κB [57]. Overall, PE reduces Sa-β-gal levels, inhibits SASP, and regulates p53/p21/p16, key signaling molecules of cell cycle progression, to delay endothelial senescence and thereby control its exacerbation of inflammation, etc.
5. PE Inhibits Inflammation in Vascular Aging
Chronic, sterile, low-grade inflammation is a hallmark of the vascular aging process, which has important implications for age-related cardiovascular diseases such as atherosclerosis, microvascular dysfunction, and aneurysm formation [25]. Vascular endothelial cells and smooth muscle cells exhibit pro-inflammatory changes in senescent blood vessels, including the secretion of inflammatory cytokines such as interleukin 6 (IL-6), interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), chemokines, adhesion molecules, and matrix metalloproteinases (MMP) [25]. It was shown that PE inhibited the secretion of inflammatory factors by SASP while decreasing the levels of IL-1β, IL-6, and TNF-α. Inflammatory factors promote endothelial cell apoptosis and impair NO bioavailability through various mechanisms, including inhibiting eNOS gene expression and affecting eNOS mRNA degradation and activation, which in turn cause vascular inflammation [72,73]. Meanwhile, SASP secretes inflammatory factors while inducing inflammation in neighboring cells through paracrine secretion, which promotes the spread of tissue and organ senescence, whereas a sustained increase in inflammatory factors exacerbates extravascular matrix remodeling and atherosclerosis, accelerating plaque formation [74,75]. PE downregulates the expression of NF-κB, an important nuclear transcription factor for inflammatory cytokine expression [57], and inhibits the pro-inflammatory response induced by its signaling activation. PE also attenuates inflammatory responses by inhibiting phosphatidylinositol-3-kinase (PI3K), V-Akt murine thymoma virus oncogene homolog 1 (AKT), NF-κBp65, signal transducers and activators of transcription 3 (STAT3) protein expression, and STAT3 protein phosphorylation in the PI3K/AKT/NF-κB signaling cascade pathway [67,76]; PI3K and AKT are responsible for the activation and nuclear translocation of transcription factors (e.g., STAT, NF-κB). Furthermore, PE inhibits vascular inflammation induced by the inflammatory mediator-producing enzymes LOX-1 and intercellular adhesion molecule-1 (ICAM-1) [77]; ICAM-1 is an important cell adhesion molecule belonging to the immunoglobulin superfamily, which stimulates vascular inflammation and is activated by TNF-α expression. It also stimulates leukocyte adhesion and migration to the subendothelial region of blood vessels, leading to the recruitment of multiple monocytes to the cell membrane to form monocyte clusters and colonies. After monocyte accumulation, other inflammatory cytokines and adipocytes begin to adhere to the cell surface, leading to the narrowing of blood vessels and ultimately to vascular dysfunction [78]. Elevated blood levels of ICAM-1 can also increase the transcription of the p65 subunit of NF-κB, which exacerbate vascular inflammation [78]. PE has also been shown to significantly inhibit the expression of key enzymes of cell signaling pathways, such as COX-2 and protein kinase C. PE reduces the production of inflammatory factors such as IL-1β and TNF-α by inhibiting the expression of such enzymes [42,79], and also inhibits the JNK and p38 pathways to suppress TNF-α-induced inflammatory responses [80].
There is a complicated cross-talk between increased oxidative stress and the activation of inflammatory processes in the aging vasculature, with ROS acting as signaling molecules to activate pro-inflammatory signaling pathways such as NF-κB, which regulates the transcription of pro-inflammatory genes and enhances the expression of pro-inflammatory secretory mediators. Secondly, inflammatory factors are potent inducers of oxidative stress, such as TNF-α, which activates NADPH oxidase [81]. NF-κB activates and transcribes most of the gene targets that regulate and amplify the inflammatory response (e.g., cytokines, chemokines, apoptotic cells, and phagocytosis) while driving pro-inflammatory shifts in oxidative stress [82,83]. Studies have shown that PE upregulates the expression level of SIRT1, which interferes with pro-inflammatory molecule signaling through the inhibition of NF-κB and promotion of immunomodulatory transcription factors, and inhibits NF-κB transcription through the deacetylation of histone tails in the NF-κB promoter and of NF-κB itself [84,85,86]. Furthermore, with progressive inflammation, macrophages within the vessel wall produce more ROS, reduce NO availability, promote adhesion molecule expression, stimulate vascular smooth muscle cell hypertrophy, and activate MMP, whose upregulation induces changes in structural components of the arterial wall (e.g., decreased elastin/collagen ratio) which in turn impacts vascular remodeling, leading to reduced arterial compliance, increased stiffness, and impaired vasodilatation, which results in vascular dysfunction [24]. PE also inhibited the differentiation of THP-1 macrophages into macrophage M1, reducing ROS production and pro-inflammatory factor release, while PE-treated THP-1 macrophages released anti-inflammatory factors (e.g., IL-4, IL-10, and Arg-1) by enhancing the Nrf2/HO-1 signaling pathway [87]. In conclusion, PE inhibits vascular inflammation by decreasing the secretion of inflammatory factors (e.g., IL-1β, IL-6, and TNF-α), downregulating the expression of inflammatory mediator enzymes (e.g., LOX-1, ICAM-1), controlling the transcription of NF-κB, and mediating the release of anti-inflammatory factors from Nrf2/HO-1.
