Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats
Abstract
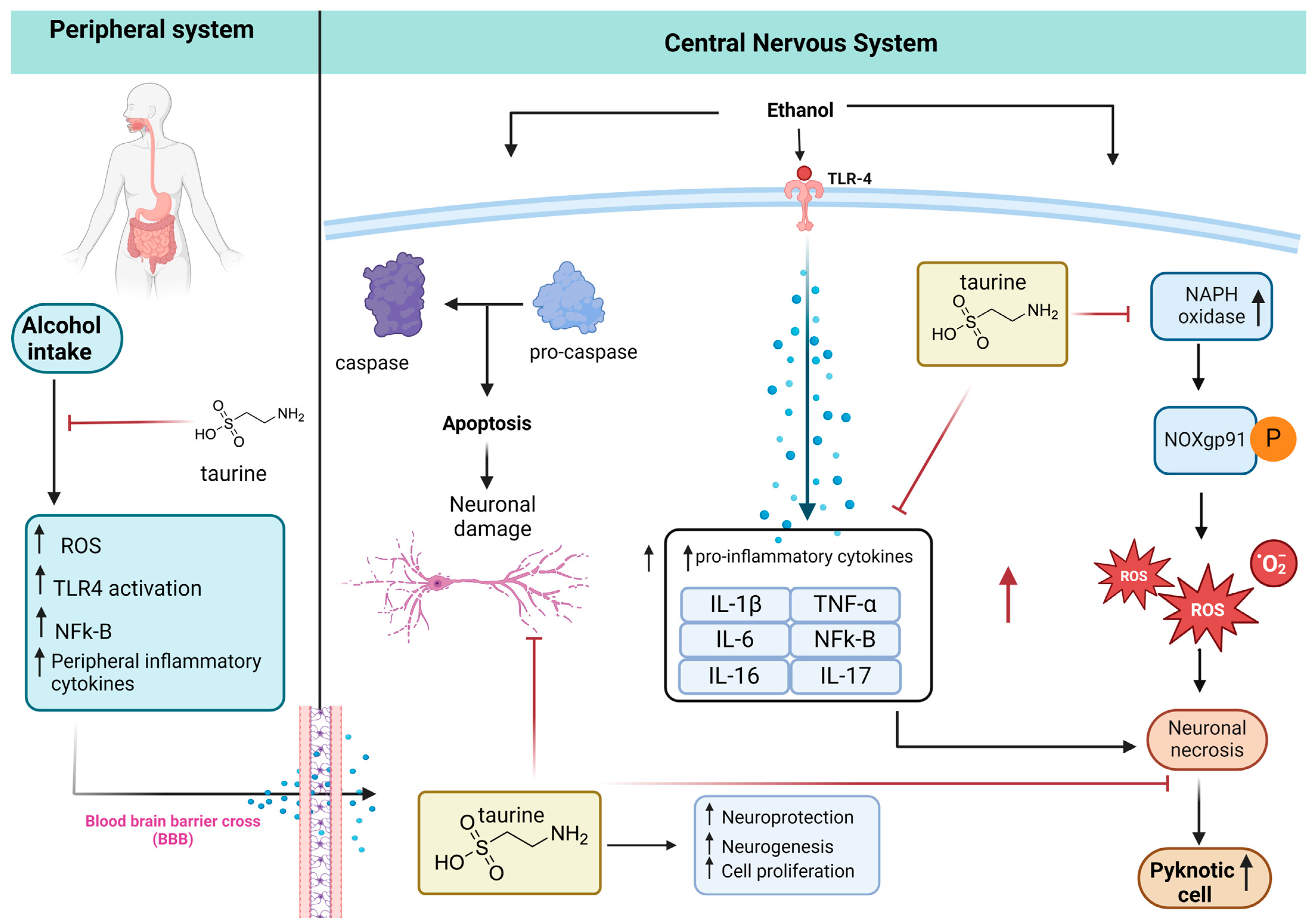
1. Introduction
2. Materials and Methods
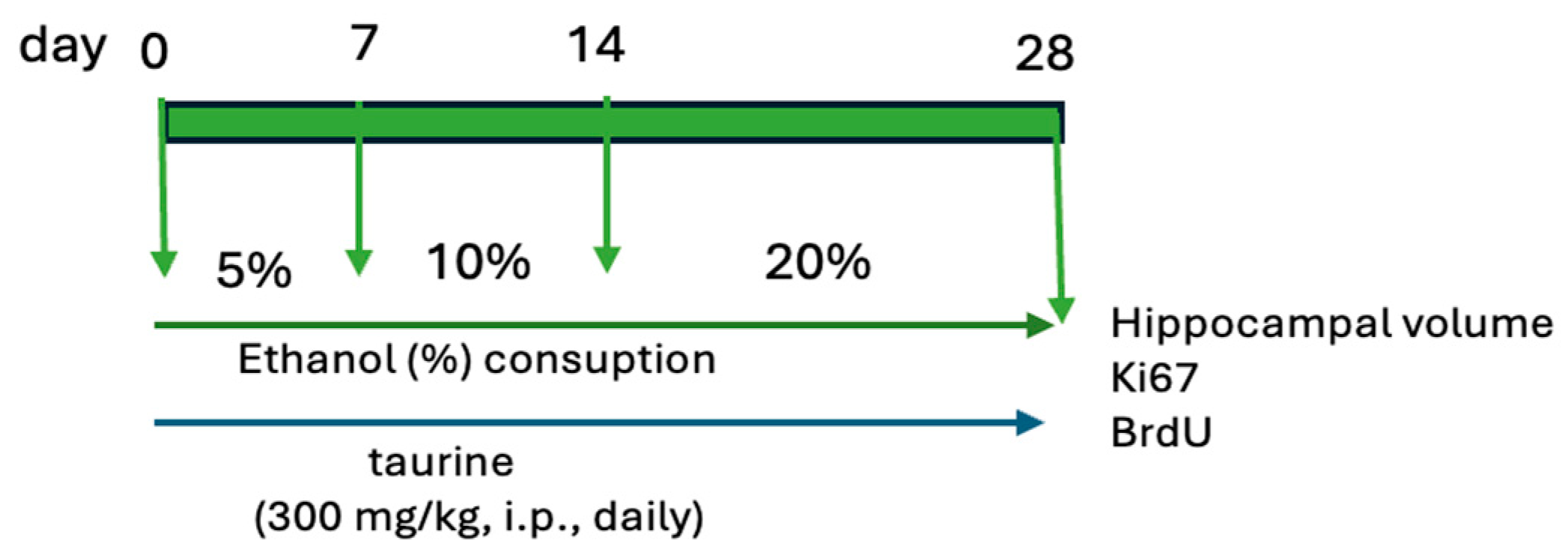
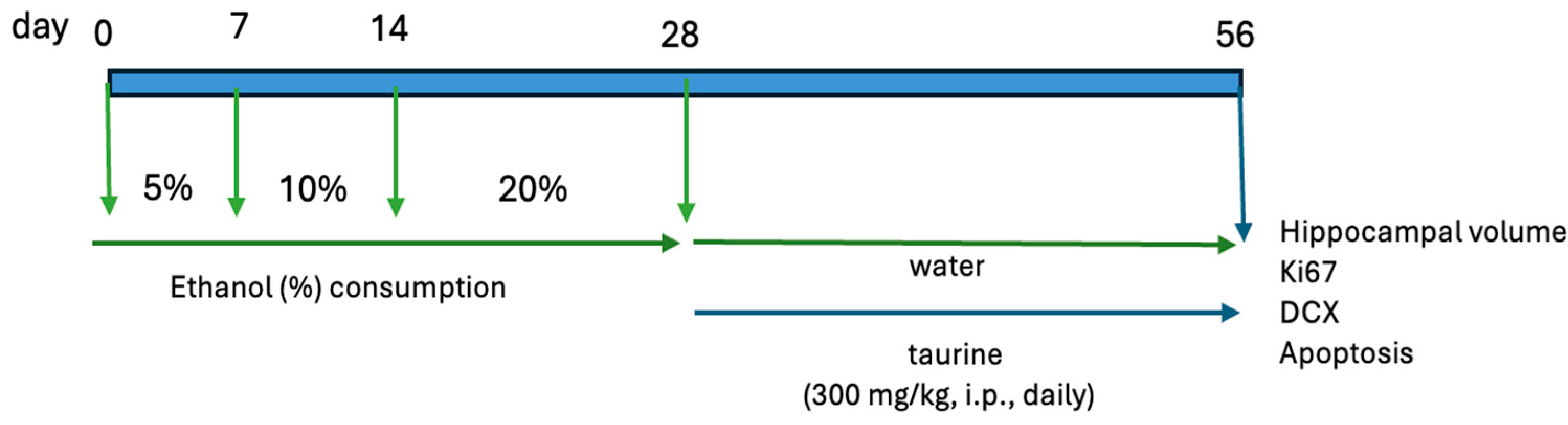
2.1. Experimental Procedure
2.1.1. Brain Slices
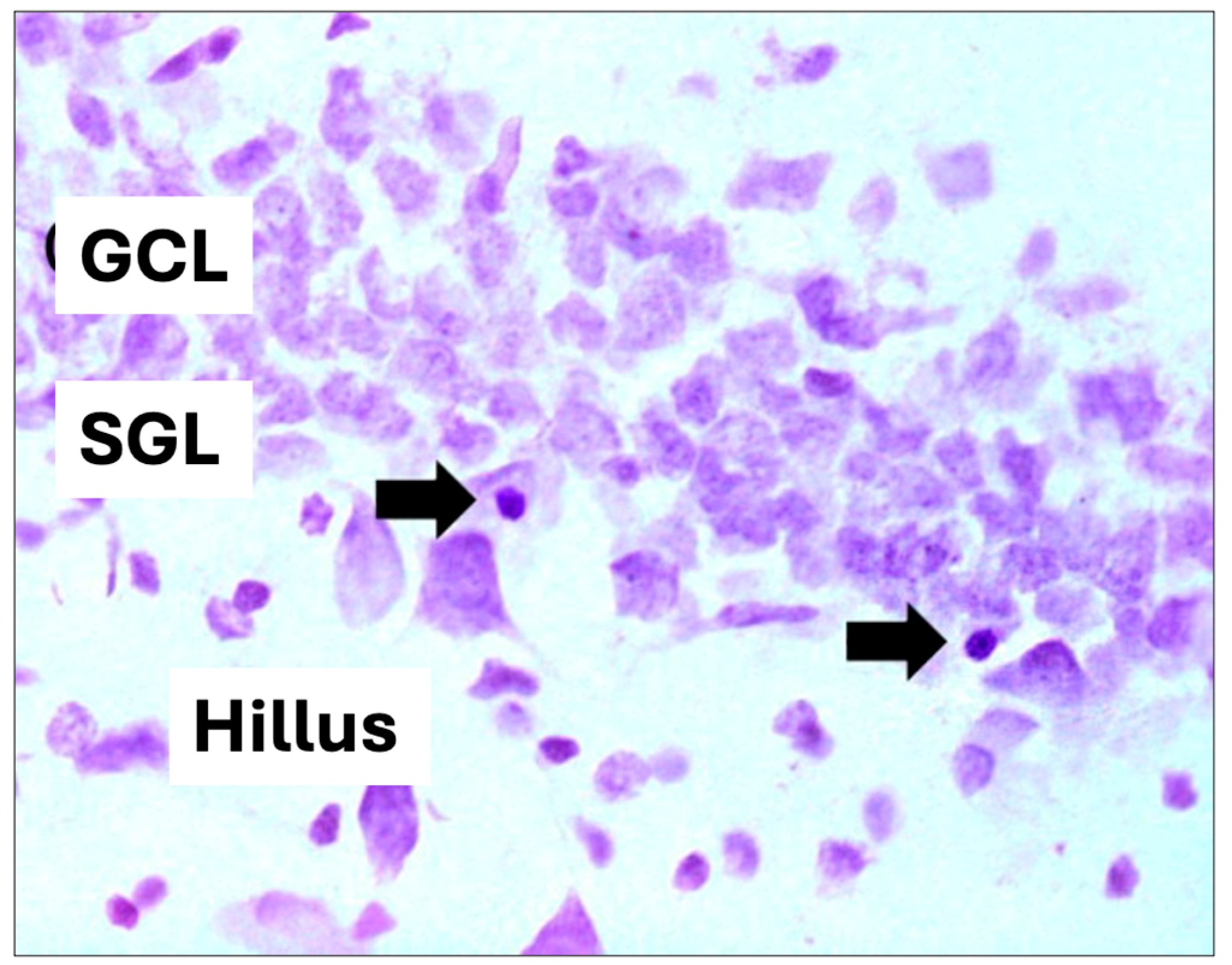
2.1.2. Hippocampal Cell Volume and Death Analysis
2.1.3. Cell Proliferation, Survival, and Neurogenesis (BrdU, Ki-67, and DCX)
2.2. Statistical Analysis
3. Results
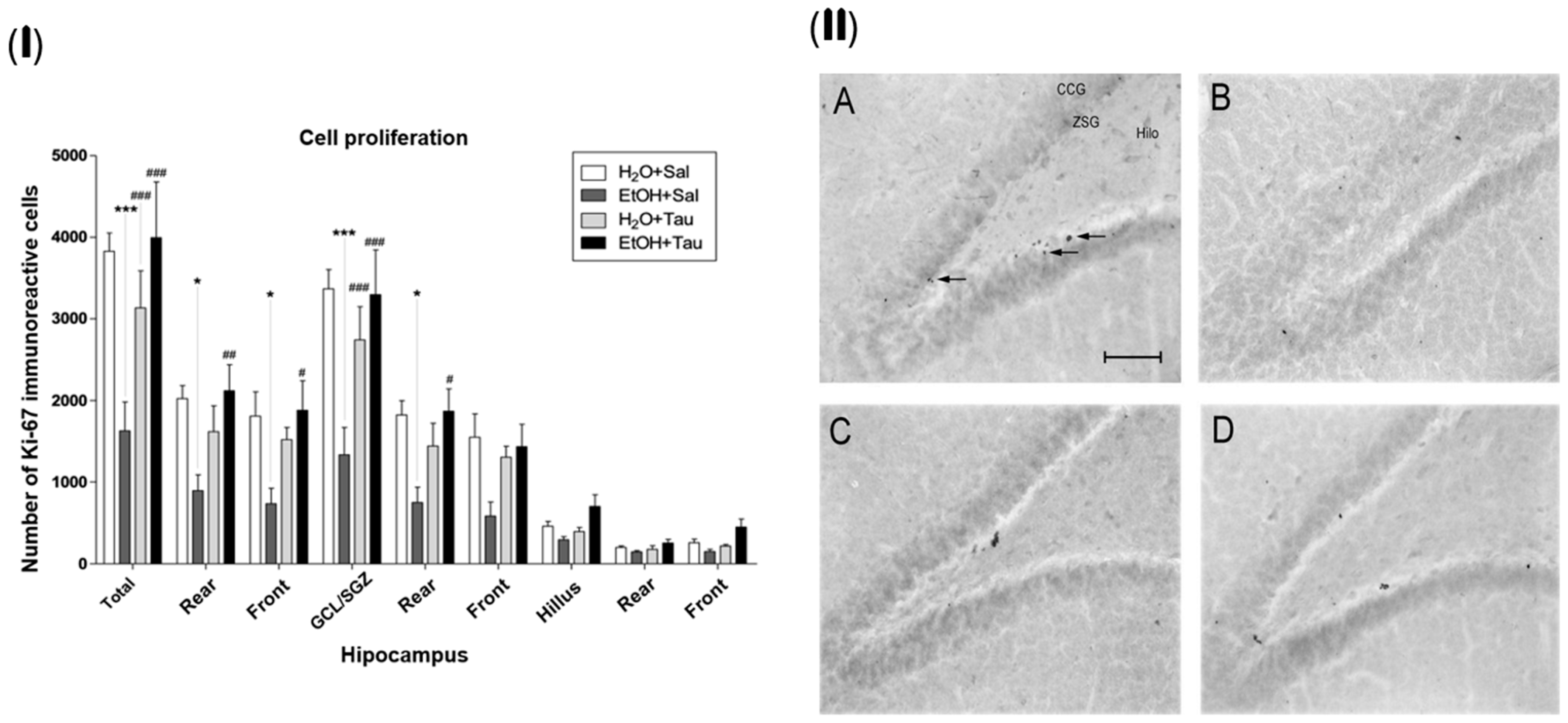
3.1. Cellular Proliferation—Ki-67
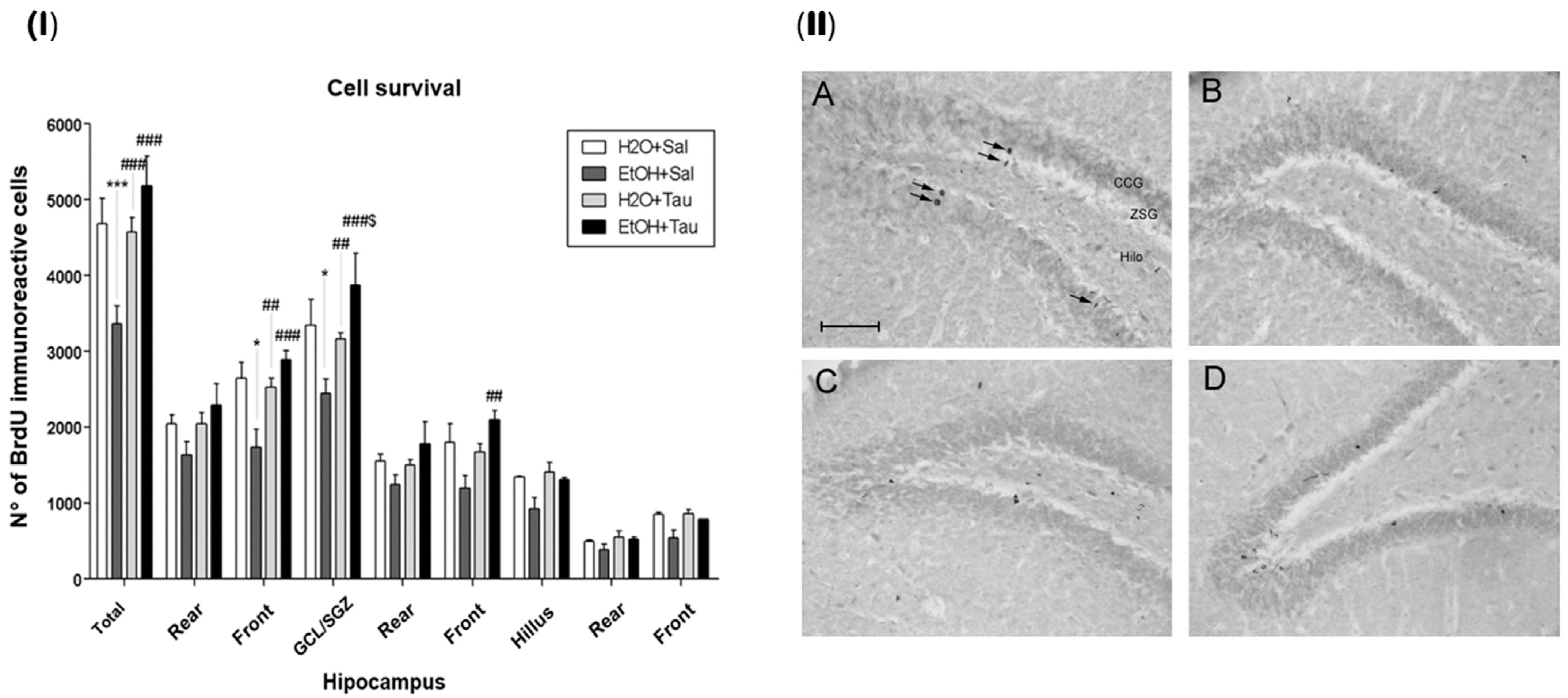
3.2. Cell Survival—BrdU
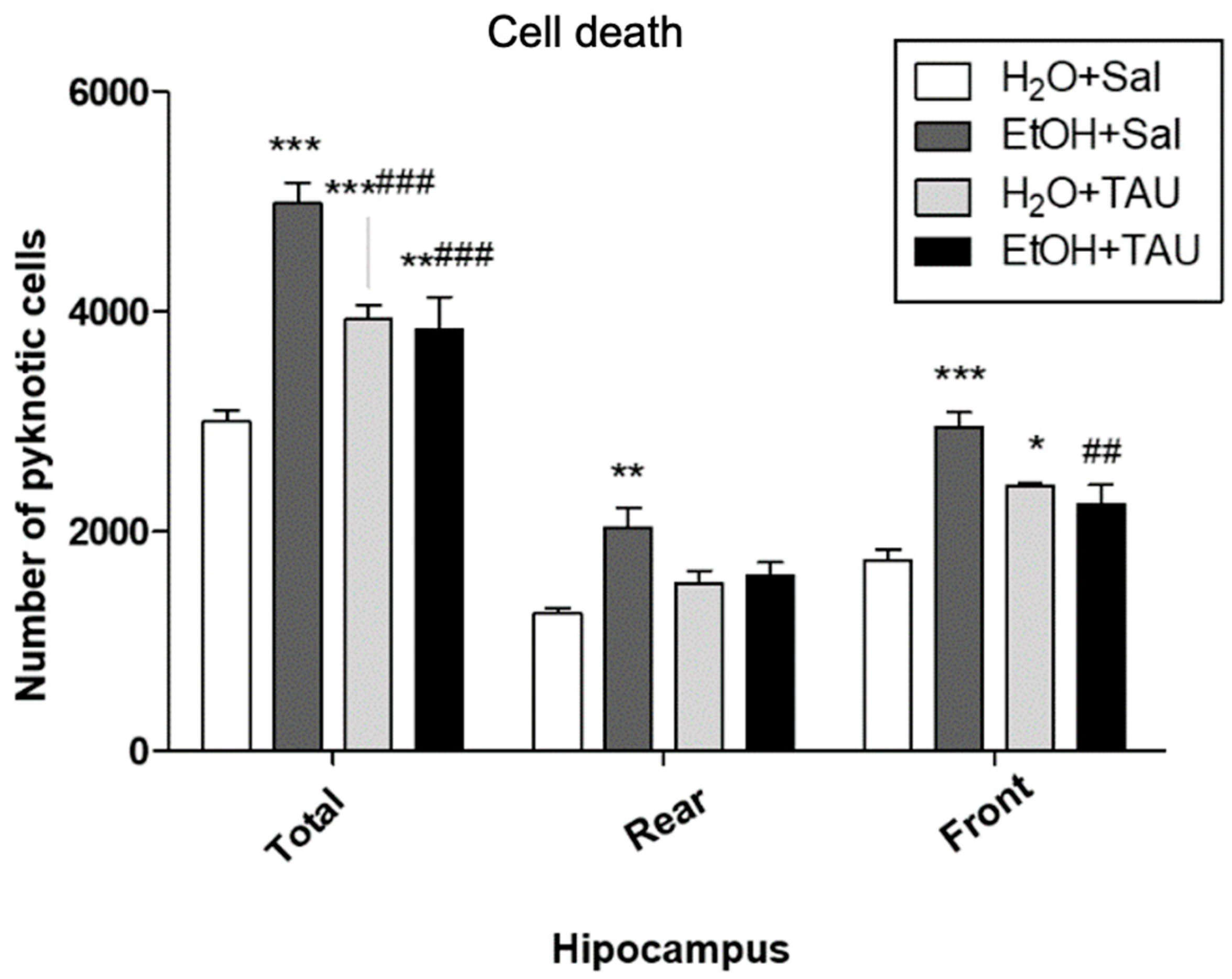
3.3. Cell Death
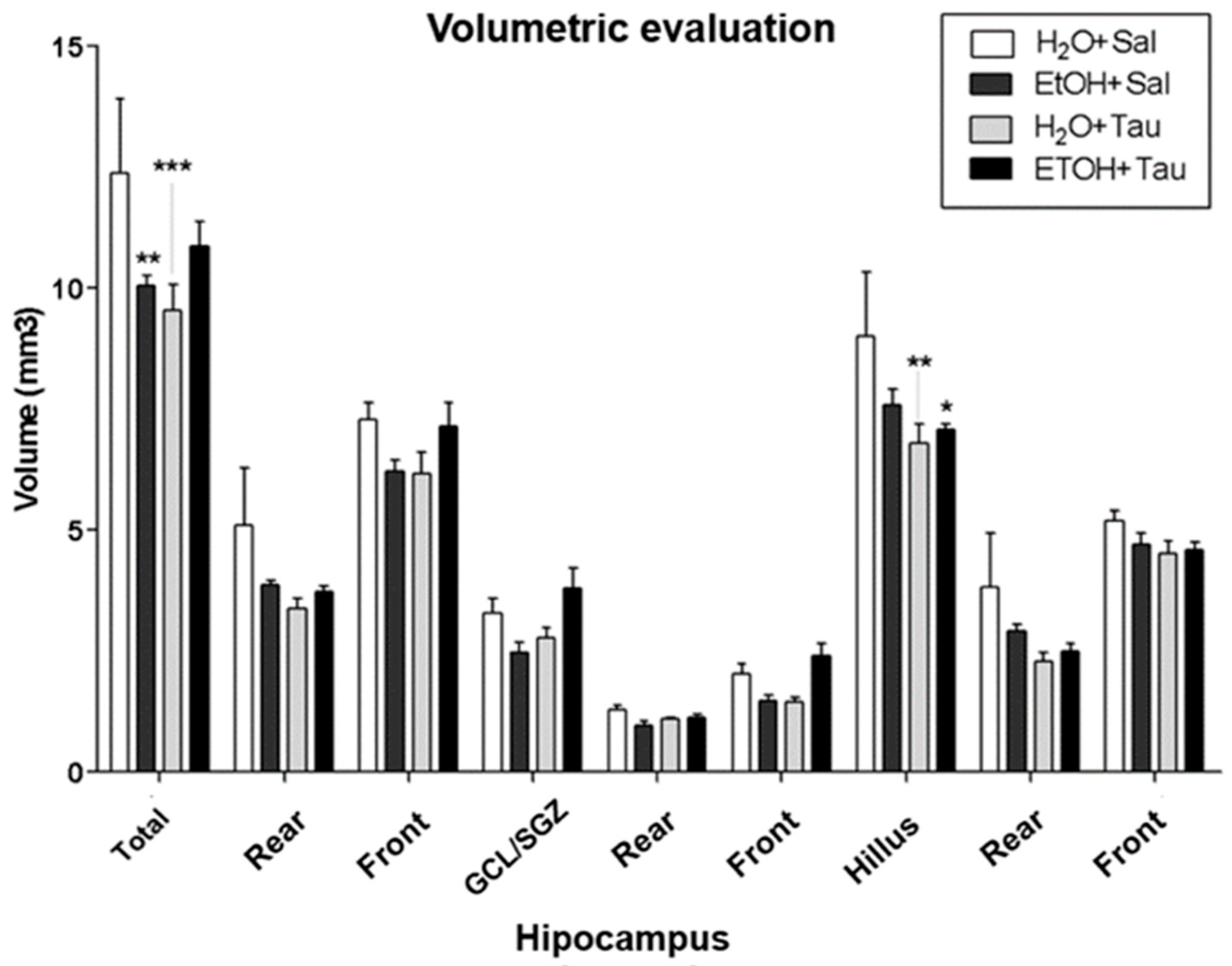
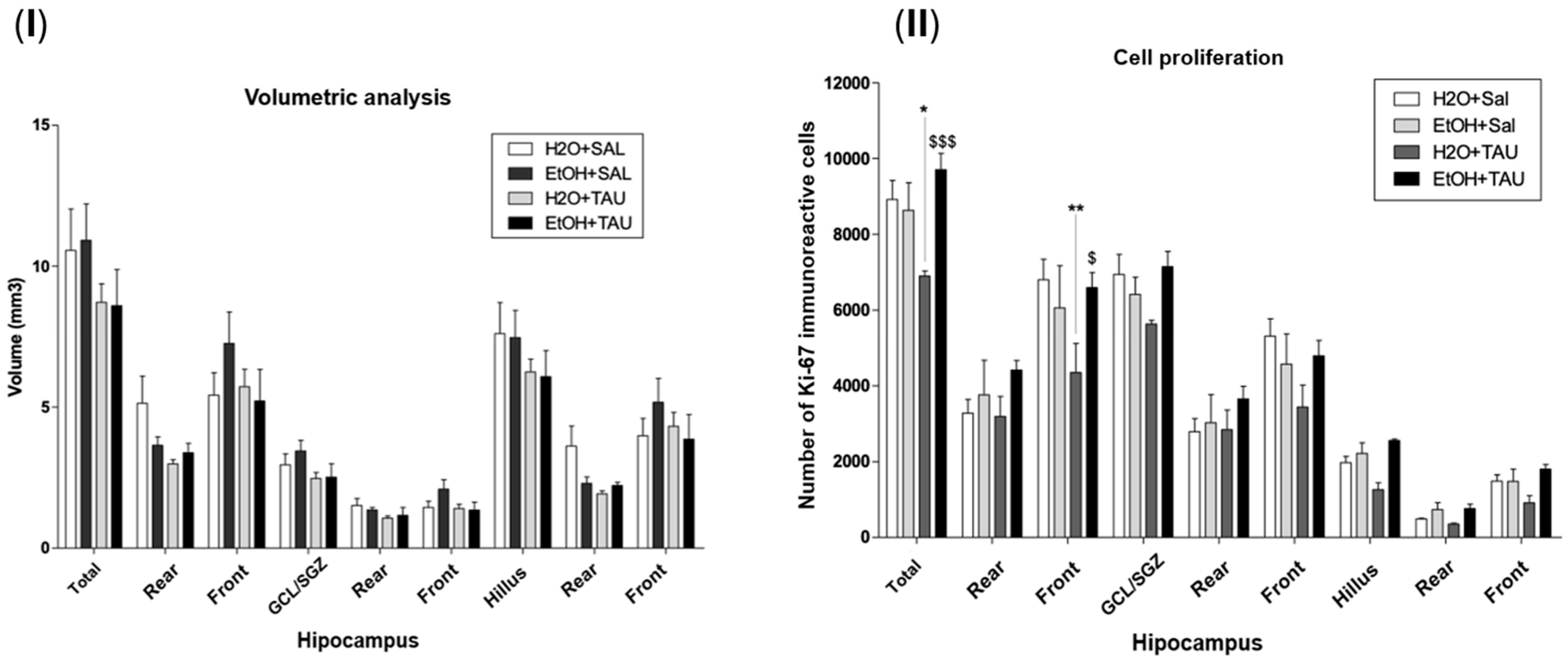
3.4. Effect of Taurine in the Reversion of Damages Caused by Chronic Ethanol Consumption
3.5. Neurogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Earliest Alcoholic Beverage in the World|Research-Penn Museum. Available online: https://www.penn.museum/research/project.php?pid=12 (accessed on 13 June 2024).
- The Lancet. Alcohol and Cancer. Lancet 2017, 390, 2215. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.; Andersen, K. Alcohol, anxiety, and depression. Ugeskr. Laeger 2022, 184, V10210816. [Google Scholar] [PubMed]
- Llamosas-Falcón, L.; Rehm, J.; Bright, S.; Buckley, C.; Carr, T.; Kilian, C.; Lasserre, A.M.; Lemp, J.M.; Zhu, Y.; Probst, C. The Relationship Between Alcohol Consumption, BMI, and Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis. Diabetes Care 2023, 46, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.R. Alcohol’s Effects on the Cardiovascular System. Alcohol Res. 2017, 38, 219–241. [Google Scholar] [PubMed]
- Wu, D.; Cederbaum, A.I. Alcohol, Oxidative Stress, and Free Radical Damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar] [PubMed]
- Levitt, D.; Luk, H.-Y.; Vingren, J. Alcohol, Resistance Exercise, and mTOR Pathway Signaling: An Evidence-Based Narrative Review. Biomolecules 2022, 13, 2. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The Appeal of a Safe Amino Acid for Skeletal Muscle Disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef]
- Kerr, J.S.; Hindmarch, I. Vlcohol, Cognitive Function and Psychomotor Performance. Rev. Environ. Health 1991, 9, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Daviet, R.; Aydogan, G.; Jagannathan, K.; Spilka, N.; Koellinger, P.D.; Kranzler, H.R.; Nave, G.; Wetherill, R.R. Associations between Alcohol Consumption and Gray and White Matter Volumes in the UK Biobank. Nat. Commun. 2022, 13, 1175. [Google Scholar] [CrossRef]
