Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Magnetic Resonance Spectroscopy
2.3. Mediterranean Diet
2.4. Assessment of Adherence to the Mediterranean Diet
2.5. Variables
2.6. Statistical Analysis
3. Results
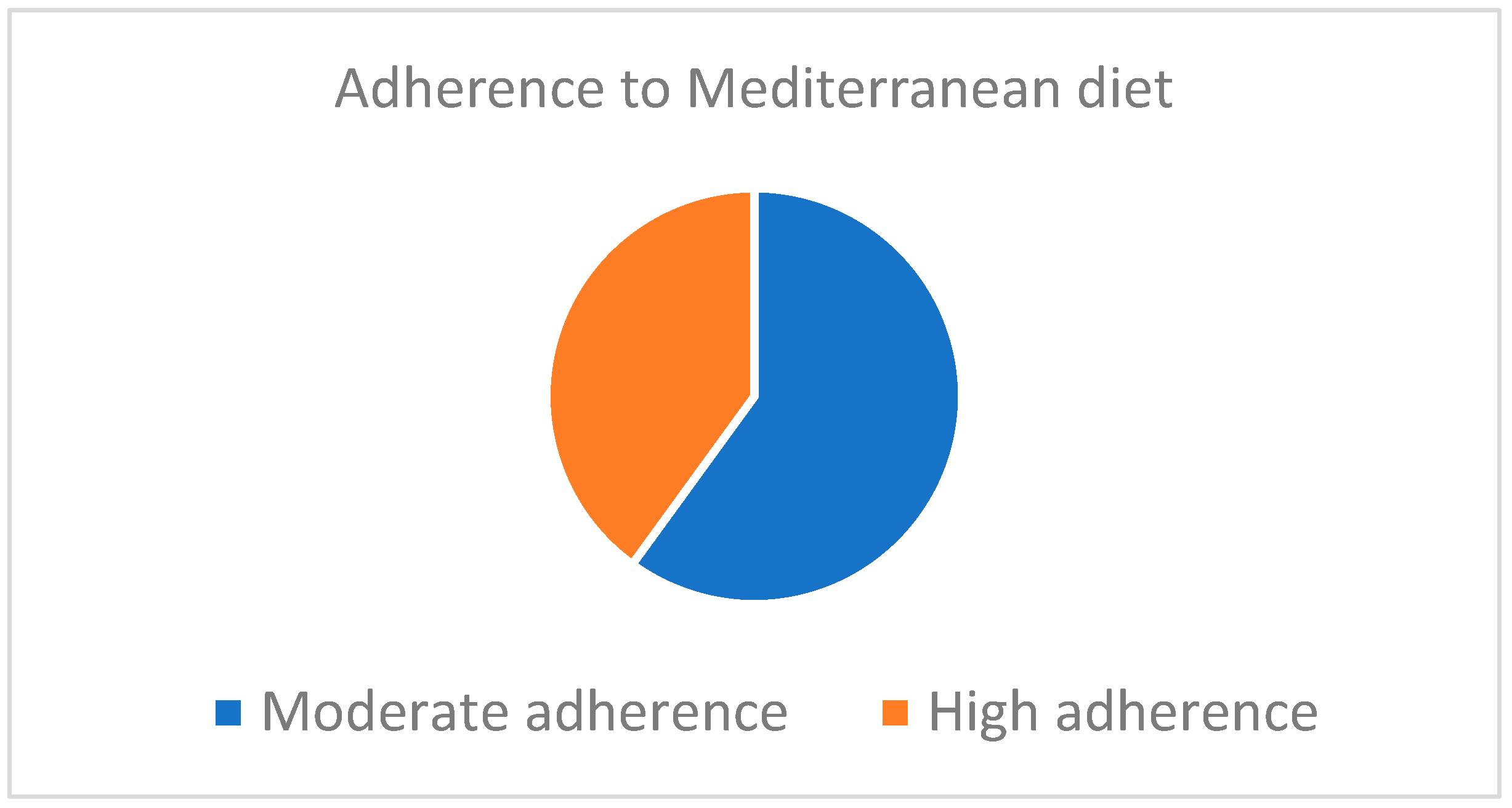
3.1. Adherence to the Mediterranean Diet
3.2. Anthropometric Measurements
3.3. MRS Measurements
3.4. Biochemical Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkins, T.; Tadkod, A.; Hepburn, I.; Schade, R.R. Nonalcoholic fatty liver disease: Diagnosis and management. Am. Fam. Physician 2013, 88, 35–42. [Google Scholar] [PubMed]
- Alves de Carvalho, M.S.; Coelho Cabral, P.; Kruze Grande de Arruda, I.; de Araújo Burgos, M.G.P.; da Silva Diniz, A.; Pernambuco, J.B.; de Lira, P.C. Risk factors associated with hepatic steatosis; a study in patients in the Northeast Brazil. Nutr. Hosp. 2012, 27, 1344–1350. [Google Scholar]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Caballeria, L.; Torán, P. The fatty liver epidemic: An analysis from the primary care. Aten. Primaria 2019, 51, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Meigs, J.B.; Sahani, D.V.; Hirschhorn, J.N.; O’Donnell, C.J.; Fox, C.S. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham Heart Study. Hepatology 2010, 51, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-M.; Huang, C.-K.; Hwang, J.-C.; Chiang, H.; Chang, C.-Y.; Lee, C.-T.; Yu, M.-L.; Lin, J.-T. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 2012, 22, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Zubiaga, L. Validation of biochemical scores for liver steatosis before and 1 year after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2019, 15, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Van Werven, J.R.; Marsman, H.A.; Nederveen, A.J.; Smits, N.J.; ten Kate, F.J.; van Gulik, T.M.; Stoker, J. Assessment of hepatic steatosis in patients undergoing liver resection: Comparison of US, CT, T1-weighted dual-echoMRimaging, and point-resolved 1H MR spectroscopy. Radiology 2010, 256, 159–168. [Google Scholar] [CrossRef]
- Alsina, M.E.; Ruiz-Tovar, J.; Bernabeu, A. Evolution of Liver Steatosis Quantified by MR Imaging and MR Spectroscopy, in Morbidly Obese Patients Undergoing Sleeve Gastrectomy: Short-Term Outcomes. Obes. Surg. 2017, 27, 1724–1728. [Google Scholar] [CrossRef]
- Costarelli, V.; Koretsi, E.; Georgitsogianni, E. Health-related quality of life of Greek adolescents: The role of the Mediterranean diet. Qual. Life Res. 2013, 22, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannos, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of theMediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Martinez, J.A.; De Irala I y Martinez-González, M.A. Determinants of the adherence to an “a priori” defined Mediterranean dietary pattern. Eur. J. Nutr. 2002, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lydakis, C.; Stefanaki, E.; Stefanaki, S.; Thalassinos, E.; Kavousanaki, M.; Lydaki, D. Correlation of blood pressure, obesity and adherence to the Mediterranean diet with indices of arterial stiffness in children. Eur. J. Pediatr. 