6. PE Improves Endothelial Dysfunction in Aging Blood Vessels
With age, a series of changes occur in blood vessels, among which ED is one of the most important clinical manifestations, being a symptom of vascular aging in the endothelial cells. ED refers to the reduction in endothelium-dependent dilatation (EDD) of endothelial cells in response to chemical or mechanical stimuli, which is manifested by functional changes in the endothelial phenotype, including the thickening of the vascular intima, alterations in the structure and function of endothelial cells, and the formation of pro-inflammatory and pro-thrombotic states [67,88]. In contrast, decreased endothelial cell capacity to synthesize and release NO, the downregulation of eNOS expression, or overproduction of ROS, impairs NO bioavailability, leading to its mediated vasodilatory function with a loss of normal vascular EDD, causing ED [89,90,91,92]. PE reduces ROS-mediated NO catabolism, upregulates eNOS expression activation to promote NO production, and increases NO bioavailability to improve vasodilation; NO, as an endothelium-derived diastolic factor, is a soluble free radical produced by eNOS and catalyzed using L-arginine as a substrate that prevents leukocyte adhesion to endothelial surfaces, as well as platelet adhesion and platelet aggregation, and inhibits the proliferation of vascular smooth muscle cells and the formation of other noncellular components that make up the matrix of the vascular wall. In addition, it has vasodilatory effects, with reduced bioavailability being a key mediator of the ED [93,94,95,96]. Simultaneous PE downregulates COX-1 and COX-2 expression levels in senescent vessels and normalizes their mediated endothelium-dependent contractile responses [97]. PE also promotes endothelial vasodilation by eliminating the ROS inhibition of EDD and reducing aging-induced vascular sclerosis by normalizing aortic wall stiffness and collagen [98]. Shahlehi et al. [99] found that PE induces vasodilation via a cholinergic pathway mediated by kaempferol 3-O-rutinoside and by muscarinic and nicotinic acetylcholine receptors present in vascular smooth muscle. In addition, PE can effectively downregulate the expression level of endothelin-1 (ET-1) and regulate the dynamic balance of active substance release from aged vascular endothelium [67]. ET-1 is an endothelium-derived constrictive factor that mediates vasoconstriction, cell proliferation and differentiation, and extracellular matrix production, and its overexpression by senescence leads to an imbalance between vasodilation and contraction, impaired EDD, and an intensification of the pro-mitotic stimulation of vascular smooth muscle cells and fibrotic processes, inducing ED [100].
