Digestive Profiles of Human Milk, Recombinant Human and Bovine Lactoferrin: Comparing the Retained Intact Protein and Peptide Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactoferrin Sample Acquisition
2.2. Iron Saturation Process
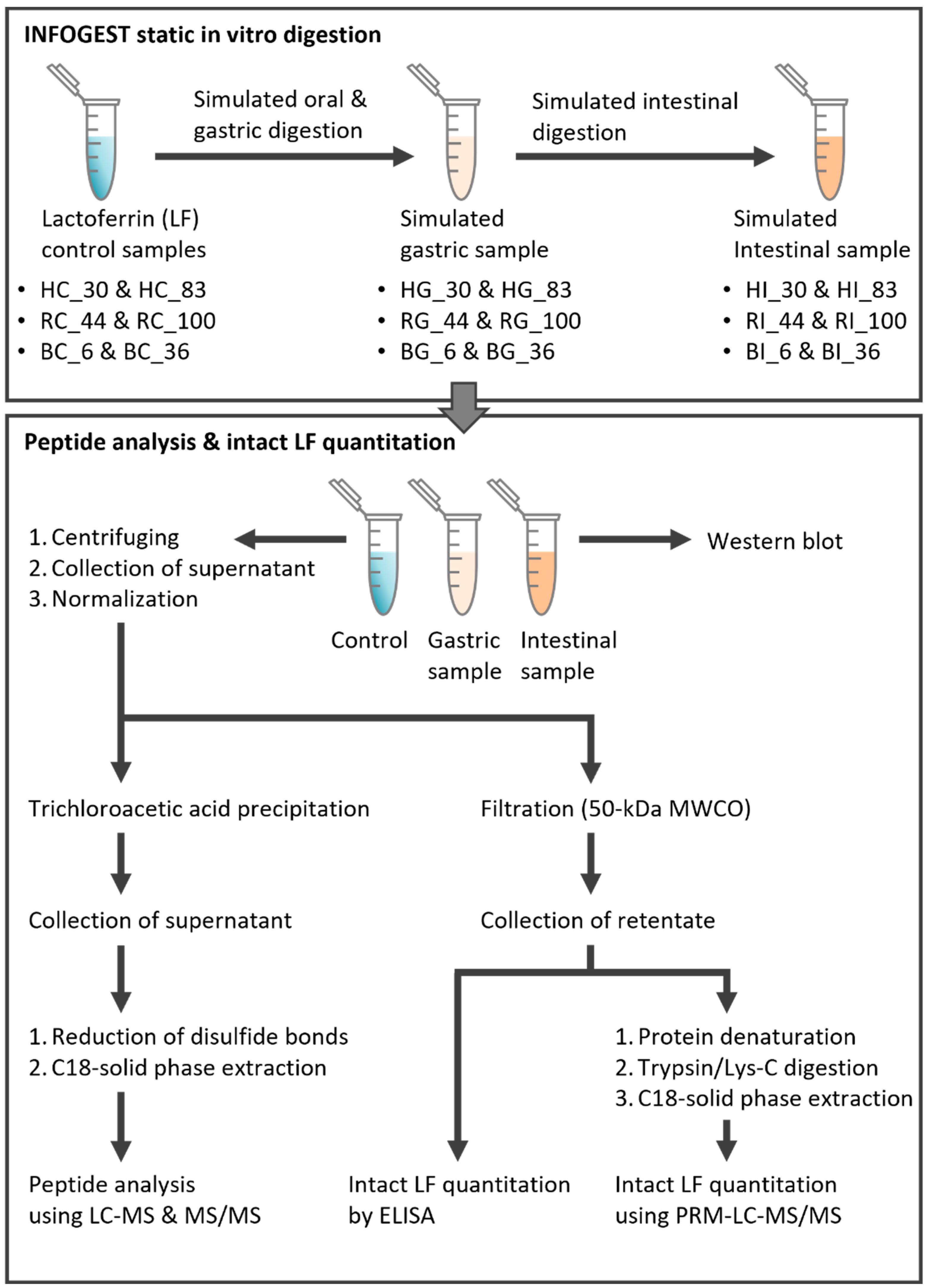
2.3. INFOGEST Static In Vitro Digestion of Lactoferrin Samples
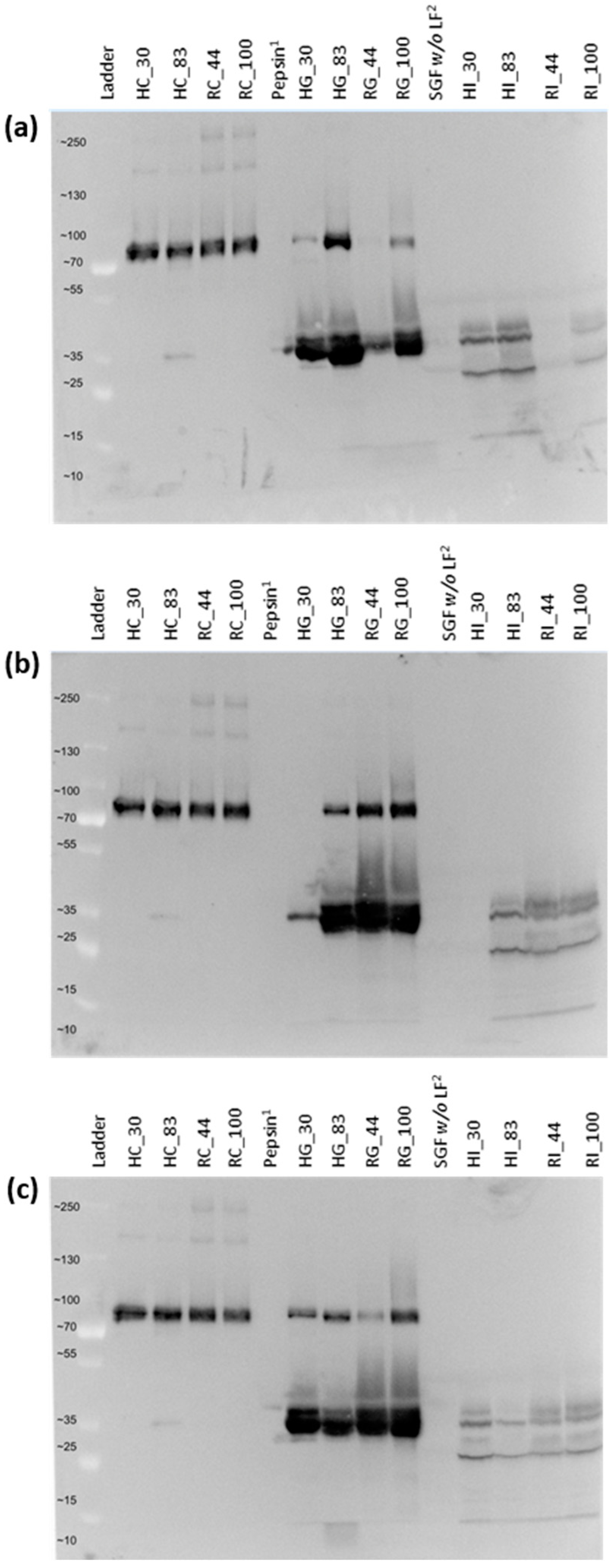
2.4. Confirmation of LF Digestion via Gel Electrophoresis and Western Blot
2.5. Extraction of the Intact LF
2.6. Determination of Intact LF Concentration by ELISA
2.7. Quantitation of the Intact LF Using LC-MS/MS
2.8. Extraction of LF-Derived Peptides in Control and Digestive Samples
2.9. Peptide Analysis Using LC-MS/MS with Data-Dependent Acquisition
2.10. Statistical Analysis
2.11. Bioactivity Prediction of LF-Derived Peptides
3. Results
3.1. Iron Saturation of the LF Samples
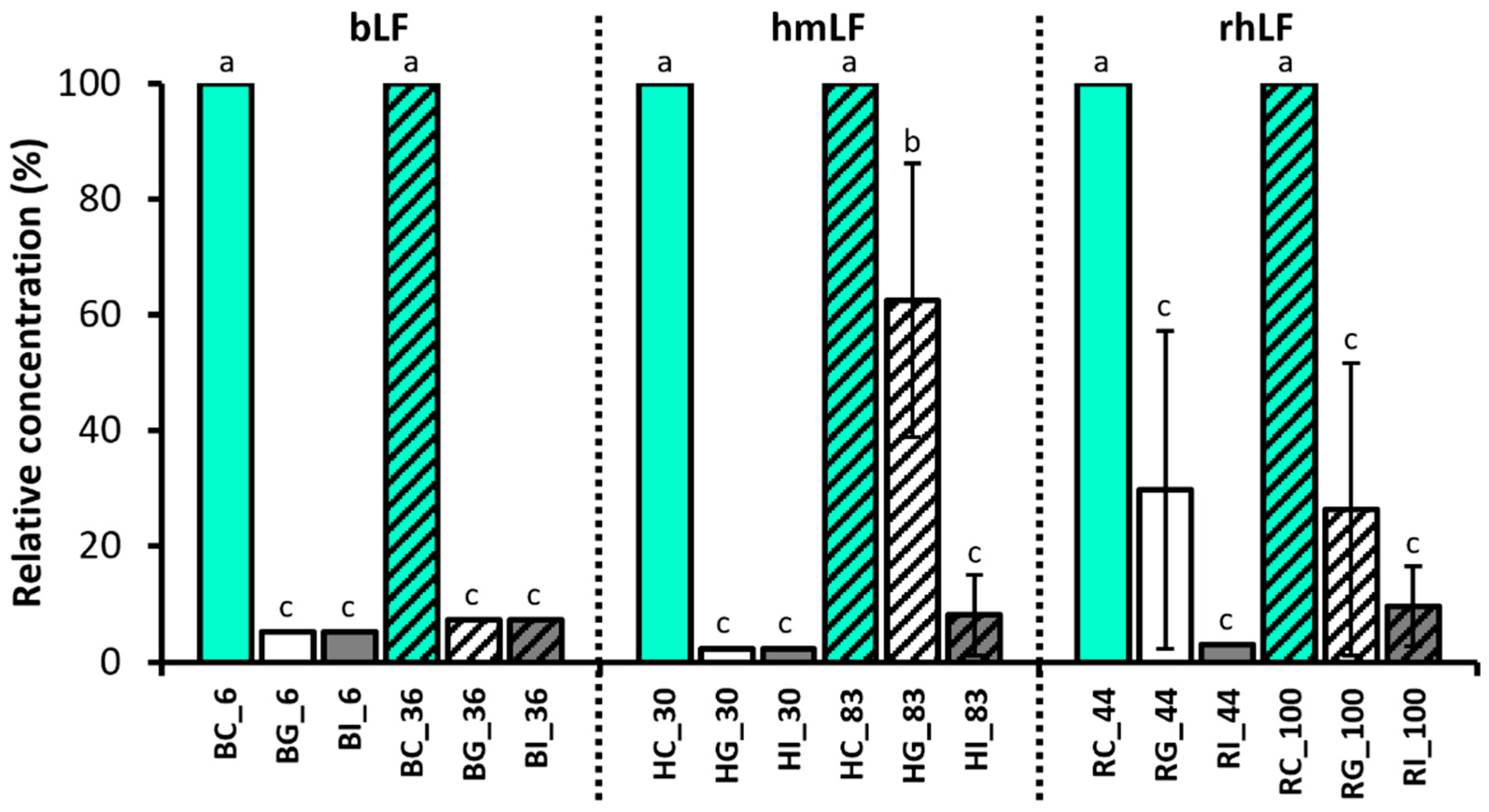
3.2. Quantitation of Intact LF in Gastric and Intestinal Samples
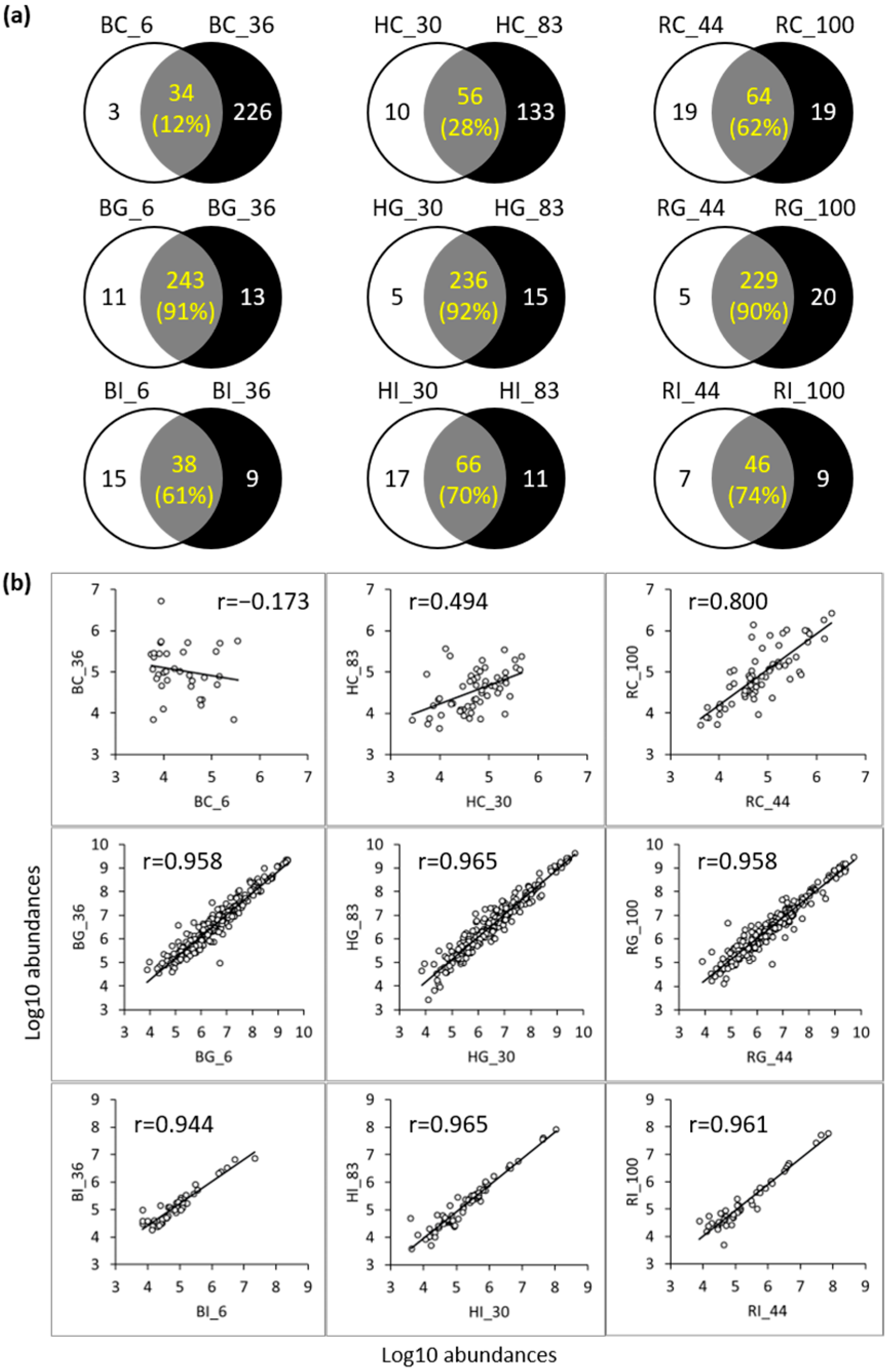
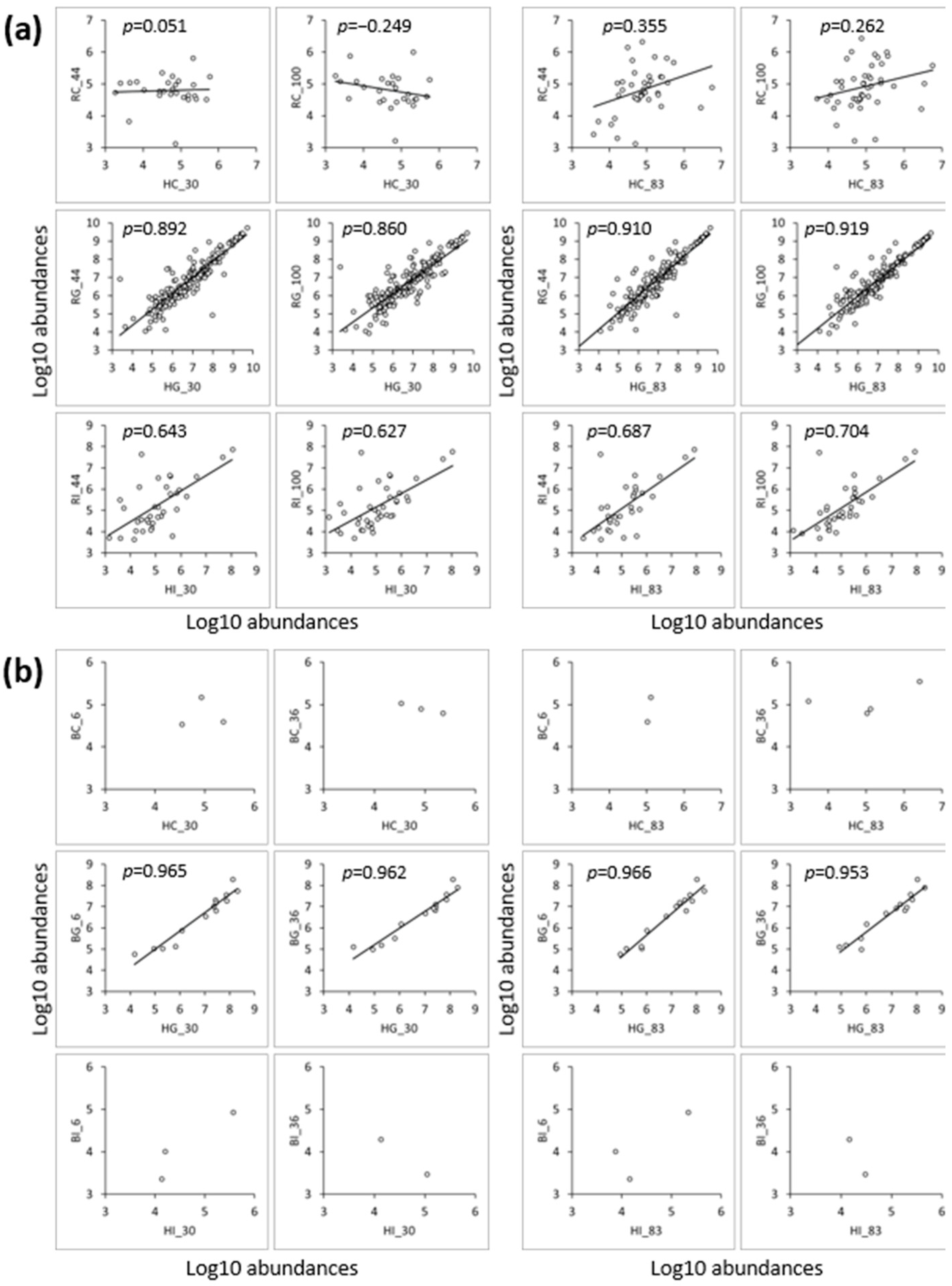
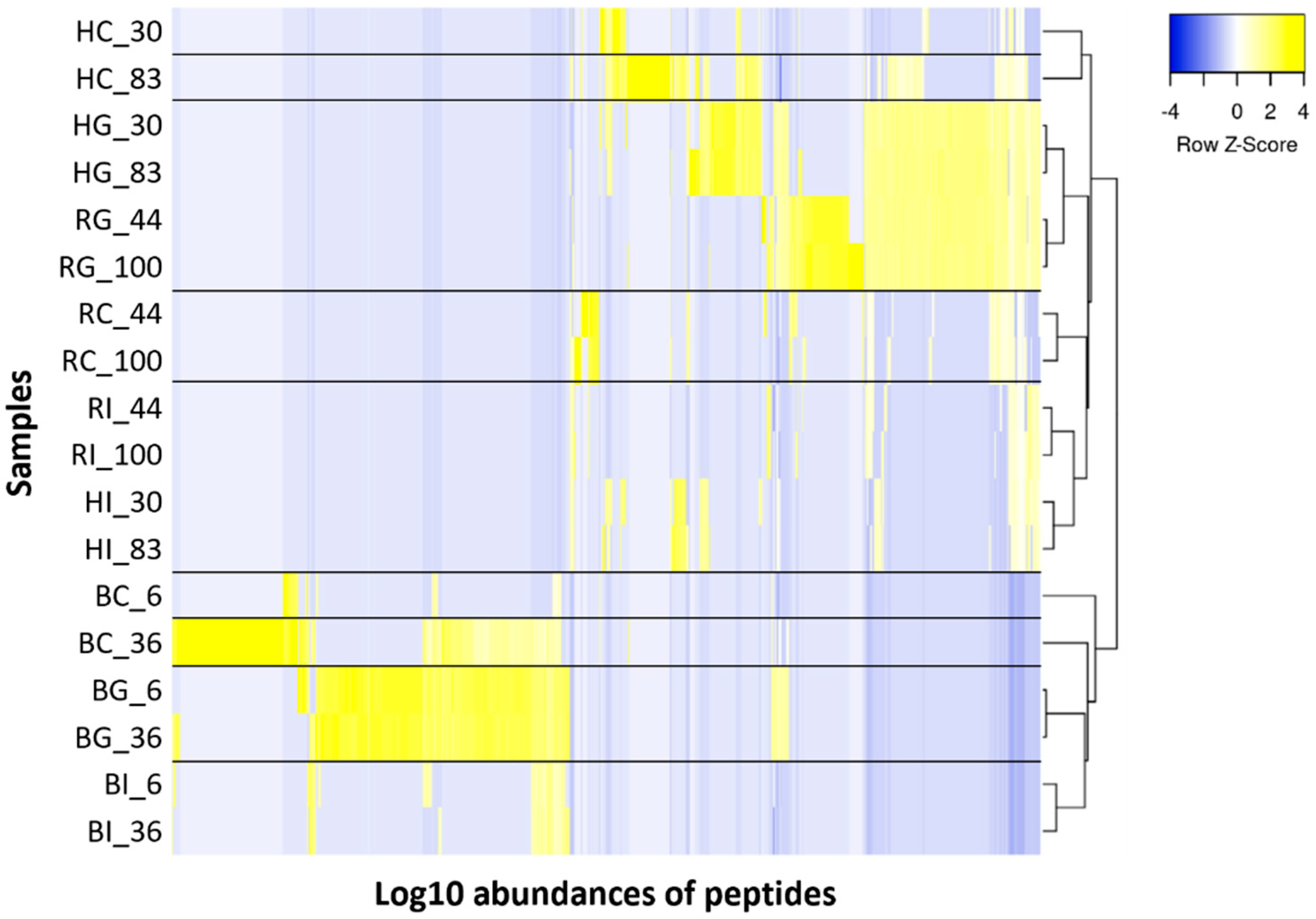
3.3. Peptide Analysis Using LC-MS and MS/MS
3.3.1. Comparison of Peptides between the Control and Digestive Samples
3.3.2. Comparison of Peptides between the Low- and High-Iron-Saturated Samples
3.3.3. Comparison of Peptides between the hmLF and rhLF or bLF Samples
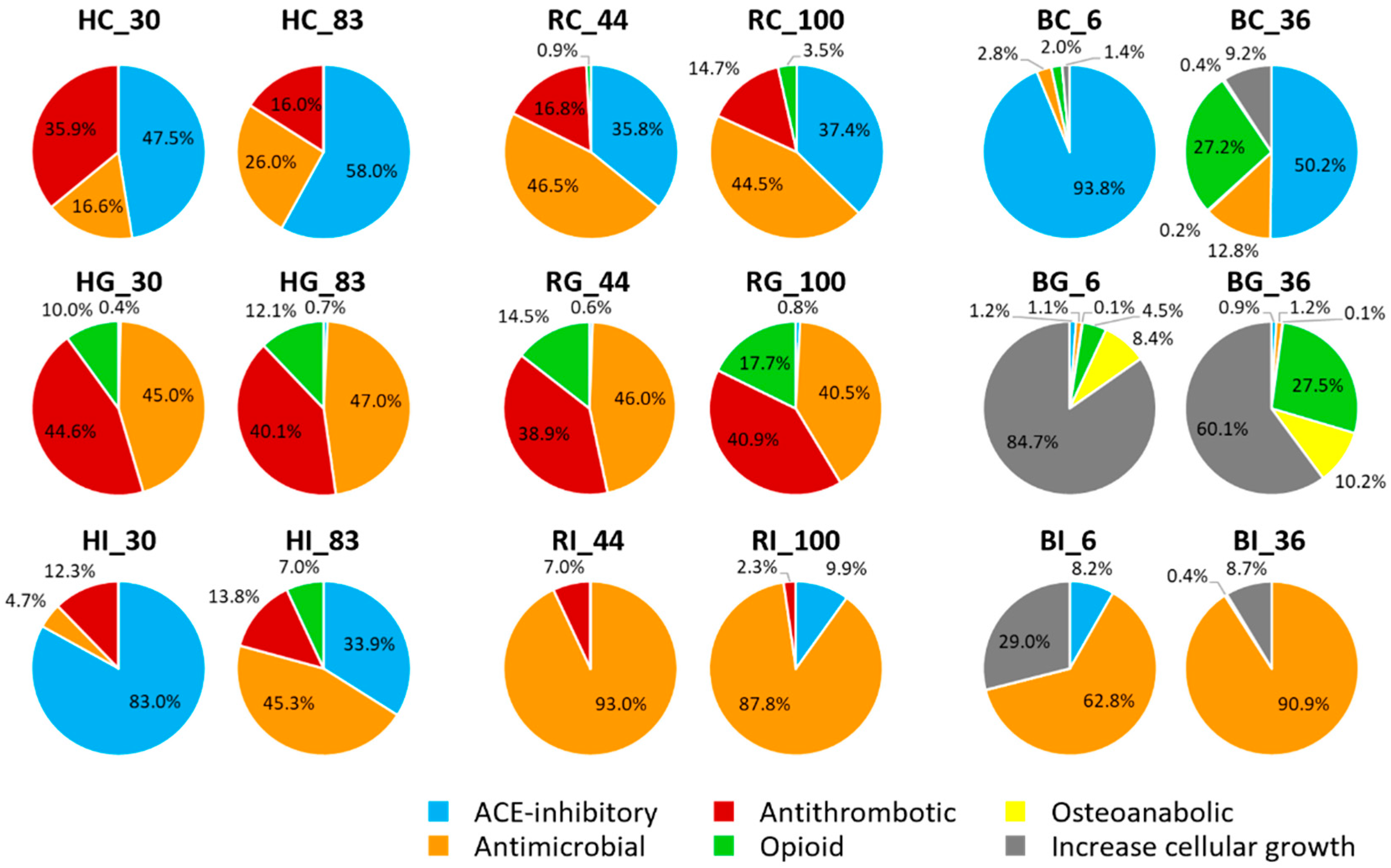
3.4. Investigation of Bioactive Peptides
4. Discussion
4.1. Quantitation of the Intact LF in Control and Digestive Samples
4.2. Peptide Analysis in Control and Digestive Samples
