Reassessing the Impact of Coffee Consumption on Liver Disease: Insights from a Large-Scale Cohort Study with IPTW Adjustment
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Patient Information Utilizing UK Biobank Cohort
2.2. Exclusion Criteria
2.3. Classification of Patient Cohorts
2.4. Inverse Probability of Treatment Weighting
2.5. Kaplan-Meier Survival Analysis
2.6. Statistical Anlysis
3. Results
3.1. Baseline Characteristics of Study Population
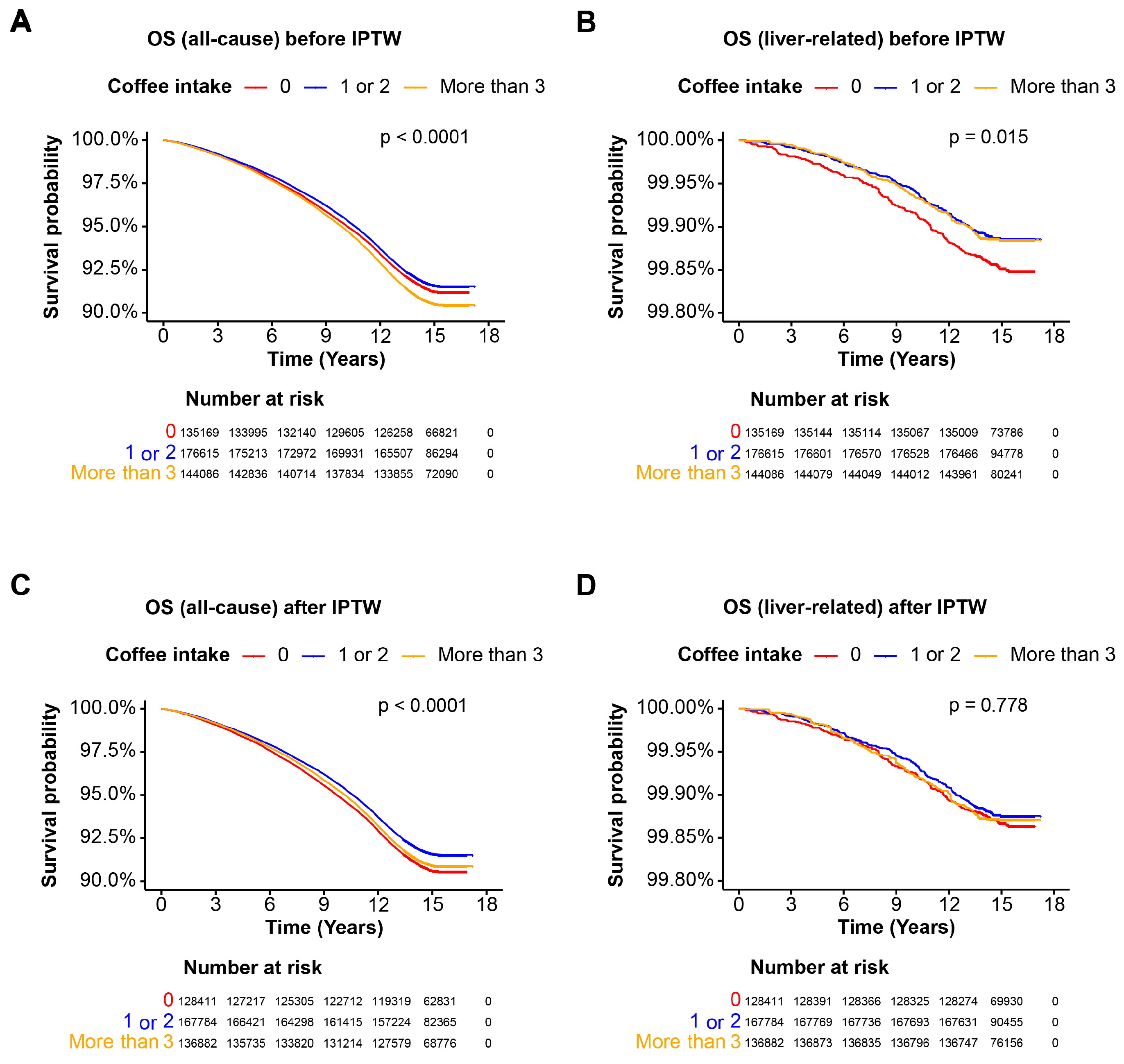
3.2. Overall Survival in the Entire Cohort
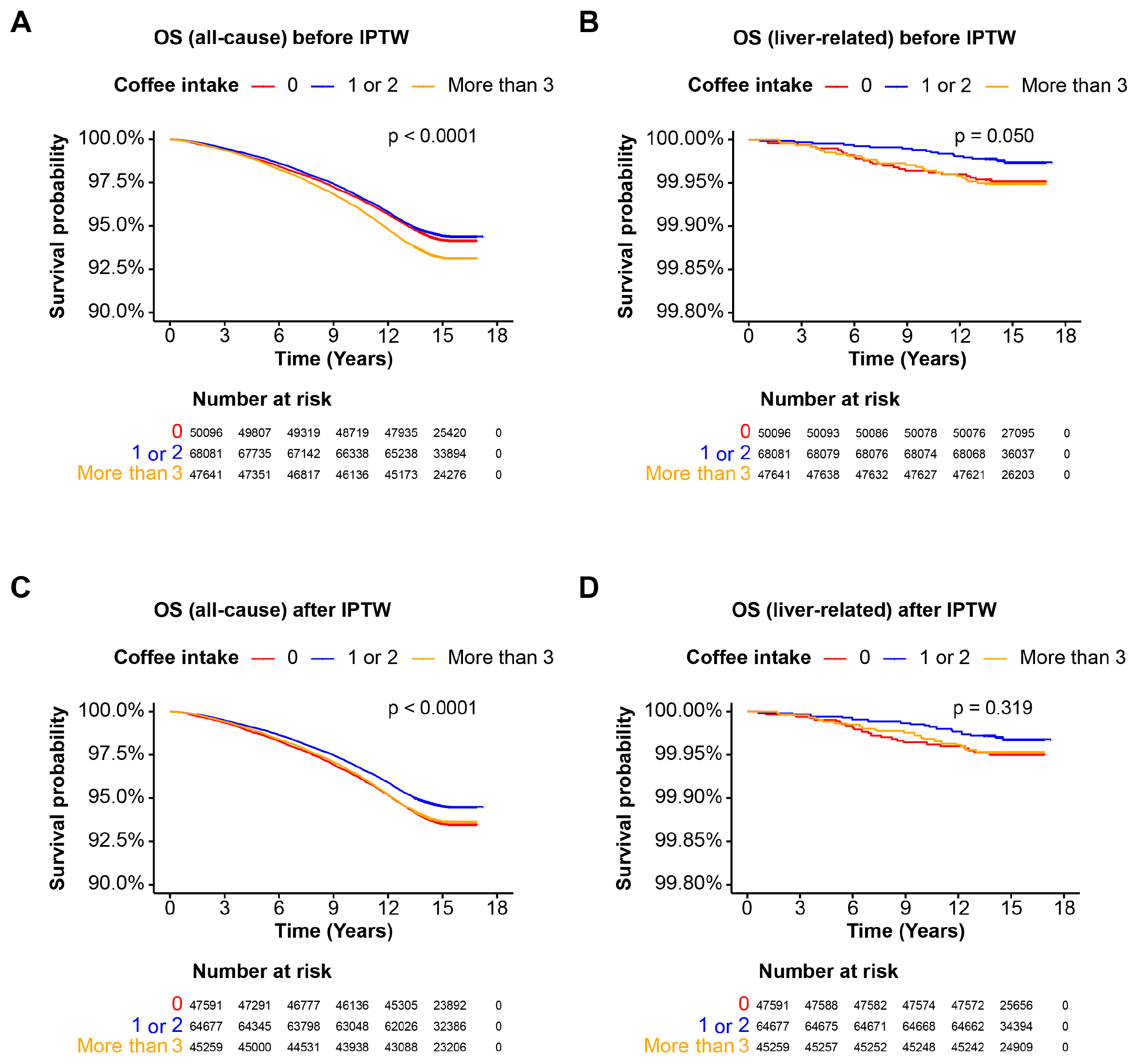
3.3. Coffee Intake and Mortality in the Cohort without Steatotic Liver Disease
3.4. Coffee Intake and Mortality in the MASLD Cohort
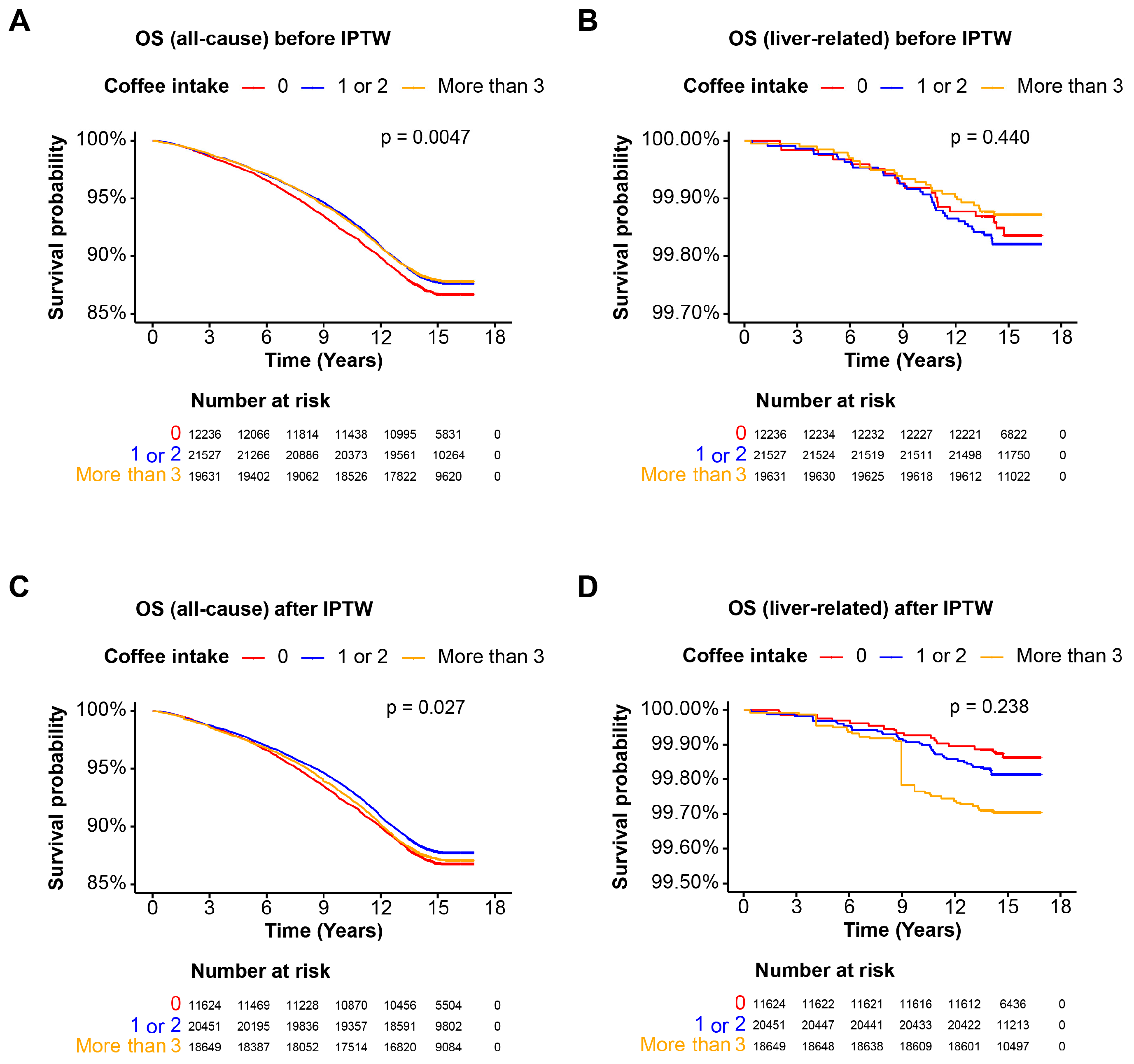
3.5. Coffee Intake and Mortality in the MetALD Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Van Son, K.; te Nijenhuis-Noort, L.; Boone, S.; Mook-Kanamori, D.; Holleboom, A.; Roos, P.; Lamb, H.; Alblas, G.; Coenraad, M.; Rosendaal, F. Prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) in a middle-aged population with overweight and normal liver enzymes, and diagnostic accuracy of noninvasive proxies. Medicine 2024, 103, e34934. [Google Scholar] [CrossRef]
- Rezaee-Zavareh, M.S.; Kim, N.; Yang, J.D. Starting the journey: Understanding the roles of complement proteins in liver diseases through mendelian randomization. Clin. Mol. Hepatol. 2024, 30, 150. [Google Scholar] [CrossRef]