Additionally, PE improves ED by modulating ion channels, attenuates the CaCl2-induced contraction of high K+-depolarizing aortic rings, and inhibits receptor-gated and voltage-dependent Ca2+ channels to reduce Ca2+ influx. This attenuates its induction of increased arterial tone and vasoconstriction, while the opening of Ca2+-activated K+ channels mediates the relaxation of the aortic rings, vasodilation, and the attenuation of vascular dysfunction [101]. In addition, eNOS uncoupling is also a key mechanism of endothelial dysfunction; in addition to oxidative stress-generated ROS leading to uncoupling, enzymatic post-translational modifications of asymmetric dimethylarginine (ADMA) and eNOS also lead to uncoupling after [102]. ADMA is a methylated arginine that reduces NO synthesis by competing for the arginine binding site of eNOS [103]. It was shown that PE promotes the phosphorylation of eNOS at Ser1177 via PI3K/Akt and induces eNOS expression in vascular aging to improve NO-mediated vasodilation [104,105]. Lee et al. [106] found that PE, in addition to reversing the d-gal-mediated decrease in eNOS serine phosphorylation, restored endothelial cell NO levels with SIRT1 expression. PE not only reversed the d-gal-mediated decrease in eNOS serine phosphorylation, but also restored endothelial NO levels with SIRT1 expression. SIRT1 affects eNOS transcription and enzymatic activity in the endothelium and regulates NO production by directly activating eNOS through deacetylation [107]; there is a positive feedback with NO, which promotes the transcription of SIRT1 [108]. Imbalance and a lack of SIRT1 regulation is a major cause of endothelial cell dysfunction and a potential mediator of age-related cardiovascular disease [109,110,111]. PE significantly increased the level of AMPK phosphorylation and its downstream gene expression [45,112], and regulates vascular senescence by activating signaling pathways such as Nrf2 and P53 through its phosphorylation of target metabolic enzymes and the regulation of the expression of related genes [113]. At the same time, SIRT1 elevates AMPK activity through the deacetylation of liver kinase B1 (LKB1), and AMPK increases SIRT1 activity by promoting NAD+ biosynthesis; an increase in the NAD+/NADH ratio can further activate SIRT1 [114,115]. In addition, PE ameliorates age-related atherosclerosis and reduces the production of advanced glycosylation end products (AGEs), while alleviating endothelial dysfunction caused by oxidative stress exacerbated by activated NADPH upon its binding to the receptor. AGEs are a heterogeneous group of bioactive molecules that are formed by nonenzymatic glycation, and their mediated cross-linking collagen and other proteins resists enzymatic degradation and interferes with collagenolysis, leading to the accumulation of collagen in the arteries with age and causing arteriosclerosis [116,117]. Atherosclerosis is defined as an increase in the velocity of the conduction wave or a change in the passive mechanical properties of the artery, resulting in a decrease in its compliance. Studies have shown that the aorta’s elasticity decreases with age, leading to impaired capacitance, an inability to properly promote blood flow and maintain diastolic pressures, etc., which ultimately affects cardiovascular disease in a number of ways [70,118]. In addition, AGEs inhibit reverse cholesterol transport by downregulating the expression of ATP-binding membrane cassette transporter proteins A1 and G1 (ABCA1 and ABCG1) on monocytes. Additionally, AGEs play a role in modifying extracellular matrix molecules through glycosylation and cross-linking alterations, thereby contributing to the advancement of atherosclerotic lesions [119,120]. Collectively, PE can induce vasodilation to restore EDD and improve endothelial dysfunction by promoting eNOS phosphorylation, increasing NO bioavailability, upregulating SIRT1 transcript expression levels, and decreasing AGEs production.
7. Summary and Prospects
Taken together, PE exerts a protective effect against vascular aging through the following five points; the proposed mechanism of action is shown in Figure 1 and Table 1. (1) Scavenging ROS by enhancing antioxidant enzyme activity, activating antioxidant signaling pathways, and reducing COX-1 and COX-2 expression levels to mitigate oxidative stress; (2) Increasing mitochondrial content, restoring respiratory chain complex activity, and decreasing mtROS production to maintain mitochondrial redox homeostasis and to alleviate mitochondrial dysfunction due to aging; (3) Reducing the proportion of Sa-β-gal positive cells, modulating critical cellular senescence pathways, and suppressing SASP secretion to delay endothelial senescence; (4) Regulating NF-κB, the SIRT1 pathway and the downregulation of the secretory expression of inflammatory factors to inhibit inflammation; (5) Restoring endothelium-dependent dilation, upregulating the NOS phosphorylation expression level, inducing NO production and reducing the superoxide-mediated NO catabolism to improve vascular endothelial dysfunction. However, the molecular mechanisms involved in the role of PE in protecting against vascular senescence and the interconnections between the mechanisms still need to be further investigated. Also, the efficacy of some of the PE currently referenced in this article has only been confirmed in their experiments and has not yet been confirmed in clinical applications.