- Kamal, H.; Tan, G.C.; Ibrahim, S.F.; Shaikh, M.F.; Mohamed, I.N.; Mohamed, R.M.P.; Hamid, A.A.; Ugusman, A.; Kumar, J. Alcohol Use Disorder, Neurodegeneration, Alzheimer’s and Parkinson’s Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity. Front. Cell Neurosci. 2020, 14, 282. [Google Scholar] [CrossRef]
- Graham, K. Theories of Intoxicated Aggression. Can. J. Behav. Sci./Rev. Can. Des Sci. Comport. 1980, 12, 141–158. [Google Scholar] [CrossRef]
- Sontate, K.V.; Rahim Kamaluddin, M.; Naina Mohamed, I.; Mohamed, R.M.P.; Shaikh, M.F.; Kamal, H.; Kumar, J. Alcohol, Aggression, and Violence: From Public Health to Neuroscience. Front. Psychol. 2021, 12, 699726. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.Y.; Smith, M.M.; Sherry, S.B.; Seno, M.; Moore, M.L.; Stewart, S.H. Alcohol Use and Death by Suicide: A Meta-analysis of 33 Studies. Suicide Life Threat. Behav. 2022, 52, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Dopico, J.G.; Sabate, M.; Gonzalez-Hernandez, T.; Rodriguez, M. Substantia Nigra Osmoregulation: Taurine and ATP Involvement. Am. J. Physiol. Cell Physiol. 2007, 292, C1934–C1941. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism Underlying the Antioxidant Activity of Taurine: Prevention of Mitochondrial Oxidant Production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Wu, H.; Jin, Y.; Wei, J.; Sha, D.; Prentice, H.; Lee, H.-H.; Lin, C.-H.; Lee, Y.-H.; Yang, L.-L. Mechanism of Neuroprotective Function of Taurine. Adv. Exp. Med. Biol. 2009, 643, 169–179. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, A. Taurine Increases Mitochondrial Buffering of Calcium: Role in Neuroprotection. Amino Acids 2008, 34, 321–328. [Google Scholar] [CrossRef]
- Paula-Lima, A.C.; De Felice, F.G.; Brito-Moreira, J.; Ferreira, S.T. Activation of GABA(A) Receptors by Taurine and Muscimol Blocks the Neurotoxicity of Beta-Amyloid in Rat Hippocampal and Cortical Neurons. Neuropharmacology 2005, 49, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, Y.; Gu, Y.; Xu, C. Anti-Inflammatory Mechanism of Taurine against Ischemic Stroke Is Related to down-Regulation of PARP and NF-κB. Amino Acids 2012, 42, 1735–1747. [Google Scholar] [CrossRef]
- Leon, R.; Wu, H.; Jin, Y.; Wei, J.; Buddhala, C.; Prentice, H.; Wu, J.-Y. Protective Function of Taurine in Glutamate-Induced Apoptosis in Cultured Neurons. J. Neurosci. Res. 2009, 87, 1185–1194. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine Suppresses Doxorubicin-Triggered Oxidative Stress and Cardiac Apoptosis in Rat via up-Regulation of PI3-K/Akt and Inhibition of P53, P38-JNK. Biochem. Pharmacol. 2011, 81, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine Deficiency as a Driver of Aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.S.; Roeske, W.R.; Bressler, R. Characterization of a Carrier-Mediated Transport System for Taurine in the Fetal Mouse Heart In Vitro. J. Clin. Investig. 