- Hagström, H.; Hegmar, H.; Moreno, C. Interactions between the metabolic syndrome and alcohol consumption increases the risk of liver disease. United Eur. Gastroenterol. J. 2024, 12, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Shahzil, M.; Moond, V.; Shahzad, M.; Thandavaram, A.; Sehar, A.; Waseem, H.; Siddiqui, T.; Dahiya, D.S.; Patel, P. Non-Pharmacological Approach to Diet and Exercise in Metabolic-Associated Fatty Liver Disease: Bridging the Gap between Research and Clinical Practice. J. Pers. Med. 2024, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- National Coffee Association. The 2020 National Coffee Data Trends Report—The “Atlas of American Coffee”; National Coffee Association: Houston, TX, USA, 2021. [Google Scholar]
- Rodríguez-Artalejo, F.; López-García, E. Coffee consumption and cardiovascular disease: A condensed review of epidemiological evidence and mechanisms. J. Agric. Food Chem. 2017, 66, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.; Roderick, P.; Buchanan, R.; Fallowfield, J.; Hayes, P.; Parkes, J. Systematic review with meta-analysis: Coffee consumption and the risk of cirrhosis. Aliment. Pharmacol. Ther. 2016, 43, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012, 55, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, B.H.; Park, J.-W. Preventive strategy for nonalcoholic fatty liver disease-related hepatocellular carcinoma. Clin. Mol. Hepatol. 2023, 29, S220. [Google Scholar] [CrossRef] [PubMed]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A.; Feld, J.J.; Lavoie, É.G.; Fausther, M. How does coffee prevent liver fibrosis? Biological plausibility for recent epidemiological observations. Hepatology 2014, 60, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and anti-inflammatory activity of coffee brew evaluated after simulated gastrointestinal digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.D.; Brahmbhatt, M.; Tahan, A.C.; Ibdah, J.A.; Tahan, V. Coffee: The magical bean for liver diseases. World J. Hepatol. 