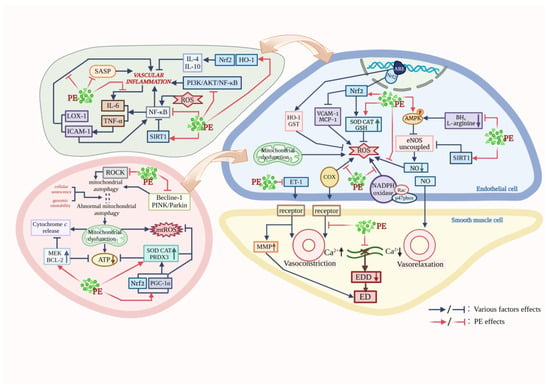
Figure 1.
Pathways of PE protection against vascular aging. (This figure was created using BioRender).

Table 1.
Molecular mechanisms underlying the protective effects of PE on vascular aging.
Author Contributions
Conceptualization, Y.L. and Z.Z. (Zeru Zhangand); validation, Y.L. and L.S.; writing—original draft preparation, Y.L. and Z.Z. (Zeru Zhangand); writing—review and editing, Y.L., Z.Z. (Zhi Zeng), Y.Z. and L.F.; visualization, Y.L. and W.Z.; supervision, Y.H., S.C., S.Y. and L.S.; project administration, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors express thanks to all persons who contributed to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Y.; Qi, Y.; Lu, W. Endogenous Vasoactive Peptides and Vascular Aging-Related Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 1534470. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yan, J.; Zhao, Y.; Yu, Z.; Tian, S.; Khan, A.H.; Zhu, Y.; Wu, A.; Zhang, C.; Tian, X.L. Vascular Aging: Assessment and Intervention. Clin. Interv. Aging 2023, 18, 1373–1395. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; He, F.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Interplay of Objective Sleep Duration and Cardiovascular and Cerebrovascular Diseases on Cause-Specific Mortality. J. Am. Heart Assoc. 2019, 8, e013043. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Rossi, M.; Rodriguez, S.; Munk, R.; Khadeer, M.; Abdelmohsen, K.; Gorospe, M.; Ferrucci, L. Identification of gingerenone A as a novel senolytic compound. PLoS ONE 2022, 17, e0266135. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Rossi, R.; Mainardi, E.; Vizzarri, F.; Corino, C. Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants 2023, 13, 39. [Google Scholar] [CrossRef]
- Shailaja, M.; Damodara, G.K.; Vishakh, K.; Suchetha, K.N. Anti-aging Role of Curcumin by Modulating the Inflammatory Markers in Albino Wistar Rats. J. Natl. Med. Assoc. 2017, 109, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Boersma, M.G.; Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; van Zanden, J.J.; Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Hemagirri, M.; Sasidharan, S. Biology of aging: Oxidative stress and RNA oxidation. Mol. Biol. Rep. 2022, 49, 5089–5105. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Juni, R.P.; Duckers, H.J.; Vanhoutte, P.M.; Virmani, R.; Moens, A.L. Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 2013, 61, 1471–1481. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, Y.; Zhang, Z.; Vallurupalli, S.; Mehta, J.L. Xanthine Oxidase Induces Foam Cell Formation through LOX-1 and NLRP3 Activation. Cardiovasc. Drugs. Ther. 2017, 31, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Paneni, F.; Diaz, C.C.; Libby, P.; Luscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Diers, A.R.; Broniowska, K.A.; Hogg, N. Nitrosative stress and redox-cycling agents synergize to cause mitochondrial dysfunction and cell death in endothelial cells. Redox Biol. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Liaudet, L.; Vassalli, G.; Pacher, P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front. Biosci. 2009, 14, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.T.; Berk, B.C. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 711–717. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS(*+) assay. J. Agric. Food. Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant health effects of aged garlic extract. J. Nutr. 2001, 131, 1010S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Uygur, R.; Yagmurca, M.; Alkoc, O.A.; Genc, A.; Songur, A.; Ucok, K.; Ozen, O.A. Effects of quercetin and fish n-3 fatty acids on testicular injury induced by ethanol in rats. Andrologia 2014, 46, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.U.; Kang, S.Y.; Park, H.Y.; Sung, S.H.; Lee, E.J.; Kim, S.Y.; Kim, Y.C. Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes. J. Pharm. Pharmacol. 