2012, 171, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Boix, E.; Bozhychko, M.; Campo, J.M.D.; Martínez, R.; Bonete, J.M.; Calpena, R. Adherencia pre y postoperatoria a la dieta mediterránea y su efecto sobre la pérdida de peso y el perfil lipídico en pacientes obesos mórbidos sometidos a gastrectomía vertical como procedimiento bariátrico. Nutr. Hosp. 2014, 30, 756–762. [Google Scholar]
- Mokhtare, M.; Abdi, A.; Sadeghian, A.M.; Sotoudeheian, M.; Namazi, A.; Sikaroudi, M.K. Investigation about the correlation between the severity of metabolic-associated fatty liver disease and adherence to the Mediterranean diet. Clin. Nutr. ESPEN 2023, 58, 221–227. [Google Scholar] [CrossRef]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef]
- Gastaldo, I.; Casas, R.; Moizé, V. Clinical Impact of Mediterranean Diet Adherence before and after Bariatric Surgery: A Narrative Review. Nutrients 2022, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Petramala, L.; Di Renzo, L.; Sarlo, F.; Della Rocca, D.G.; Rizzo, M.; Fondacaro, V.; Iacopino, L.; Pepine, C.J.; De Lorenzo, A. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2013, 50, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Routh and the mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, M.; Lerotić, I.; Barsić, N.; Tomašić, V.; Jukić, L.V.; Velagić, V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J. Gastroenterol. 2007, 13, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; de Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postpradial lipemia in non-alcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Toledo, F.G.; Sniderman, A.D.; Kelley, D.E. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care 2006, 29, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.; Allende, H.; Lecube, A.; Salcedo, M.T.; A Baena-Fustegueras, J.; Fort, J.M.; Rivero, J.; Ferrer, R.; Catalán, R.; Pardina, E.; et al. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J. Hepatol. 2012, 4, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Pontiroli, A.E.; Benetti, A.; Folini, L.; Merlotti, C.; Frige, F. Other aspects of bariatric surgery: Liver steatosis, ferritin and cholesterol metabolism. Nutr. Hosp. 2013, 28, 104–108. [Google Scholar] [PubMed]
- Cefalu, W.T.; Rubino, F.; Cummings, D.E. Metabolic surgery for type 2diabetes: Changing the landscape of diabetes care. Diabetes Care 2016, 39, 857–860. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Camastra, S.; Astiarraga, B.; Nannipieri, M.; Castro-Perez, J.; Xie, D.; Wang, L.; Chakravarthy, M.; Haeusler, R.A. Increased bile acidsynthesis and deconjugation after biliopancreatic diversion. Diabetes 2015, 64, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Aylwin, S.J.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut hormone profilesfollowing bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Libby, A.E.; Bales, E.; Orlicky, D.J.; McManaman, J.L. Perilipin-2deletion impairs hepatic lipid accumulation by interfering with sterol regula-tory element-binding protein (SREBP) activation and altering the hepaticlipidome. J. Biol. Chem 2016, 291, 24231–24246. [Google Scholar] [CrossRef] [PubMed]