1978, 61, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Hansen, D.B. Regulation of Taurine Transport Systems by Protein Kinase CK2 in Mammalian Cells. Cell Physiol. Biochem. 2011, 28, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Roig-Pérez, S.; Moretó, M.; Ferrer, R. Transepithelial Taurine Transport in Caco-2 Cell Monolayers. J. Membr. Biol. 2005, 204, 85–92. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological Role of Taurine—From Organism to Organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.R.; Clements, J.R.; Wu, J.Y.; Beitz, A.J. Colocalization of Taurine- and Cysteine Sulfinic Acid Decarboxylase-like Immunoreactivity in the Hippocampus of the Rat. Synapse 1989, 4, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Chan-Palay, V.; Lin, C.T.; Palay, S.; Yamamoto, M.; Wu, J.Y. Taurine in the Mammalian Cerebellum: Demonstration by Autoradiography with [3H]Taurine and Immunocytochemistry with Antibodies against the Taurine-Synthesizing Enzyme, Cysteine-Sulfinic Acid Decarboxylase. Proc. Natl. Acad. Sci. USA 1982, 79, 2695–2699. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Tamai, I. Sodium- and Chloride-Dependent Transport of Taurine at the Blood-Brain Barrier. Adv. Exp. Med. Biol. 1996, 403, 385–391. [Google Scholar] [CrossRef]
- Kang, Y.S. Taurine Transport Mechanism through the Blood-Brain Barrier in Spontaneously Hypertensive Rats. Adv. Exp. Med. Biol. 2000, 483, 321–324. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Ohtsuki, S.; Takanaga, H.; Tomi, M.; Hosoya, K.-I.; Terasaki, T. Regulation of Taurine Transport at the Blood-Brain Barrier by Tumor Necrosis Factor-Alpha, Taurine and Hypertonicity. J. Neurochem. 2002, 83, 1188–1195. [Google Scholar] [CrossRef]
- Rafiee, Z.; García-Serrano, A.M.; Duarte, J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients 2022, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Physiological Actions of Taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Kontro, P.; Oja, S.S. Co-operativity in Sodium-Independent Taurine Binding to Brain Membranes in the Mouse. Neuroscience 1987, 23, 567–570. [Google Scholar] [CrossRef]
- Urquhart, N.; Perry, T.L.; Hansen, S.; Kennedy, J. Passage of Taurine into Adult Mammalian Brain. J. Neurochem. 1974, 22, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Menzie, J.; Pan, C.; Prentice, H.; Wu, J.-Y. Taurine and Central Nervous System Disorders. Amino Acids 2014, 46, 31–46. [Google Scholar] [CrossRef]
- Agrawal, H.C.; Davis, J.M.; Himwich, W.A. Developmental Changes in Mouse Brain: Weight, Water Content and Free Amino Acids. J. Neurochem. 1968, 15, 917–923. [Google Scholar] [CrossRef]