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Joo, M.-J.; Lee, Y.-S.; Kim, M.-G. Effects of coffee consumption on insulin resistance and sensitivity: A meta-analysis. Nutrients 2021, 13, 3976. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; White, D.; Kanwal, F.; Ramsey, D.; Mittal, S.; Tavakoli-Tabasi, S.; Kuzniarek, J.; El-Serag, H.B. Coffee and caffeine are associated with decreased risk of advanced hepatic fibrosis among patients with hepatitis C. Clin. Gastroenterol. Hepatol. 2015, 13, 1521–1531.e1523. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Wu, G.; Chen, L.; Hu, P.; Ren, H.; Hu, H. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: A meta-analysis. PLoS ONE 2015, 10, e0142457. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Intake, Cups/Day | SMD | Coffee Intake, Cups/Day | SMD | |||||
| None, n = 135,169 | 1 or 2, n = 176,615 | More than 3, n = 144,086 | None, n = 128,410 | 1 or 2, n = 167,784 | More than 3, n = 136,882 | |||
| Sex (Male) | 56,913 (42.1%) | 77,148 (43.7%) | 71,266 (49.5%) | 0.124 | 58,220 (45.3%) | 75,596 (45.1%) | 61,841 (45.2%) | 0.001 |
| Age at recruitment | 55.71 ± 8.20 | 57.21 ± 8.06 | 56.53 ± 8.01 | 0.099 | 56.58 ± 8.09 | 56.53 ± 8.20 | 56.60 ± 8.01 | 0.006 |
| Smoking status | 0.175 | 0.002 | ||||||
| Current | 12,517 (9.3%) | 13,862 (7.8%) | 20,825 (14.5%) | 13,367 (10.4%) | 17,512 (10.4%) | 14,253 (10.4%) | ||
| Previous | 42,599 (31.5%) | 61,882 (35.0%) | 52,121 (36.2%) | 44,290 (34.5%) | 57,659 (34.4%) | 47,115 (34.4%) | ||
| Never | 79,932 (59.2%) | 100,871 (57.1%) | 71,140 (49.4%) | 70,720 (55.1%) | 92,614 (55.2%) | 75,514 (55.2%) | ||