2000, 52, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The Glutathione System: A Journey from Cyanobacteria to Higher Eukaryotes. Antioxidants 2023, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, R.; Neves, K.B.; Montezano, A.C.; Harvey, A.; Carneiro, F.S.; Touyz, R.M.; Tostes, R.C. Internal Pudental Artery Dysfunction in Diabetes Mellitus Is Mediated by NOX1-Derived ROS-, Nrf2-, and Rho Kinase-Dependent Mechanisms. Hypertension 2016, 68, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Sosnowska, D.; Wang, M.; Monticone, R.E.; Telljohann, R.; Pinto, J.T.; de Cabo, R.; Sonntag, W.E.; et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-kappaB activation in the nonhuman primate Macaca mulatta. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2011, 66, 866–875. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Sosnowska, D.; Gautam, T.; Koncz, P.; Losonczy, G.; Ballabh, P.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol.-Heart Circul. Physiol. 2011, 301, H363–H372. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.A.; De Luca, C.; Mikhal’Chik, E.; Korkina, L.G. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid. Redox Signal. 2012, 16, 314–328. [Google Scholar] [CrossRef]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFkappaB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.J.; Lopez-Nicolas, J.M.; Gandia-Herrero, F.; Garcia-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Sewell, R.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Huang, J.; Klarich, D.S.; Zhao, Y.; Pourafshar, S.; Arjmandi, B.H.; Salazar, G. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct. 2016, 7, 4175–4187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, M.; Cheng, C.; Liu, D.; Wu, L.; Zhu, J.; Qian, X. Ginsenoside Rb1 reduces H2O2-induced HUVEC dysfunction by stimulating the sirtuin-1/AMP-activated protein kinase pathway. Mol. Med. Rep. 2020, 22, 247–256. [Google Scholar] [CrossRef]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol.-Heart Circul. Physiol. 2014, 307, H292–H306. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Jimenez, R.; Pinto, J.T.; Ballabh, P.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Ungvari, Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech. Ageing Dev. 2009, 130, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Martin-Padura, I.; de Nigris, F.; Giorgio, M.; Mansueto, G.; Somma, P.; Condorelli, M.; Sica, G.; De Rosa, G.; Pelicci, P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc. Natl. Acad. Sci. USA 2003, 100, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Gupte, S.; Chander, P.N.; Edwards, J.G.; Csiszar, A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol.-Heart Circul. Physiol. 2008, 294, H2121–H2128. [Google Scholar] [CrossRef]
- van der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T.; et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000, 192, 1731–1744. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, Y.; Wang, D.; Zhou, Y.; Zhang, P.; Wu, J.; Chen, J.; Qiu, J.; Zhou, J. Zhen-Wu-Tang Induced Mitophagy to Protect Mitochondrial Function in Chronic Glomerulonephritis via PI3K/AKT/mTOR and AMPK Pathways. Front. Pharmacol. 2021, 12, 777670. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Zhang, J.; Reinhart, G.A.; Chew, B.P. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Anim. Sci. 2013, 91, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Pongkan, W.; Takatori, O.; Ni, Y.; Xu, L.; Nagata, N.; Chattipakorn, S.C.; Usui, S.; Kaneko, S.; Takamura, M.; Sugiura, M.; et al. beta-Cryptoxanthin exerts greater cardioprotective effects on cardiac ischemia-reperfusion injury than astaxanthin by attenuating mitochondrial dysfunction in mice. Mol. Nutr. Food Res. 2017, 61, 1601077. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant Fortification of the Diet for Anti-Ageing Effects: A Review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Muller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol.-Heart Circul. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, C.; Di, T.; Chen, L.; Zhao, W.; Wei, L.; Zhou, S.; Wu, X.; Wang, G.; Zhang, Y. Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells. Bioengineered 2022, 13, 3486–3502. [Google Scholar] [CrossRef]
- Rath, N.; Olson, M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012, 13, 900–908. [Google Scholar] [CrossRef]
- Prerna, K.; Dubey, V.K. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int. J. Biol. Macromol. 2022, 204, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef]
