Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters
Abstract
1. Introduction
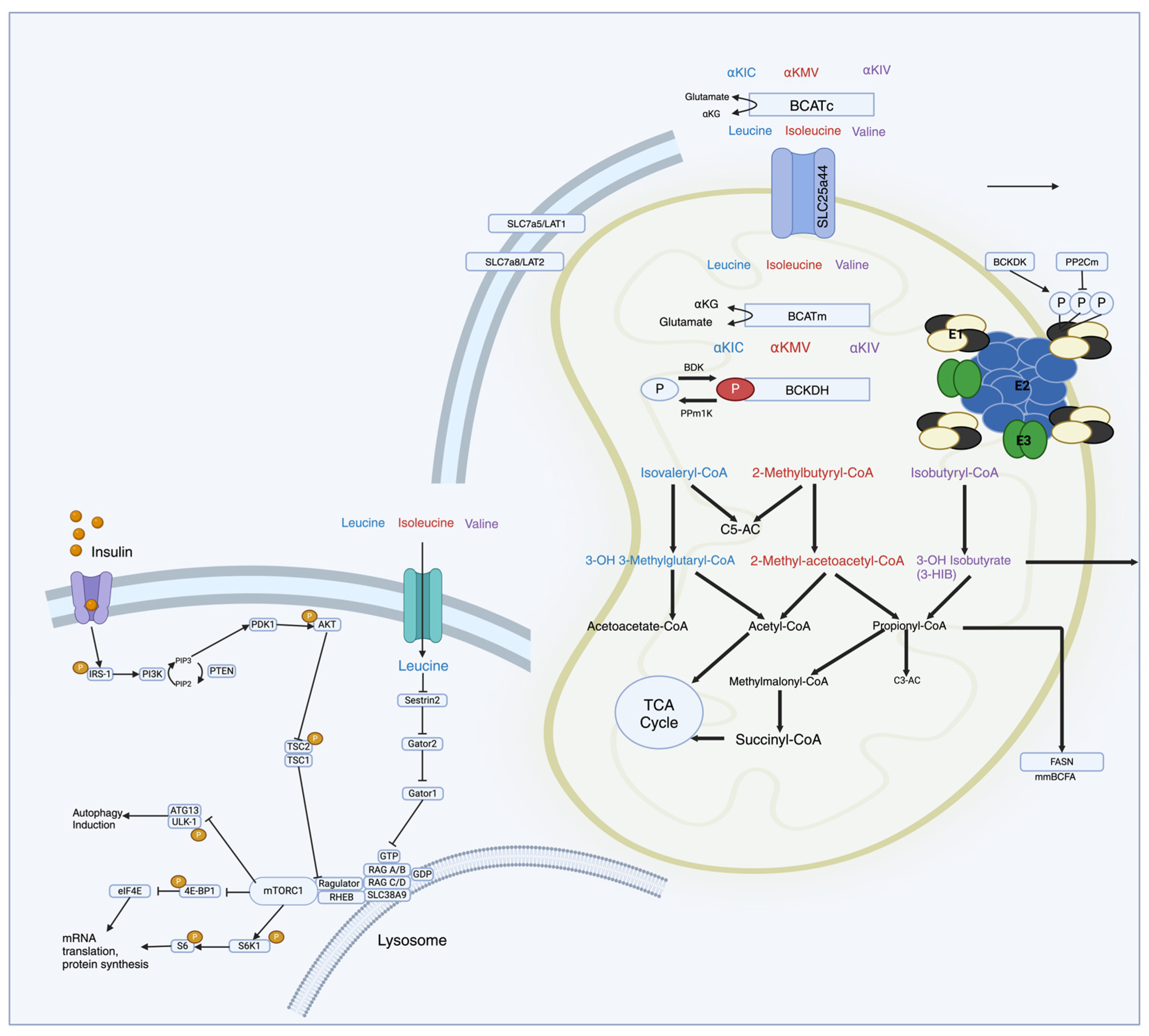
2. Branched-Chain Amino Acid Synthesis, Metabolism, and Catabolites
2.1. Transamination
2.2. Decarboxylation
2.3. ATP Generation
3. Branched-Chain Amino Acid-Regulated Signaling Pathways
3.1. mTOR and Upstream Regulators
3.2. Downstream Regulators
4. Branched-Chain Amino Acid in Cardiovascular Disease
4.1. Heart Failure
4.2. Cardiometabolic Disease
4.3. Hypertension
4.4. Atherosclerosis and Coronary Artery Disease
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, W.; Lu, K.; Zhuang, Z.; Wang, X.; Tang, X.; Huang, T.; Gao, P.; Wang, Y.; Du, J. Mendelian Randomization Analysis Provides Insights into the Pathogenesis of Serum Levels of Branched-Chain Amino Acids in Cardiovascular Disease. Metabolites 2023, 13, 403. [Google Scholar] [CrossRef]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of Branched-Chain Amino Acid Metabolism in Type 2 Diabetes, Obesity, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, J.; Cembrowska, P.; Sypniewska, G.; Stefanska, A. The Association between Branched-Chain Amino Acids (BCAAs) and Cardiometabolic Risk Factors in Middle-Aged Caucasian Women Stratified According to Glycemic Status. Nutrients 2021, 13, 3307. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin Action, Type 2 Diabetes, and Branched-Chain Amino Acids: A Two-Way Street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Walejko, J.M.; Christopher, B.A.; Crown, S.B.; Zhang, G.-F.; Pickar-Oliver, A.; Yoneshiro, T.; Foster, M.W.; Page, S.; Van Vliet, S.; Ilkayeva, O.; et al. Branched-Chain α-Ketoacids Are Preferentially Reaminated and Activate Protein Synthesis in the Heart. Nat. Commun. 2021, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Alfaqih, M.A.; Melhem, N.Y.; Khabour, O.F.; Al-Dwairi, A.; Elsalem, L.; Alsaqer, T.G.; Allouh, M.Z. Normalization of Vitamin D Serum Levels in Patients with Type Two Diabetes Mellitus Reduces Levels of Branched Chain Amino Acids. Medicina 2022, 58, 1267. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Wu, X.; Duan, W.; Feng, R.; Li, Y.; Sun, C. Dietary Intakes of Branched-Chained Amino Acid and Risk for Type 2 Diabetes in Adults: The Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases Study. Can. J. Diabetes 2018, 42, 484–492.e7. [Google Scholar] [CrossRef]
- Bonvini, A.; Coqueiro, A.Y.; Tirapegui, J.; Calder, P.C.; Rogero, M.M. Immunomodulatory Role of Branched-Chain Amino Acids. Nutr. Rev. 2018, 76, 840–856. [Google Scholar] [CrossRef]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429.e4. [Google Scholar] [CrossRef]
- Bar-Tana, J. Type 2 Diabetes—Unmet Need, Unresolved Pathogenesis, mTORC1-Centric Paradigm. Rev. Endocr. Metab. Disord. 2020, 21, 613–629. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Cho, K. Lipid Metabolism, Inflammation, and Foam Cell Formation in Health and Metabolic Disorders: Targeting mTORC1. J. Mol. Med. 2021, 99, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Rohini, A.; Agrawal, N.; Kumar, H.; Kumar, V. Emerging Role of Branched Chain Amino Acids in Metabolic Disorders: A Mechanistic Review. PharmaNutrition 2018, 6, 47–54. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; De Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched Chain Amino Acids, Aging and Age-Related Health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2018, 81, 139–164. [Google Scholar] [CrossRef]