| Body mass index (kg/m2) | 27.42 ± 4.98 | 27.09 ± 4.65 | 27.77 ± 4.77 | 0.095 | 27.41 ± 4.90 | 27.42 ± 4.88 | 27.44 ± 4.65 | 0.004 |
| Waist circumference (cm) | 89.80 ± 13.65 | 89.32 ± 13.22 | 91.45 ± 13.50 | 0.105 | 90.18 ± 13.57 | 90.17 ± 13.54 | 90.21 ± 13.36 | 0.002 |
| Type 2 diabetes | 15,501 (11.5%) | 16,891 (9.6%) | 15,147 (10.5%) | 0.042 | 13,420 (10.5%) | 17,595 (10.5%) | 14,333 (10.5%) | 0.001 |
| Dyslipidemia | 37,979 (28.1%) | 50,189 (28.4%) | 41,802 (29.0%) | 0.016 | 36,765 (28.6%) | 47,851 (28.5%) | 39,220 (28.7%) | 0.002 |
| Hypertension | 57,056 (42.2%) | 72,461 (41.0%) | 59,335 (41.2%) | 0.013 | 53,377 (41.6%) | 69,540 (41.4%) | 56,971 (41.6%) | 0.002 |
| ALT (U/L) | 23.29 ± 14.00 | 23.16 ± 13.47 | 23.61 ± 13.92 | 0.022 | 23.40 ± 13.90 | 23.38 ± 13.54 | 23.45 ± 14.53 | 0.003 |
| GGT (U/L) | 37.84 ± 43.61 | 35.81 ± 38.09 | 36.00 ± 35.93 | 0.033 | 36.77 ± 37.48 | 36.56 ± 40.37 | 37.24 ± 47.28 | 0.011 |
| Platelet (109/L) | 254.67 ± 61.08 | 252.01 ± 59.26 | 253.92 ± 59.60 | 0.059 | 253.36 ± 60.64 | 253.41 ± 60.18 | 253.41 ± 59.25 | 0.001 |
| Albumin (g/L) | 4.51 ± 0.27 | 4.53 ± 0.26 | 4.53 ± 0.26 | 0.030 | 4.52 ± 0.26 | 4.52 ± 0.26 | 4.52 ± 0.26 | 0.002 |
| Clinical Characteristics | Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Intake, Cups/Day | SMD | Coffee Intake, Cups/Day | SMD | |||||
| None, n = 50,096 | 1 or 2, n = 68,081 | More than 3, n = 47,641 | None, n = 47,591 | 1 or 2, n = 64,677 | More than 3, n = 45,259 | |||
| Sex (Male) | 10,797 (21.6%) | 15,068 (22.1%) | 12,049 (25.3%) | 0.059 | 10,971 (23.1%) | 14,770 (22.8%) | 10,402 (23.0%) | 0.003 |
| Age at recruitment | 53.99 ± 8.25 | 55.83 ± 8.23 | 55.36 ± 8.12 | 0.149 | 55.16 ± 8.23 | 55.11 ± 8.32 | 55.18 ± 8.15 | 0.005 |