- Sturman, J.A.; Gaull, G.E. Taurine in the Brain and Liver of the Developing Human and Monkey. J. Neurochem. 1975, 25, 831–835. [Google Scholar] [CrossRef]
- Miller, T.J.; Hanson, R.D.; Yancey, P.H. Developmental Changes in Organic Osmolytes in Prenatal and Postnatal Rat Tissues. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 45–56. [Google Scholar] [CrossRef]
- Shivaraj, M.C.; Marcy, G.; Low, G.; Ryu, J.R.; Zhao, X.; Rosales, F.J.; Goh, E.L.K. Taurine Induces Proliferation of Neural Stem Cells and Synapse Development in the Developing Mouse Brain. PLoS ONE 2012, 7, e42935. [Google Scholar] [CrossRef]
- Wu, G.; Yang, J.; Lin, S.; Feng, Y.; Yang, Q.; Lv, Q.; Hu, J. Taurine and Chinese Traditional Medicine Accelerate Alcohol Metabolism in Mice. Adv. Exp. Med. Biol. 2013, 776, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Benítez, R.; Ramos-Mandujano, G.; Pasantes-Morales, H. Taurine Stimulates Proliferation and Promotes Neurogenesis of Mouse Adult Cultured Neural Stem/Progenitor Cells. Stem Cell Res. 2012, 9, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Chen, H. Antenatal Taurine Supplementation for Improving Brain Ultrastructure in Fetal Rats with Intrauterine Growth Restriction. Neuroscience 2011, 181, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Crestani, C.C.; Lopes Da Silva, A.; Scopinho, A.A.; Ruginsk, S.G.; Uchoa, E.T.; Correa, F.M.A.; Elias, L.L.K.; Antunes-Rodrigues, J.; Resstel, L.B.M. Cardiovascular Alterations at Different Stages of Hypertension Development during Ethanol Consumption: Time-Course of Vascular and Autonomic Changes. Toxicol. Appl. Pharmacol. 2014, 280, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.G.; Yague, A.G.; Johnsen-Soriano, S.; Bosch-Morell, F.; Collado-Morente, L.; Muriach, M.; Romero, F.J.; Garcia-Verdugo, J.M. Selective Impairment of Hippocampal Neurogenesis by Chronic Alcoholism: Protective Effects of an Antioxidant. Proc. Natl. Acad. Sci. USA 2003, 100, 7919–7924. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The Utility of Ki-67 and BrdU as Proliferative Markers of Adult Neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef]
- Crane, A.M.; Bhattacharya, S.K. The Use of Bromodeoxyuridine Incorporation Assays to Assess Corneal Stem Cell Proliferation. Methods Mol. Biol. 2013, 1014, 65–70. [Google Scholar] [CrossRef]
- Tateno, M.; Saito, T. Biological Studies on Alcohol-Induced Neuronal Damage. Psychiatry Investig. 2008, 5, 21–27. [Google Scholar] [CrossRef]