- Motomura, W.; Inoue, M.; Ohtake, T.; Takahashi, N.; Nagamine, M.; Tanno, S.; Kohgo, Y.; Okumura, T. Upregulation of ADRP in fatty liver in humanand liver steatosis in mice fed with high fat diet. Biochem. Biophys. Res. Commun. 2006, 340, 1111–1118. [Google Scholar] [CrossRef]
- Angelini, G.; Castagneto-Gissey, L.; Casella-Mariolo, J.; Caristo, M.E.; Russo, M.F.; Lembo, E.; Verrastro, O.; Stefanizzi, G.; Marini, P.L.; Casella, G.; et al. Duodenal-jejunal bypass improves nonalcoholic fatty liver diseaseindependently of weight loss in rodents with diet-induced obesity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 319, G502–G511. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Velasco, N.; Contreras, A.; Grassi, B. The Mediterranean diet, hepatic steatosis and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive component oleuropein promotes β-cell insulin secretion and protects β-cells from amylin amyloid-induced cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, Y.; Mena, V.; Priken, K. Effect of the Mediterranean diet on histological indicators and imaging tests in non-alcoholic fatty liver disease. Gastroenterol. Hepatol. 2022, 45, 350–360. [Google Scholar] [CrossRef] [PubMed]
| Vegetables | Spinach, chard, aubergines, watercress, endive, lettuce, cauliflower, mushrooms, leeks, asparagus, endive, cabbage, cucumber, peppers, tomatoes, green beans, beetroot, carrot, artichoke, or Brussels sprouts |
| Cereals | Pasta, semolina, rice, tapioca, potato, bread, or unsweetened biscuits |
| Legumes | Lentils, chickpeas, or beans |
| Fruits | Apple, pear, orange, peach, kiwi, peach, melon, watermelon, or strawberry |
| Protein sources | White fish, chicken, turkey, rabbit, beef, or eggs |
| Dairy products | Skimmed milk, skimmed yogurts, or fresh cheese |
| Fat source | Olive oil |
| Moderate Adherence | High Adherence | |
|---|---|---|
| 1. Have a fruit or fruit juice every day. | 100% | 100% |
| 2. You eat a second fruit every day. | 75% | 87.5% |
| 3. You eat fresh, raw, salad, or cooked vegetables regularly once a day. | 75% | 87.5% |
| 4. You eat fresh fish regularly (at least 2 or 3 times a week). | 50% | 75% |
| 5. You go once or more per week to a fast-food center (e.g., hamburger restaurant). | 16.7% | 0% |
| 6. Likes pulses and eats them more than once a week. | 33.3% | 50% |
| 7. Eats pasta or rice almost every day (5 days or more per week). | 25% | 25% |
| 8. Eats a cereal or cereal derivative (bread, toast, etc.) for breakfast. | 100% | 100% |
| 9. Eat nuts regularly (at least 2 or 3 times a week). | 16.7% | 37.5% |
| 10. Use olive oil at home. | 100% | 100% |
| 11. Do not eat breakfast. | 0% | 0% |
| 12. Have dairy for breakfast (milk or yogurt, etc.). | 25% | 50% |
| 13. Eats industrial pastries for breakfast. | 8.3% | 0% |
| 14. Eat 2 yogurts and/or 40 g of cheese every day. | 25% | 50% |