- Bai, X.; Long, X.; Song, F.; Chen, B.; Sheng, C.; Tang, C.; Li, L.; Zhang, J.; Zhang, R.; Zhang, J.; et al. High Doses of Rosuvastatin Induce Impaired Branched-chain Amino Acid Catabolism and Lead to Insulin Resistance. Exp. Physiol. 2023, 108, 961–974. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and Branched-Chain Amino Acids: What Is the Link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, W.-J.; Yang, J.; Zhao, S.-S.; Liu, H.-X. The Role of Branched-Chain Amino Acids and Branched-Chain α-Keto Acid Dehydrogenase Kinase in Metabolic Disorders. Front. Nutr. 2022, 9, 932670. [Google Scholar] [CrossRef]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Long, Z.-X.; Zhang, Y.-J.; Luo, C.-Y.; Yan, L.-T.; Gao, W.-Y.; Li, H. The Chemical Mechanisms of the Enzymes in the Branched-Chain Amino Acids Biosynthetic Pathway and Their Applications. Biochimie 2021, 184, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Vander Heiden, M.G. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Supruniuk, E.; Żebrowska, E.; Chabowski, A. Branched Chain Amino Acids—Friend or Foe in the Control of Energy Substrate Turnover and Insulin Sensitivity? Crit. Rev. Food Sci. Nutr. 2023, 63, 2559–2597. [Google Scholar] [CrossRef] [PubMed]
- Yahsi, B.; Gunaydin, G. Immunometabolism—The Role of Branched-Chain Amino Acids. Front. Immunol. 2022, 13, 886822. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Guo, X.; Wang, Q.; Liu, Y.; Wang, R.-T.; Gao, C.; Li, C.-Y.; Li, C.-X.; Tao, L. PP2Cm Overexpression Alleviates MI/R Injury Mediated by a BCAA Catabolism Defect and Oxidative Stress in Diabetic Mice. Eur. J. Pharmacol. 2020, 866, 172796. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, M.; Ning, F.; Zhou, S.; Hu, X.; Xin, H.; Reilly, S.; Zhang, X. Emerging Role for Branched-Chain Amino Acids Metabolism in Fibrosis. Pharmacol. Res. 2023, 187, 106604. [Google Scholar] [CrossRef]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diab Rep. 2018, 18, 76. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-Chain Amino Acids as Critical Switches in Health and Disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of Branched-Chain Amino Acid Metabolism in the Pathogenesis of Obesity and Type 2 Diabetes-Related Metabolic Disturbances BCAA Metabolism in Type 2 Diabetes. Nutr. Diabetes 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Trautman, M.E.; Richardson, N.E.; Lamming, D.W. Protein Restriction and Branched-chain Amino Acid Restriction Promote Geroprotective Shifts in Metabolism. Aging Cell 2022, 21, e13626. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y. Branched Chain Amino Acid Metabolic Reprogramming in Heart Failure. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Zhuravlev, A.; Orekhov, N.A.; Kalmykov, V.; Orekhov, A.N. Modulating mTOR Signaling as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1153. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.S.; Rivera, C.N.; Vaughan, R.A. Branched-Chain Amino Acids and Mitochondrial Biogenesis: An Overview and Mechanistic Summary. Mol. Nutr. Food Res. 2022, 66, 2200109. [Google Scholar] [CrossRef] [PubMed]
- Kogot-Levin, A.; Riahi, Y.; Abramovich, I.; Mosenzon, O.; Agranovich, B.; Kadosh, L.; Ben-Haroush Schyr, R.; Kleiman, D.; Hinden, L.; Cerasi, E.; et al. Mapping the Metabolic Reprogramming Induced by Sodium-Glucose Cotransporter 2 Inhibition. JCI Insight 2023, 8, e164296. [Google Scholar] [CrossRef] [PubMed]