| Smoking status | 0.200 | 0.002 | ||||||
| Current | 4101 (8.2%) | 4665 (6.9%) | 6899 (14.5%) | 4542 (9.6%) | 6162 (9.5%) | 4311 (9.5%) | ||
| Previous | 48,402 (29.2%) | 13,244 (26.5%) | 20,333 (29.9%) | 13,950 (29.3%) | 18,895 (29.2%) | 13,227 (29.2%) | ||
| Never | 32,630 (65.3%) | 43,083 (63.3%) | 25,917 (54.4%) | 29,064 (61.1%) | 39,620 (61.3%) | 27,721 (61.3%) | ||
| Body mass index (kg/m2) | 23.42 ± 2.39 | 23.47 ± 2.30 | 23.72 ± 2.36 | 0.085 | 23.53 ± 2.38 | 23.53 ± 2.31 | 23.53 ± 2.36 | 0.002 |
| Waist circumference (cm) | 77.26 ± 7.13 | 77.51 ± 7.07 | 78.12 ± 7.10 | 0.081 | 77.62 ± 7.12 | 77.61 ± 7.07 | 77.63 ± 7.16 | 0.002 |
| Type 2 diabetes | 1569 (3.1%) | 1800 (2.6%) | 1461 (3.1%) | 0.019 | 1398 (2.9%) | 1883 (2.9%) | 1333 (2.9%) | 0.001 |
| Dyslipidemia | 7229 (14.4%) | 10,893 (16.0%) | 7612 (16.0%) | 0.029 | 7410 (15.6%) | 10,009 (15.5%) | 7049 (15.6%) | 0.002 |
| Hypertension | 12,195 (24.3%) | 17,089 (25.1%) | 11,776 (24.7%) | 0.012 | 11,816 (24.8%) | 15,977 (24.7%) | 11,250 (24.9%) | 0.002 |
| ALT (U/L) | 17.27 ± 7.57 | 17.59 ± 7.61 | 17.44 ± 7.95 | 0.027 | 17.48 ± 7.78 | 17.47 ± 7.43 | 17.52 ± 8.66 | 0.004 |
| GGT (U/L) | 21.77 ± 13.64 | 21.57 ± 12.69 | 21.48 ± 13.09 | 0.015 | 21.66 ± 12.60 | 21.59 ± 12.97 | 21.80 ± 16.58 | 0.010 |
| Platelet (109/L) | 253.99 ± 59.53 | 252.97 ± 57.87 | 256.13 ± 59.28 | 0.036 | 254.21 ± 59.48 | 254.17 ± 58.95 | 254.19 ± 58.41 | <0.001 |
| Albumin (g/L) | 4.51 ± 0.26 | 4.54 ± 0.26 | 4.54 ± 0.26 | 0.070 | 4.53 ± 0.26 | 4.53 ± 0.26 | 4.53 ± 0.26 | <0.001 |
| Clinical Characteristics | Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Intake, Cups/Day | SMD | Coffee Intake, Cups/Day | SMD | |||||