4.3. Investigation of Bioactive Peptides of bLF, hmLF and rhLF across Digestion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garcia-Montoya, I.A.; Cendon, T.S.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Conesa, C.; Calvo, M.; Sanchez, L. Recombinant human lactoferrin: A valuable protein for pharmaceutical products and functional foods. Biotechnol. Adv. 2010, 28, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Andrés, M.T.; Fierro, J.F.; Yeaman, M.R. The γ-core motif correlates with antimicrobial activity in cysteine-containing kaliocin-1 originating from transferrins. Biochim. Biophys. Acta 2007, 1768, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Du, X.; Jiang, R. Biological activities of commercial bovine lactoferrin sources. Biochem. Cell Biol. 2021, 99, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Abad, I.; Serrano, L.; Graikini, D.; Perez, M.D.; Grasa, L.; Sanchez, L. Effect of in vitro gastrointestinal digestion on the antibacterial activity of bioactive dairy formulas supplemented with lactoferrin against Cronobacter sakazakii. Biometals 2023, 36, 667–681. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Kawakami, H.; Lonnerdal, B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am. J. Physiol. 1991, 261, G841–G846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Du, X.; Lonnerdal, B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.M.; Boone, R.L.; Lu, J.; Spicer, S.K.; Guevara, M.A.; Moore, R.E.; Chambers, S.A.; Manning, S.D.; Dent, L.; Marshall, D.; et al. Analysis of antimicrobial and antibiofilm activity of human milk lactoferrin compared to bovine lactoferrin against multidrug resistant and susceptible Acinetobacter baumannii clinical isolates. ACS Infect. Dis. 2021, 7, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Talbott, R.D.; Batema, R.P. Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J. Med. Microbiol. 1999, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Han, X.; Shan, Y.J.; Zhang, L.W.; Du, M.; Liu, M.; Yi, H.X.; Ma, Y. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J. Dairy Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath-Deutsch, R.; Dallas, D.C.; Besada-Lombana, P.; Katz, L.; Conze, D.; Kruger, C.; Clark, A.J.; Peterson, R.; Malinczak, C.A. A review of the safety evidence on recombinant human lactoferrin for use as a food ingredient. Food Chem. Toxicol. 2024, 189, 114727. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.J.; Dallas, D.C. Bioactive milk peptides: An updated comprehensive overview and database. Crit. Rev. Food Sci. Nutr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Brines, R.D.; Brock, J.H. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta 1983, 759, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cummings, C.; Osuala, U.A.; Yennawar, N.H.; Namitz, K.E.W.; Hellner, B.; Besada-Lombana, P.B.; Peterson, R.D.; Clark, A.J. Characterization of recombinant human lactoferrin expressed in Komagataella Phaffii. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Boesman-Finkelstein, M.; Finkelstein, R.A. Sequential purification of lactoferrin, lysozyme and secretory immunoglobulin A from human milk. FEBS Lett. 1982, 144, 1–5. [Google Scholar] [CrossRef]
- Briscoe, M. Determination of heavy metals in food by inductively coupled plasma–mass spectrometry: First action 2015.01. J. AOAC Int. 2019, 98, 1113–1120. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Anaya, Y.; Martinez, R.R.; Goodman, R.E.; Johnson, P.; Vajpeyi, S.; Lu, X.; Peterson, R.; Weyers, S.M.; Breen, B.; Newsham, K.; et al. Evaluation of the potential food allergy risks of recombinant human lactoferrin expressed in Komagataella phaffii. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Liang, N.; Koh, J.; Kim, B.J.; Ozturk, G.; Barile, D.; Dallas, D.C. Structural and functional changes of bioactive proteins in donor human milk treated by vat-pasteurization, retort sterilization, ultra-high-temperature sterilization, freeze-thawing and homogenization. Front. Nutr. 2022, 9, 926814. [Google Scholar] [CrossRef]
- Kim, B.J.; Lueangsakulthai, J.; Sah, B.N.P.; Scottoline, B.; Dallas, D.C. Quantitative analysis of antibody survival across the infant digestive tract using mass spectrometry with parallel reaction monitoring. Foods 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Koh, J.; Liang, N.J.; Yang, J.; Ozturk, G.; Barile, D.; Dallas, D.C. Effect of vat pasteurization, ultra-high temperature sterilization, retort sterilization and homogenization on soluble proteins in donor human milk detected via proteomics. Lwt-Food Sci. Technol. 2023, 182, 114842. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Kuhfeld, R.F.; Eshpari, H.; Kim, B.J.; Kuhfeld, M.R.; Atamer, Z.; Dallas, D.C. Identification of bitter peptides in aged Cheddar cheese by crossflow filtration-based fractionation, peptidomics, statistical screening and sensory analysis. Food Chem. 2024, 439, 138111. [Google Scholar] [CrossRef]
- Tani, F.; Iio, K.; Chiba, H.; Yoshikawa, M. Isolation and characterization of opioid antagonist peptides derived from human lactoferrin. Agric. Biol. Chem. 1990, 54, 1803–1810. [Google Scholar]
- Sienkiewicz-Szlapka, E.; Jarmolowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Iwan, M. Contents of agonistic and antagonistic opioid peptides in different cheese varieties. Int. Dairy J. 2009, 19, 258–263. [Google Scholar] [CrossRef]