- Bu, L.L.; Yuan, H.H.; Xie, L.L.; Guo, M.H.; Liao, D.F.; Zheng, X.L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 5160. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Liao, S.; Huang, R.; Jiang, Y.; Fei, J.; Cai, L.; Zhang, K. Canthaxanthin Attenuates the Vascular Aging or Endothelial Cell Senescence by Inhibiting Inflammation and Oxidative Stress in Mice. Front. Biosci. 2023, 28, 367. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, J.; Zhu, J.; Wang, Y.; Wang, G.Z.; Wan, L.; Huang, D.; Guo, J.C.; Qi, Y.J.; Hu, Y.D. Mechanism of Huangqin Qingre Chubi Capsules regulating p53/p21 998 signaling pathway to delay chondrocyte senescence in osteoarthritic rats. China J. Chin. 2024, 49, 3330–3339. [Google Scholar] [CrossRef]
- Sa, G.; Das, T. Anti cancer effects of curcumin: Cycle of life and death. Cell Div. 2008, 3, 14. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Ogrodnik, M.B.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Montecucco, F. Inflammation in arterial diseases. IUBMB Life 2015, 67, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Kitabatake, M.; Ouji-Sageshima, N.; Yasui, S.; Mochida, N.; Nakano, R.; Kasahara, K.; Tomoda, K.; Yano, H.; Kayano, S.I.; et al. Persimmon-derived tannin has bacteriostatic and anti-inflammatory activity in a murine model of Mycobacterium avium complex (MAC) disease. PLoS ONE 2017, 12, e0183489. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Coppe, J.P.; Lam, E.W. Cellular Senescence: The Sought or the Unwanted? Trends Mol. Med. 2018, 24, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Shiina, K.; Matsumoto-Nakano, C.; Ninomiya, T.; Komatsu, S.; Kimura, K.; Chikamori, T.; Yamashina, A. The Contribution of Inflammation to the Development of Hypertension Mediated by Increased Arterial Stiffness. J. Am. Heart Assoc. 2017, 6, e005729. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, S.; Zhuo, B.; Hu, J.; Shi, Y.; Zhao, J.; Tang, J.; Hu, X.; Wei, S. Comparison of Three Species of Rhubarb in Inhibiting Vascular Endothelial Injury via Regulation of PI3K/AKT/NF-kappaB Signaling Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 8979329. [Google Scholar] [CrossRef]
- Cherniack, E.P. The potential influence of plant polyphenols on the aging process. Forsch. Komplementmed. 2010, 17, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food. Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef]
- Zhou, P.; Lu, S.; Luo, Y.; Wang, S.; Yang, K.; Zhai, Y.; Sun, G.; Sun, X. Attenuation of TNF-alpha-Induced Inflammatory Injury in Endothelial Cells by Ginsenoside Rb1 via Inhibiting NF-kappaB, JNK and p38 Signaling Pathways. Front. Pharmacol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Labinskyy, N.; Rivera, A.; Xiangmin, Z.; Smith, K.; Csiszar, A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol.-Heart Circul. Physiol. 2007, 293, H37–H47. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Saito, T.; Ogihara, T.; Ishigaki, Y.; Yamada, T.; Imai, J.; Uno, K.; Gao, J.; Kaneko, K.; Shimosawa, T.; et al. Blockade of the nuclear factor-kappaB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 2012, 125, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, L.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature. Oncotarget 2016, 7, 19201–19213. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.L. Foxo in the immune system. Oncogene 2008, 27, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Zhao, B.; Wei, H.; Yang, Y.; Yin, H.; Gao, J.; Zhao, W.; Kong, W.; Ge, G.; Lei, T. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine 2023, 112, 154667. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.L. The Study on Mechanism of Traditional Chinese Drug of Supplementing Qi and Activating Blood Circulation Delay Vascualr Aging Caused by Aging and Hypertension. Ph.D. Dissertation, China Academy of Chinese Medical Sciences, Beijing, China, 2011. Available online: https://kns.cnki.net/kcms2/article/abstract?v=0rU-DchPtstpP50t9b-jlXUwouWu_51rWsXzYym-4xsgCpenz15HF0SgIPYJNjoQAoP0XD_HAe-_-tN9qgxsY-0QtKahIzj8OJCEWR2IxINMsWWR-bYQFuJCQUOm1zI7&uniplatform=NZKPT&language=CHS (accessed on 4 March 2024). (In Chinese).