- Sakai, R.; Ukai, W.; Sohma, H.; Hashimoto, E.; Yamamoto, M.; Ikeda, H.; Saito, T. Attenuation of Brain Derived Neurotrophic Factor (BDNF) by Ethanol and Cytoprotective Effect of Exogenous BDNF against Ethanol Damage in Neuronal Cells. J. Neural Transm. 2005, 112, 1005–1013. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Taranukhin, A.G.; Taranukhina, E.Y.; Saransaari, P.; Pelto-Huikko, M.; Podkletnova, I.M.; Oja, S.S. Taurine Protects Cerebellar Neurons of the External Granular Layer against Ethanol-Induced Apoptosis in 7-Day-Old Mice. Amino Acids 2012, 43, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Taranukhin, A.G.; Taranukhina, E.Y.; Saransaari, P.; Podkletnova, I.M.; Pelto-Huikko, M.; Oja, S.S. Neuroprotection by Taurine in Ethanol-Induced Apoptosis in the Developing Cerebellum. J. Biomed. Sci. 2010, 17 (Suppl. 1), S12. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-H.; Fang, W.-L.; Li, A.F.-Y.; Liang, P.-H.; Wu, C.-W.; Shyr, Y.-M.; Yang, M.-H. Caspase-3, a Key Apoptotic Protein, as a Prognostic Marker in Gastric Cancer after Curative Surgery. Int. J. Surg. 2018, 52, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species-Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Kreisman, N.R.; Olson, J.E. Taurine Enhances Volume Regulation in Hippocampal Slices Swollen Osmotically. Neuroscience 2003, 120, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P.R.; Stack, E.C.; Gallagher, M. Morphometric Studies of the Aged Hippocampus: I. Volumetric Analysis in Behaviorally Characterized Rats. J. Comp. Neurol. 1999, 403, 459–470. [Google Scholar] [CrossRef]
- Driscoll, I.; Howard, S.R.; Stone, J.C.; Monfils, M.H.; Tomanek, B.; Brooks, W.M.; Sutherland, R.J. The Aging Hippocampus: A Multi-Level Analysis in the Rat. Neuroscience 2006, 139, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Gebara, E.; Udry, F.; Sultan, S.; Toni, N. Taurine Increases Hippocampal Neurogenesis in Aging Mice. Stem Cell Res. 2015, 14, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Nixon, K.; Kim, D.H.; Potts, E.N.; He, J.; Crews, F.T. Distinct Cell Proliferation Events during Abstinence after Alcohol Dependence: Microglia Proliferation Precedes Neurogenesis. Neurobiol. Dis. 2008, 31, 218–229. [Google Scholar] [CrossRef]
- Nixon, K.; Crews, F.T. Temporally Specific Burst in Cell Proliferation Increases Hippocampal Neurogenesis in Protracted Abstinence from Alcohol. J. Neurosci. 2004, 24, 9714–9722. [Google Scholar] [CrossRef]
- Nixon, K.; Crews, F.T. Binge Ethanol Exposure Decreases Neurogenesis in Adult Rat Hippocampus. J. Neurochem. 2002, 83, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-H.; Shin, M.-C.; Jung, S.-B.; Lee, T.-H.; Bahn, G.-H.; Kwon, Y.K.; Kim, E.-H.; Kim, C.-J. Alcohol and Nicotine Reduce Cell Proliferation and Enhance Apoptosis in Dentate Gyrus. Neuroreport 2002, 13, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Nixon, K.; Shetty, A.K.; Crews, F.T. Chronic Alcohol Exposure Reduces Hippocampal Neurogenesis and Dendritic Growth of Newborn Neurons. Eur. J. Neurosci. 