- Superti, F.; Siciliano, R.; Rega, B.; Giansanti, F.; Valenti, P.; Antonini, G. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Et Biophys. Acta 2001, 1528, 107–115. [Google Scholar] [CrossRef]
- Shi, P.; Fan, F.; Chen, H.; Xu, Z.; Cheng, S.; Lu, W.; Du, M. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J. Dairy Sci. 2020, 103, 3950–3960. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.; Vogel, H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 2009, 91, 141–154. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, M.I.; Groenink, J.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, S.; Theron, L.; Jimenez-Barrios, P.; Olalla-Herrera, M.; Recio, I.; Miralles, B. Protein profile and simulated digestive behavior of breast milk from overweight and normal weight mothers. Foods 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Elwakiel, M.; Boeren, S.; Wang, W.; Schols, H.A.; Hettinga, K.A. Degradation of proteins from colostrum and mature milk from Chinese mothers using an in vitro infant digestion model. Front. Nutr. 2020, 7, 162. [Google Scholar] [CrossRef]
- David-Birman, T.; Mackie, A.; Lesmes, U. Impact of dietary fibers on the properties and proteolytic digestibility of lactoferrin nano-particles. Food Hydrocoll. 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Nojima, Y.; Suzuki, Y.; Yoshida, K.; Abe, F.; Shiga, T.; Takeuchi, T.; Sugiyama, A.; Shimizu, H.; Sato, A. Lactoferrin conjugated with 40-kDa branched poly(ethylene glycol) has an improved circulating half-life. Pharm. Res. 2009, 26, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, G.; Shani Levi, C.; Levi Tal, S.; Lesmes, U. Emulsions stabilization by lactoferrin nano-particles under in vitro digestion conditions. Food Hydrocoll. 2013, 33, 264–272. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef]