| None, n = 72,873 | 1 or 2, n = 87,007 | More than 3, n = 76,814 | None, n = 69,195 | 1 or 2, n = 82,657 | More than 3, n = 72,973 | |||
| Sex (Male) | 37,568 (51.6%) | 47,082 (54.1%) | 44,923 (58.5%) | 0.093 | 38,049 (55.0%) | 45,277 (54.8%) | 39,981 (54.8%) | 0.003 |
| Age at recruitment | 56.45 ± 8.06 | 57.74 ± 7.93 | 56.74 ± 7.96 | 0.108 | 57.05 ± 7.98 | 56.99 ± 8.10 | 57.05 ± 7.91 | 0.005 |
| Smoking status | 0.164 | 0.002 | ||||||
| Current | 6736 (9.2%) | 6863 (7.9%) | 10,770 (14.0%) | 7138 (10.3%) | 8553 (10.3%) | 7535 (10.3%) | ||
| Previous | 23,419 (32.2%) | 30,838 (35.4%) | 28,046 (36.5%) | 24,141 (34.9%) | 28,754 (34.8%) | 25,400 (34.8%) | ||
| Never | 42,682 (58.6%) | 49,306 (56.7%) | 37,998 (49.5%) | 37,916 (54.8%) | 45,350 (54.9%) | 40,039 (54.9%) | ||
| Body mass index (kg/m2) | 29.97 ± 4.68 | 29.62 ± 4.41 | 30.06 ± 4.50 | 0.066 | 29.87 ± 4.62 | 29.89 ± 4.63 | 29.89 ± 4.37 | 0.003 |
| Waist circumference (cm) | 97.21 ± 11.06 | 96.79 ± 10.70 | 98.22 ± 11.00 | 0.087 | 97.40 ± 11.02 | 97.41 ± 11.04 | 97.41 ± 10.77 | 0.001 |
| Type 2 diabetes | 12,484 (17.1%) | 12,823 (14.7%) | 11,628 (15.1%) | 0.044 | 10,784 (15.6%) | 12,940 (15.7%) | 11,358 (15.6%) | 0.002 |
| Dyslipidemia | 26,082 (35.8%) | 31,135 (35.8%) | 27,077 (35.3%) | 0.008 | 24,708 (35.7%) | 29,440 (35.6%) | 26,020 (35.7%) | 0.001 |
| Hypertension | 37,902 (52.0%) | 43,942 (50.5%) | 37,654 (49.0%) | 0.04 | 35,011 (50.6%) | 41,744 (50.5%) | 36,899 (50.6%) | 0.001 |
| ALT (U/L) | 26.56 ± 15.40 | 26.45 ± 14.85 | 26.47 ± 15.03 | 0.005 | 26.52 ± 15.16 | 26.53 ± 14.75 | 26.57 ± 15.97 | 0.002 |
| GGT (U/L) | 44.29 ± 46.16 | 42.31 ± 41.63 | 41.22 ± 38.47 | 0.048 | 42.82 ± 40.33 | 42.64 ± 42.86 | 43.10 ± 47.99 | 0.007 |