2005, 21, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Eaves, D.W.; Smith, A.R.; Nixon, K. Alcohol Inhibition of Neurogenesis: A Mechanism of Hippocampal Neurodegeneration in an Adolescent Alcohol Abuse Model. Hippocampus 2010, 20, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.C.; Bullock, M.R.; Shelton, K.L. Chronic Ethanol Consumption Transiently Reduces Adult Neural Progenitor Cell Proliferation. Brain Res. 2004, 1011, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Salin, K.; Auer, S.K.; Rey, B.; Selman, C.; Metcalfe, N.B. Variation in the Link between Oxygen Consumption and ATP Production, and Its Relevance for Animal Performance. Proc. Biol. Sci. 2015, 282, 20151028. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Nevado-Holgado, A.; Whiley, L.; Snowden, S.G.; Soininen, H.; Kloszewska, I.; Mecocci, P.; Tsolaki, M.; Vellas, B.; Thambisetty, M.; et al. Association between Plasma Ceramides and Phosphatidylcholines and Hippocampal Brain Volume in Late Onset Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 809–817. [Google Scholar] [CrossRef]
- García-Suástegui, W.A.; Ramos-Chávez, L.A.; Rubio-Osornio, M.; Calvillo-Velasco, M.; Atzin-Méndez, J.A.; Guevara, J.; Silva-Adaya, D. The Role of CYP2E1 in the Drug Metabolism or Bioactivation in the Brain. Oxid. Med. Cell Longev. 2017, 2017, 4680732. [Google Scholar] [CrossRef]
- Huf, F.; Gutierres, J.M.; da Silva, G.N.; Zago, A.M.; Koenig, L.F.C.; Fernandes, M.C. Neuroprotection Elicited by Taurine in Sporadic Alzheimer-like Disease: Benefits on Memory and Control of Neuroinflammation in the Hippocampus of Rats. Mol. Cell Biochem. 2023. [Google Scholar] [CrossRef]
- Liu, C.; He, P.; Guo, Y.; Tian, Q.; Wang, J.; Wang, G.; Zhang, Z.; Li, M. Taurine Attenuates Neuronal Ferroptosis by Regulating GABAB/AKT/GSK3β/β-Catenin Pathway after Subarachnoid Hemorrhage. Free Radic. Biol. Med. 2022, 193, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qu, J.; Li, Q.; Cui, M.; Wang, J.; Zhang, K.; Liu, X.; Feng, H.; Chen, Y. Taurine Supplementation Reduces Neuroinflammation and Protects against White Matter Injury after Intracerebral Hemorrhage in Rats. Amino Acids 2018, 50, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, S.; He, J.; Lu, G.; Xie, Z.; Han, H. Roles of Taurine in Cognitive Function of Physiology, Pathologies and Toxication. Life Sci. 2019, 231, 116584. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Ahmad, K.; Khan, M.S.A.; Bhat, M.A.; Almatroudi, A.; Rahman, S.; Jan, A.T. Expedition into Taurine Biology: Structural Insights and Therapeutic Perspective of Taurine in Neurodegenerative Diseases. Biomolecules 2020, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F. Interactions between Taurine and Ethanol in the Central Nervous System. Amino Acids 2002, 23, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Gao, H.