- Furlund, C.B.; Ulleberg, E.K.; Devold, T.G.; Flengsrud, R.; Jacobsen, M.; Sekse, C.; Holm, H.; Vegarud, G.E. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J. Dairy Sci. 2013, 96, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Dallas, D.C. Systematic examination of protein extraction, proteolytic glycopeptide enrichment and MS/MS fragmentation techniques for site-specific profiling of human milk N-glycoproteins. Talanta 2021, 224, 121811. [Google Scholar] [CrossRef]
- Hua, S.; Nwosu, C.C.; Strum, J.S.; Seipert, R.R.; An, H.J.; Zivkovic, A.M.; German, J.B.; Lebrilla, C.B. Site-specific protein glycosylation analysis with glycan isomer differentiation. Anal. Bioanal. Chem. 2012, 403, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, N.; Kane, M.; Joshi, L.; Hickey, R.M. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology 2014, 24, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Valk-Weeber, R.L.; Eshuis-de Ruiter, T.; Dijkhuizen, L.; van Leeuwen, S.S. Dynamic temporal variations in bovine lactoferrin glycan structures. J. Agric. Food Chem. 2020, 68, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Leeuwen, S.S.V.; Schoemaker, R.J.; Timmer, C.J.; Kamerling, J.P.; Dijkhuizen, L. Use of Wisteria floribunda agglutinin affinity chromatography in the structural analysis of the bovine lactoferrin N-linked glycosylation. Biochim. Biophys. Acta 2012, 1820, 1444–1455. [Google Scholar] [CrossRef]