- Satomi, S.; Morio, A.; Miyoshi, H.; Nakamura, R.; Tsutsumi, R.; Sakaue, H.; Yasuda, T.; Saeki, N.; Tsutsumi, Y.M. Branched-Chain Amino Acids-Induced Cardiac Protection against Ischemia/Reperfusion Injury. Life Sci. 2020, 245, 117368. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights into the Role of mTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. mTOR Signaling: Recent Progress. Int. J. Mol. Sci. 2024, 25, 2587. [Google Scholar] [CrossRef]
- Suhara, T.; Baba, Y.; Shimada, B.K.; Higa, J.K.; Matsui, T. The mTOR Signaling Pathway in Myocardial Dysfunction in Type 2 Diabetes Mellitus. Curr. Diab Rep. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ye, W.-R.; Shi, W.; Yin, P.; Chen, C.; He, Y.-B.; Chen, M.-F.; Zu, X.-B.; Cai, Y. Perfect Match: mTOR Inhibitors and Tuberous Sclerosis Complex. Orphanet J. Rare Dis. 2022, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting Autophagy in Obesity: From Pathophysiology to Management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ji, L.; Hu, J.; Zhao, Y.; Johnston, L.J.; Zhang, X.; Ma, X. Functional Amino Acids and Autophagy: Diverse Signal Transduction and Application. Int. J. Mol. Sci. 2021, 22, 11427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.-F. mTOR Signalling: The Molecular Interface Connecting Metabolic Stress, Aging and Cardiovascular Diseases: mTOR in Metabolic and Age-Related Cardiovascular Diseases. Obes. Rev. 2012, 13, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. The Complex Network of mTOR Signalling in the Heart. Cardiovasc. Res. 2022, 118, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, K.; Wei, J.; Lin, Y.; Liu, Y. The Contradictory Role of Branched-Chain Amino Acids in Lifespan and Insulin Resistance. Front. Nutr. 2023, 10, 1189982. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Sharma, S. Mammalian Target of Rapamycin (mTOR) as a Potential Therapeutic Target in Various Diseases. Inflammopharmacology 2017, 25, 293–312. [Google Scholar] [CrossRef]
- Bae, J.; Paltzer, W.G.; Mahmoud, A.I. The Role of Metabolism in Heart Failure and Regeneration. Front. Cardiovasc. Med. 2021, 8, 702920. [Google Scholar] [CrossRef]
- Xiang, L.; Nie, J.; Wang, L.; Wang, Y.; Shi, J.; Wei, J.; Lau, C.-W.; Cai, Z.; Huang, Y. Integrated Metabolomics Analysis of the Effect of PPARδ Agonist GW501516 on Catabolism of BCAAs and Carboxylic Acids in Diabetic Mice. Chin. Chem. Lett. 2021, 32, 2197–2202. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR Control of Metabolism and Cardiovascular Functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched Chain Amino Acids Exacerbate Myocardial Ischemia/Reperfusion Vulnerability via Enhancing GCN2/ATF6/PPAR-α Pathway-Dependent Fatty Acid Oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Castagneto-Gissey, L.; Angelini, G.; Mingrone, G.; Cavarretta, E.; Tenori, L.; Licari, C.; Luchinat, C.; Tiepner, A.L.; Basso, N.; Bornstein, S.R.; et al. The Early Reduction of Left Ventricular Mass after Sleeve Gastrectomy Depends on the Fall of Branched-Chain Amino Acid Circulating Levels. eBioMedicine 2022, 76, 103864. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.S.; Manolis, T.A.; Manolis, A.A. Ketone Bodies and Cardiovascular Disease: An Alternate Fuel Source to the Rescue. Int. J. Mol. Sci. 2023, 24, 3534. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, J.; Hu, S. Metabolic Remodeling of Substrate Utilization during Heart Failure Progression. Heart Fail. Rev. 2019, 24, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Buttrick, P.; Rosenzweig, A.; Abel, E.D.; Allen, L.A.; Bristow, M.; Das, S.; DeVore, A.D.; Drakos, S.G.; Fang, J.C.; et al. Heart Failure Strategically Focused Research Network: Summary of Results and Future Directions. J. Am. Heart Assoc. 