-Y.; Liu, J. The Role of Taurine in Improving Neural Stem Cells Proliferation and Differentiation. Nutr. Neurosci. 2017, 20, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Agartz, I.; Brag, S.; Franck, J.; Hammarberg, A.; Okugawa, G.; Svinhufvud, K.; Bergman, H. MR Volumetry during Acute Alcohol Withdrawal and Abstinence: A Descriptive Study. Alcohol. Alcohol. 2003, 38, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Maynard, M.E.; Leasure, J.L. Exercise Enhances Hippocampal Recovery Following Binge Ethanol Exposure. PLoS ONE 2013, 8, e76644. [Google Scholar] [CrossRef] [PubMed]
- Will, B.; Galani, R.; Kelche, C.; Rosenzweig, M.R. Recovery from Brain Injury in Animals: Relative Efficacy of Environmental Enrichment, Physical Exercise or Formal Training (1990–2002). Prog. Neurobiol. 2004, 72, 167–182. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Dorado-Martínez, C.; Borgonio-Pérez, G.; Hiriart-Urdanivia, M.; Verdugo-Diaz, L.; Durán-Vázquez, A.; Colin-Baranque, L.; Avila-Costa, M.R. Effects of Taurine on Ozone-Induced Memory Deficits and Lipid Peroxidation Levels in Brains of Young, Mature, and Old Rats. Environ. Res. 2000, 82, 7–17. [Google Scholar] [CrossRef]
- Wu, G.; Matsuwaki, T.; Tanaka, Y.; Yamanouchi, K.; Hu, J.; Nishihara, M. Taurine Counteracts the Suppressive Effect of Lipopolysaccharide on Neurogenesis in the Hippocampus of Rats. Adv. Exp. Med. Biol. 2013, 775, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, L.S.; Volpe, N.P.; El Idrissi, A. Taurine Effects on Emotional Learning and Memory in Aged Mice: Neurochemical Alterations and Differentiation in Auditory Cued Fear and Context Conditioning. Adv. Exp. Med. Biol. 2013, 775, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cha, Y.-N. Taurine Chloramine Produced from Taurine under Inflammation Provides Anti-Inflammatory and Cytoprotective Effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.-I.; Kang, I.S.; Lee, J.S.; Lee, J.-K.; Kim, C. Taurine Chloramine-Mediated Nrf2 Activation and HO-1 Induction Confer Protective Effects in Astrocytes. Antioxidants 2024, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Jamshidzadeh, A.; Niknahad, H.; Mardani, E.; Ommati, M.M.; Azarpira, N.; Khodaei, F.; Zarei, A.; Ayarzadeh, M.; Mousavi, S.; et al. Effect of Taurine on Chronic and Acute Liver Injury: Focus on Blood and Brain Ammonia. Toxicol. Rep. 2016, 3, 870–879. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mozaffari, M.S. Role of Taurine in the Vasculature: An Overview of Experimental and Human Studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar]