- Fujiyama, K.; Sakai, Y.; Misaki, R.; Yanagihara, I.; Honda, T.; Anzai, H.; Seki, T. N-Linked glycan structures of human lactoferrin produced by transgenic rice. Biosci. Biotechnol. Biochem. 2004, 68, 2565–2570. [Google Scholar] [CrossRef]
- Teng, C.T.; Gladwell, W. Single nucleotide polymorphisms (SNPs) in human lactoferrin gene. Biochem. Cell Biol. 2006, 84, 381–384. [Google Scholar] [CrossRef]

| Sample Name 1 | Lactoferrin Type | Digestion Stage | Iron Saturation (%) |
|---|---|---|---|
| HC_30 | Human milk (native) | Control | 29.9 |
| HG_30 | Gastric | ||
| HI_30 | Intestinal | ||
| HC_83 | Control | 82.5 | |
| HG_83 | Gastric | ||
| HI_83 | Intestinal | ||
| RC_44 | Recombinant human | Control | 43.7 |
| RG_44 | Gastric | ||
| RI_44 | Intestinal | ||
| RC_100 | Control | 100.0 | |
| RG_100 | Gastric | ||
| RI_100 | Intestinal | ||
| BC_6 | Bovine (native) | Control | 6.2 |
| BG_6 | Gastric | ||
| BI_6 | Intestinal | ||
| BC_36 | Control | 35.9 | |
| BG_36 | Gastric | ||
| BI_36 | Intestinal |
| Sample Name 1 | ||||||
|---|---|---|---|---|---|---|
| HC_30 | HG_30 | HI_30 | HC_83 | HG_83 | HI_83 | |
| Counts of peptides 2 | 5 | 7 | 5 | 5 | 7 | 5 |
| Counts of bioactive functions 3 | ||||||
| ACE-inhibitory | 2 | 1 | 1 | 2 | 2 | 1 |
| Antimicrobial | 2 | 12 | 3 | 2 | 12 | 1 |
| Opioid | 2 | 4 | 2 | 2 | 4 | 3 |
| Osteoanabolic | 0 | 3 | 0 | 0 | 3 | 1 |
| RC_44 | RG_44 | RI_44 | RC_100 | RG_100 | RI_100 | |
| Counts of peptides | 8 | 7 | 3 | 8 | 9 | 5 |
| Counts of bioactive functions | ||||||
| ACE-inhibitory | 3 | 2 | 0 | 3 | 4 | 1 |
| Antimicrobial | 5 | 14 | 3 | 3 | 14 | 6 |
| Opioid | 3 | 3 | 1 | 2 | 3 | 1 |
| Osteoanabolic | 1 | 3 | 0 | 1 | 3 | 0 |
| BC_6 | BG_6 | BI_6 | BC_36 | BG_36 | BI_36 | |
| Counts of peptides | 5 | 17 | 4 | 14 | 17 | 5 |
| Counts of bioactive functions | ||||||