| Platelet (109/L) | 254.40 ± 61.11 | 256.37 ± 62.40 | 252.85 ± 60.77 | 0.038 | 254.34 ± 61.69 | 254.43 ± 61.55 | 254.41 ± 60.11 | 0.001 |
| Albumin (g/L) | 4.50 ± 0.27 | 4.51 ± 0.26 | 4.52 ± 0.26 | 0.054 | 4.51 ± 0.27 | 4.51 ± 0.26 | 4.52 ± 0.26 | 0.002 |
| Clinical Characteristics | Before IPTW | After IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Coffee Intake, Cups/Day | SMD | Coffee Intake, Cups/Day | SMD | |||||
| None, n = 12,236 | 1 or 2, n = 21,527 | More than 3, n = 19,631 | None, n = 11,624 | 1 or 2, n = 20,451 | More than 3, n = 18,649 | |||
| Sex (Male) | 8548 (69.9%) | 14,998 (69.7%) | 14,294 (72.8%) | 0.046 | 8255 (71.0%) | 14,478 (70.8%) | 13,214 (70.9%) | 0.003 |
| Age at recruitment | 58.31 ± 7.44 | 59.50 ± 7.21 | 58.55 ± 7.42 | 0.108 | 58.87 ± 7.28 | 58.85 ± 7.47 | 58.86 ± 7.35 | 0.002 |
| Smoking status | 0.103 | 57.05 ± 7.98 | 0.003 | |||||
| Current | 1680 (13.7%) | 2334 (10.8%) | 3156 (16.1%) | 1569 (13.5%) | 2757 (13.5%) | 2521 (13.5%) | ||
| Previous | 5936 (48.5%) | 10,711 (49.8%) | 9250 (47.1%) | 5664 (48.7%) | 9921 (48.5%) | 9049 (48.5%) | ||
| Never | 4620 (37.8%) | 8482 (39.4%) | 7225 (36.8%) | 4392 (37.8%) | 7773 (38.0%) | 7080 (38.0%) | ||
| Body mass index (kg/m2) | 28.54 ± 3.79 | 28.35 ± 3.60 | 28.64 ± 3.65 | 0.053 | 28.52 ± 3.75 | 28.51 ± 3.71 | 28.52 ± 3.60 | 0.002 |
| Waist circumference (cm) | 96.97 ± 10.09 | 96.44 ± 9.75 | 97.29 ± 9.84 | 0.057 | 96.92 ± 9.98 | 96.88 ± 9.93 | 96.91 ± 9.81 | 0.003 |
| Type 2 diabetes | 1461 (11.9%) | 2268 (10.5%) | 2058 (10.5%) | 0.031 | 1271 (10.9%) | 2230 (10.9%) | 2050 (11.0%) | 0.002 |
| Dyslipidemia | 4688 (38.3%) | 8161 (37.9%) | 7113 (36.2%) | 0.027 | 4358 (37.5%) | 7649 (37.4%) | 7013 (37.6%) | 0.003 |