| 15. Eat sweets and candies every day. | 0% | 0% |
| Moderate Adherence (n = 12) | High Adherence (n = 8) | p | |
|---|---|---|---|
| Preoperative weight | 107.9 ± 17.9 | 106.5 ± 18.3 | NS |
| Preoperative BMI | 41.7 ± 3.5 | 41.1 ± 3.3 | NS |
| Postoperative weight | 78.1 ± 8.6 | 70.7 ± 7.4 | 0.048 |
| Postoperative BMI | 30.2 ± 2.7 | 27.3 ± 2.5 | 0.041 |
| Percentage of Excess weight loss | 68.9 ± 5.0 | 85.7 ± 5.4 | 0.027 |
| Moderate Adherence (n = 12) | High Adherence (n = 8) | p | |
|---|---|---|---|
| Preoperative PLC (%) | 14.5 ± 9.5 | 13.8 ± 9.3 | NS |
| Postoperative PLC (%) | 4.5 ± 1.8 | 3.3 ± 1.6 | 0.045 |
| D | <0.001 | <0.001 |
| Preoperative | Postoperative | p | |
|---|---|---|---|
| AST (U/L) | 26.3 ± 18.1 | 17.7 ± 9.2 | 0.025 |
| ALT (U/L) | 36.9 ± 19.6 | 19.1 ± 10.6 | 0.019 |
| Total cholesterol (mg/dL) | 221.2 ± 39.5 | 205.2 ± 22.7 | 0.16 |
| Triglyceride (mg/dL) | 178.2 ± 31.5 | 95.3 ± 16.3 | 0.001 |
| HDL-cholesterol (mg/dL) | 46.5 ± 12.3 | 61.4 ± 13.8 | 0.001 |
| LDL-cholesterol (mg/dL) | 128.7 ± 23.4 | 117 ± 21.1 | 0.216 |
| Fasting glucose (mg/dL) | 106.5 ± 34.2 | 84.9 ± 18.7 | 0.001 |
| Glycated hemoglobin (%) | 6.5 ± 1.7 | 5.1 ± 0.6 | 0.011 |
| Moderate Adherence | High Adherence | p | |
|---|---|---|---|
| AST (U/L) | 19.2 ± 9.5 | 16.3 ± 8.9 | 0.048 |
| ALT (U/L) | 21.0 ± 10.8 | 17.1 ± 10.4 | 0.042 |
| Total cholesterol (mg/dL) | 206.0 ± 22.7 | 204.6 ± 22.5 | 0.341 |
| Triglyceride (mg/dL) | 99.4 ± 16.4 | 91.3 ± 16.0 | 0.037 |
| HDL-cholesterol (mg/dL) | 60.2 ± 13.7 | 62.8 ± 13.9 | 0.089 |
| LDL-cholesterol (mg/dL) | 118.1 ± 21.1 | 116.1 ± 21.0 | 0.395 |
| Fasting glucose (mg/dL) | 87.8 ± 18.8 | 81.9 ± 18.5 | 0.044 |
| Glycated hemoglobin (%) | 5.2 ± 0.7 | 5.1 ± 0.6 | 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Tovar, J.; Llavero, C.; Rodriguez-Ortega, M.; De Castro, N.M.; Martín-Crespo, M.C.; Escobar-Aguilar, G.; Martin-Nieto, A.; Gonzalez, G. Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients 2024, 16, 2280. https://doi.org/10.3390/nu16142280
Ruiz-Tovar J, Llavero C, Rodriguez-Ortega M, De Castro NM, Martín-Crespo MC, Escobar-Aguilar G, Martin-Nieto A, Gonzalez G. Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients. 2024; 16(14):2280. https://doi.org/10.3390/nu16142280
Chicago/Turabian StyleRuiz-Tovar, Jaime, Carolina Llavero, Maria Rodriguez-Ortega, Nuria M. De Castro, Maria Cristina Martín-Crespo, Gema Escobar-Aguilar, Ana Martin-Nieto, and Gilberto Gonzalez. 2024. "Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet" Nutrients 16, no. 14: 2280. https://doi.org/10.3390/nu16142280
APA StyleRuiz-Tovar, J., Llavero, C., Rodriguez-Ortega, M., De Castro, N. M., Martín-Crespo, M. C., Escobar-Aguilar, G., Martin-Nieto, A., & Gonzalez, G. (2024). Improvement of Metabolic-Associated Fatty Liver Disease by Magnetic Resonance Spectroscopy in Morbidly Obese Women Undergoing Roux-en-Y Gastric Bypass, following a Postoperative Mediterranean-like Diet. Nutrients, 16(14), 2280. https://doi.org/10.3390/nu16142280