- Mason, B.J.; Heyser, C.J. Acamprosate: A Prototypic Neuromodulator in the Treatment of Alcohol Dependence. CNS Neurol. Disord. Drug Targets 2010, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kalk, N.J.; Lingford-Hughes, A.R. The Clinical Pharmacology of Acamprosate. Brit J. Clin. Pharma 2014, 77, 315–323. [Google Scholar] [CrossRef]
- Madamba, S.G.; Schweitzer, P.; Zieglgänsberger, W.; Siggins, G.R. Acamprosate (Calcium Acetylhomotaurinate) Enhances the N-Methyl-D-Aspartate Component of Excitatory Neurotransmission in Rat Hippocampal CA1 Neurons in Vitro. Alcohol. Clin. Exp. Res. 1996, 20, 651–658. [Google Scholar] [CrossRef]
- De Witte, P.; Littleton, J.; Parot, P.; Koob, G. Neuroprotective and Abstinence-Promoting Effects of Acamprosate: Elucidating the Mechanism of Action. CNS Drugs 2005, 19, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Pulcinelli, R.R.; De Paula, L.F.; Nietiedt, N.A.; Bandiera, S.; Hansen, A.W.; Izolan, L.D.R.; Almeida, R.F.; Gomez, R. Taurine Enhances Voluntary Alcohol Intake and Promotes Anxiolytic-like Behaviors in Rats. Alcohol 2020, 88, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Páez, A.; Marichal-Cancino, B.A.; Sánchez-Castillo, H.; Vázquez-León, P. Acute Taurine Reduced Alcohol Intake and Preference in Alcohol-Experienced, but Not in Alcohol-Näive Rats by Central Mechanisms. Behav. Brain Res. 2024, 463, 114892. [Google Scholar] [CrossRef] [PubMed]
| Groups | ||||
|---|---|---|---|---|
| H2O/Sal | EtOH/Sal | H2O/TAU | EtOH/TAU | |
| Total | 679 ± 56 | 411 ± 43 *** | 546 ± 7 * | 580 ± 119 ### |
| Rear | 267 ± 55 | 209 ± 56 | 321 ± 27 | 260 ± 59 |
| Front | 412 ± 3 | 202 ± 15 ** | 225 ± 21 | 321 ± 90 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodella, P.; Boreski, D.; Luz, M.A.M.; Gabriel, E.A.; Takase, L.F.; Chin, C.M. Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats. Nutrients 2024, 16, 1973. https://doi.org/10.3390/nu16121973
Rodella P, Boreski D, Luz MAM, Gabriel EA, Takase LF, Chin CM. Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats. Nutrients. 2024; 16(12):1973. https://doi.org/10.3390/nu16121973
Chicago/Turabian StyleRodella, Patricia, Diogo Boreski, Marcus Alexandre Mendes Luz, Edmo Atique Gabriel, Luiz Fernando Takase, and Chung Man Chin. 2024. "Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats" Nutrients 16, no. 12: 1973. https://doi.org/10.3390/nu16121973
APA StyleRodella, P., Boreski, D., Luz, M. A. M., Gabriel, E. A., Takase, L. F., & Chin, C. M. (2024). Taurine Neuroprotection and Neurogenesis Effect in Chronic Ethanol-Induced Rats. Nutrients, 16(12), 1973. https://doi.org/10.3390/nu16121973