| Hypertension | 6988 (57.1%) | 11,430 (53.1%) | 9905 (50.5%) | 0.089 | 6169 (53.1%) | 10,845 (53.0%) | 9911 (53.1%) | 0.002 |
| ALT (U/L) | 28.48 ± 16.69 | 27.52 ± 15.87 | 27.41 ± 15.70 | 0.044 | 27.90 ± 16.57 | 27.75 ± 15.97 | 28.11 ± 17.77 | 0.014 |
| GGT (U/L) | 65.09 ± 74.15 | 54.59 ± 56.78 | 50.78 ± 49.29 | 0.153 | 56.93 ± 55.06 | 56.08 ± 64.77 | 58.39 ± 103.83 | 0.029 |
| Platelet (109/L) | 247.37 ± 58.86 | 245.66 ± 56.98 | 247.10 ± 57.46 | 0.020 | 246.42 ± 58.14 | 246.54 ± 57.46 | 246.19 ± 57.67 | 0.004 |
| Albumin (g/L) | 4.53 ± 0.26 | 4.54 ± 0.26 | 4.54 ± 0.26 | 0.041 | 4.54 ± 0.26 | 4.54 ± 0.26 | 4.54 ± 0.26 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Chang, Y.; Jeong, S.W.; Jang, J.Y.; Ryu, T. Reassessing the Impact of Coffee Consumption on Liver Disease: Insights from a Large-Scale Cohort Study with IPTW Adjustment. Nutrients 2024, 16, 2020. https://doi.org/10.3390/nu16132020
Yang K, Chang Y, Jeong SW, Jang JY, Ryu T. Reassessing the Impact of Coffee Consumption on Liver Disease: Insights from a Large-Scale Cohort Study with IPTW Adjustment. Nutrients. 2024; 16(13):2020. https://doi.org/10.3390/nu16132020
Chicago/Turabian StyleYang, Keungmo, Young Chang, Soung Won Jeong, Jae Young Jang, and Tom Ryu. 2024. "Reassessing the Impact of Coffee Consumption on Liver Disease: Insights from a Large-Scale Cohort Study with IPTW Adjustment" Nutrients 16, no. 13: 2020. https://doi.org/10.3390/nu16132020
APA StyleYang, K., Chang, Y., Jeong, S. W., Jang, J. Y., & Ryu, T. (2024). Reassessing the Impact of Coffee Consumption on Liver Disease: Insights from a Large-Scale Cohort Study with IPTW Adjustment. Nutrients, 16(13), 2020. https://doi.org/10.3390/nu16132020