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Ferri, C. Cardiovascular risk and endothelial dysfunction: The preferential route for atherosclerosis. Curr. Pharm. Biotechnol. 2011, 12, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Di Giosia, P.; De Feo, M.; Fellini, E.; Cheli, P.; Ferri, L.; Ferri, C. Tea, flavonoids, and cardiovascular health: Endothelial protection. Am. J. Clin. Nutr. 2013, 98, 1660S–1666S. [Google Scholar] [CrossRef] [PubMed]
- Idris-Khodja, N.; Auger, C.; Koch, E.; Schini-Kerth, V.B. Crataegus special extract WS((R))1442 prevents aging-related endothelial dysfunction. Phytomedicine 2012, 19, 699–706. [Google Scholar] [CrossRef]
- Anter, E.; Thomas, S.R.; Schulz, E.; Shapira, O.M.; Vita, J.A.; Keaney, J.J. Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J. Biol. Chem. 2004, 279, 46637–46643. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Hutton, D.A.; Brunt, V.E.; VanDongen, N.S.; Ziemba, B.P.; Casso, A.G.; Greenberg, N.T.; Mercer, A.N.; Rossman, M.J.; Campisi, J.; et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am. J. Physiol.-Heart Circul. Physiol. 2021, 321, H185–H196. [Google Scholar] [CrossRef]
- Shahlehi, S.; Petalcorin, M. Activation of cholinergic pathway induced vasodilation in rat aorta using aqueous and methanolic leaf extracts of Gynura procumbens. Biomed. Pharmacother. 2021, 143, 112066. [Google Scholar] [CrossRef]
- Trindade, M.; Oigman, W.; Fritsch, N.M. Potential Role of Endothelin in Early Vascular Aging. Curr. Hypertens. Rev. 2017, 13, 33–40. [Google Scholar] [CrossRef]
- Luo, T.; Chen, Z.; Wang, F.; Yin, S.; Liu, P.; Zhang, J.; Yang, Z. Endothelium-Independent Vasodilatory Effects of Isodillapiolglycol Isolated from Ostericum citriodorum. Molecules 2020, 25, 885. [Google Scholar] [CrossRef]
- Chen, C.A.; Wang, T.Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.; Chen, Y.R.; Druhan, L.J.; Zweier, J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Islam, K.N.; Polhemus, D.J.; Donnarumma, E.; Brewster, L.P.; Tao, Y.X.; Goodchild, T.T.; Lefer, D.J. Sustained release nitrite therapy results in myocardial protection in a porcine model of metabolic syndrome with peripheral vascular disease. Am. J. Physiol.-Heart Circul. Physiol. 2015, 309, H305–H317. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Andriambeloson, E.; Diebolt, M.; Andriantsitohaina, R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol. Res. 2004, 53, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Idris, K.N.; Chataigneau, T.; Auger, C.; Schini-Kerth, V.B. Grape-derived polyphenols improve aging-related endothelial dysfunction in rat mesenteric artery: Role of oxidative stress and the angiotensin system. PLoS ONE 2012, 7, e32039. [Google Scholar] [CrossRef]
- Lee, G.H.; Hoang, T.H.; Jung, E.S.; Jung, S.J.; Han, S.K.; Chung, M.J.; Chae, S.W.; Chae, H.J. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 2020, 19, e13279. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Li, H.; Xia, N. The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging. Front. Physiol. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Forstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide-Biol. Chem. 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Zhang, H.N.; Dai, Y.; Zhang, C.H.; Omondi, A.M.; Ghosh, A.; Khanra, I.; Chakraborty, M.; Yu, X.B.; Liang, J. Sirtuins family as a target in endothelial cell dysfunction: Implications for vascular ageing. Biogerontology 2020, 21, 495–516. [Google Scholar] [CrossRef]
- Mechchate, H.; El, A.A.; El, O.N.; El, H.N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Vanhoutte, P.M. SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Lett. 2011, 585, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, B.S.; Sindler, A.L.; Marvi, N.K.; Howell, K.L.; Zigler, M.L.; Yoshizawa, M.; Seals, D.R. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp. Gerontol. 2013, 48, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guerin, A.P. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am. Heart J. 1999, 138, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Pichna, B.A.; Shi, Y.; Bowes, A.J.; Werstuck, G.H. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid. Redox Signal. 2009, 11, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical Characterization, Free Radical Scavenging, and Cellular Antioxidant and Anti-Inflammatory Properties of a Stilbenoid-Rich Root Extract of Vitis vinifera. Oxidative Med. Cell. Longev. 2016, 2016, 8591286. [Google Scholar] [CrossRef]