| ACE-inhibitory | 2 | 8 | 1 | 7 | 8 | 0 |
| Antimicrobial | 1 | 14 | 6 | 11 | 14 | 7 |
| Antithrombotic | 0 | 1 | 0 | 1 | 1 | 0 |
| Opioid | 1 | 4 | 0 | 4 | 4 | 2 |
| Osteoanabolic | 0 | 5 | 0 | 2 | 5 | 0 |
| Increase cellular growth | 2 | 6 | 3 | 9 | 5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.J.; Kuhfeld, R.F.; Haas, J.L.; Anaya, Y.M.; Martinez, R.R.; Sah, B.N.P.; Breen, B.; Newsham, K.; Malinczak, C.-A.; Dallas, D.C. Digestive Profiles of Human Milk, Recombinant Human and Bovine Lactoferrin: Comparing the Retained Intact Protein and Peptide Release. Nutrients 2024, 16, 2360. https://doi.org/10.3390/nu16142360
Kim BJ, Kuhfeld RF, Haas JL, Anaya YM, Martinez RR, Sah BNP, Breen B, Newsham K, Malinczak C-A, Dallas DC. Digestive Profiles of Human Milk, Recombinant Human and Bovine Lactoferrin: Comparing the Retained Intact Protein and Peptide Release. Nutrients. 2024; 16(14):2360. https://doi.org/10.3390/nu16142360
Chicago/Turabian StyleKim, Bum Jin, Russell F. Kuhfeld, Joanna L. Haas, Yanisa M. Anaya, Raysa Rosario Martinez, Baidya Nath P. Sah, Bella Breen, Kahler Newsham, Carrie-Anne Malinczak, and David C. Dallas. 2024. "Digestive Profiles of Human Milk, Recombinant Human and Bovine Lactoferrin: Comparing the Retained Intact Protein and Peptide Release" Nutrients 16, no. 14: 2360. https://doi.org/10.3390/nu16142360
APA StyleKim, B. J., Kuhfeld, R. F., Haas, J. L., Anaya, Y. M., Martinez, R. R., Sah, B. N. P., Breen, B., Newsham, K., Malinczak, C.-A., & Dallas, D. C. (2024). Digestive Profiles of Human Milk, Recombinant Human and Bovine Lactoferrin: Comparing the Retained Intact Protein and Peptide Release. Nutrients, 16(14), 2360. https://doi.org/10.3390/nu16142360