- Kumar, H.; Dhalaria, R.; Guleria, S.; Cimler, R.; Sharma, R.; Siddiqui, S.A.; Valko, M.; Nepovimova, E.; Dhanjal, D.S.; Singh, R.; et al. Anti-oxidant potential of plants and probiotic spp. in alleviating oxidative stress induced by H2O2. Biomed. Pharmacother. 2023, 165, 115022. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Nakakuki, Y.; Taniguchi, M.; Kanai, S.; Nakayama, A.; Ohnishi, K.; Sakata, T.; Nohira, T.; Matsuda, J.; Baba, K.; et al. Xanthoangelols isolated from Angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. Biofactors 2011, 37, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Zhao, H.; You, P.; You, H.; Wu, H.; Shou, X.; Cheng, G. Rosmarinic acid ameliorated cardiac dysfunction and mitochondrial injury in diabetic cardiomyopathy mice via activation of the SIRT1/PGC-1alpha pathway. Biochem. Biophys. Res. Commun. 2021, 546, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Chen, Y.; Xu, X.Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD(+)/SIRT1/PGC-1alpha Signaling Pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Dai, Q.L.; He, S.; Jia, H.J.; Liu, X.G.; Hua, H.; Zhou, M.; Wang, X. Artesunate alleviates 5-fluorouracil-induced intestinal damage by suppressing cellular senescence and enhances its antitumor activity. Discov. Oncol. 2023, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Dubey, V.; Luqman, S. Activation of Caspase-3 by Terpenoids and Flavonoids in Different Types of Cancer Cells. Curr. Top. Med. Chem. 2020, 20, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Choi, M.Y.; Ko, M.S.; Jeong, J.M.; Kim, Y.H.; Jang, B.H.; Sung, J.H.; Kim, M.G.; Whang, W.K.; Sim, S.S. Competitive inhibition of cytosolic Ca2+-dependent phospholipase A2 by acteoside in RBL-2H3 cells. Arch. Pharm. Res. 2012, 35, 905–910. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-kappaB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, L.; Shan, Q.; Ding, Y.; Zhang, Z.; Zhu, M.; Mao, Y. Total flavonoids from Astragalus alleviate endothelial dysfunction by activating the Akt/eNOS pathway. J. Int. Med. Res. 2018, 46, 2096–2103. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, X.; Wang, J.; Wang, C.; Ma, C.; Zhang, Y.; Li, Y.; Peng, C. Corrigendum to ‘Carthami flos extract against carbon tetrachloride-induced liver fibrosis via alleviating angiogenesis in mice’ Volume 108. https://doi.org/10.1016/j.phymed.2022.154517]. Phytomedicine 2024, 129, 154719. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Hu, N.; Zhang, L.H.; Jiang, W.; Yan, T.; Zhang, T.; Liu, A.; Zhang, Y.Q.; Zhao, J.; Shi, L.; et al. Lonicerae japonicae flos ameliorates radiotherapy-induced mesenteric artery endothelial dysfunction through GTPCH1/BH(4)/eNOS pathway. Phytomedicine 2022, 102, 154146. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Fratantonio, D.; Herrera-Bravo, J.; Sukreet, S.; Martorell, M.; Ekaterina, R.G.; Les, F.; Lopez, V.; Kumar, M.; Pentea, M.; et al. Plant-food-derived Bioactives in Managing Hypertension: From Current Findings to Upcoming Effective Pharmacotherapies. Curr. Top. Med. Chem. 2023, 23, 589–617. [Google Scholar] [CrossRef]
- Taguchi, K.; Tano, I.; Kaneko, N.; Matsumoto, T.; Kobayashi, T. Plant polyphenols Morin and Quercetin rescue nitric oxide production in diabetic mouse aorta through distinct pathways. Biomed. Pharmacother. 2020, 129, 110463. [Google Scholar] [CrossRef]
- An, F.; Bai, Y.; Xuan, X.; Bian, M.; Zhang, G.; Wei, C. 1,8-Cineole Ameliorates Advanced Glycation End Products-Induced Alzheimer’s Disease-like Pathology In Vitro and In Vivo. Molecules 2022, 27, 3913. [Google Scholar] [CrossRef]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes, D.F.J.; Lopes, D.F.J. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishnaraj, M.; Wang, K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed. Pharmacother. 2021, 144, 112336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).