2022, 11, e025517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Marrachelli, V.G.; Yang, W.-Y.; Trenson, S.; Huang, Q.-F.; Wei, F.-F.; Thijs, L.; Van Keer, J.; Monleon, D.; Verhamme, P.; et al. Diastolic Left Ventricular Function in Relation to Circulating Metabolic Biomarkers in a Population Study. Eur. J. Prev. Cardiol. 2019, 26, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Ng, S.M.; Neubauer, S.; Rider, O.J. Myocardial Metabolism in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 63–75. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, K.; Liu, F.; Lu, X.; Huang, J.; Gu, D. Association of Circulating Branched-Chain Amino Acids with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. Atherosclerosis 2022, 350, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Bertsch, T.; Volke, J.; Schmid, A.; Klingbeil, R.; Metodiev, Y.; Karaca, B.; Kim, S.-H.; Lindner, S.; Schupp, T.; et al. Narrative Review of Metabolomics in Cardiovascular Disease. J. Thorac. Dis. 2021, 13, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, R.G.; Churilla, J.R.; Josephson, S.; Molle-Rios, Z.; Hossain, M.J.; Prado, W.L.; Balagopal, P.B. Branched-Chain Amino Acids and Relationship with Inflammation in Youth With Obesity: A Randomized Controlled Intervention Study. J. Clin. Endocrinol. Metab. 2021, 106, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Zhang, L.; Shah, S.; Fukushima, A.; Wagg, C.S.; Gopal, K.; Al Batran, R.; Pherwani, S.; Ho, K.L.; Boisvenue, J.; et al. Impaired Branched Chain Amino Acid Oxidation Contributes to Cardiac Insulin Resistance in Heart Failure. Cardiovasc. Diabetol. 2019, 18, 86. [Google Scholar] [CrossRef]
- Tharrey, M.; Mariotti, F.; Mashchak, A.; Barbillon, P.; Delattre, M.; Huneau, J.-F.; Fraser, G.E. Patterns of Amino Acids Intake Are Strongly Associated with Cardiovascular Mortality, Independently of the Sources of Protein. Int. J. Epidemiol. 2019, 49, 312–321. [Google Scholar] [CrossRef]
- Jacob, K.J.; Chevalier, S.; Lamarche, M.; Morais, J.A. Leucine Supplementation Does Not Alter Insulin Sensitivity in Prefrail and Frail Older Women Following a Resistance Training Protocol. J. Nutr. 2019, 149, 959–967. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engström, G.; Östling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A Diabetes-Predictive Amino Acid Score and Future Cardiovascular Disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite Profiling and Cardiovascular Event Risk: A Prospective Study of 3 Population-Based Cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Rankin, N.; Li, Q.; Mark, P.B.; Würtz, P.; Ala-Korpela, M.; Marre, M.; Poulter, N.; Hamet, P.; Chalmers, J.; et al. Circulating Amino Acids and the Risk of Macrovascular, Microvascular and Mortality Outcomes in Individuals with Type 2 Diabetes: Results from the ADVANCE Trial. Diabetologia 2018, 61, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Smith, E.; Fernandez, C.; Melander, O. Plasma Metabolites Associate with All-Cause Mortality in Individuals with Type 2 Diabetes. Metabolites 2020, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Ribeiro, R.; Senior, A.; Hsu, B.; Hirani, V.; Blyth, F.M.; Waite, L.M.; Simpson, S.J.; Naganathan, V.; Cumming, R.G.; et al. Branched Chain Amino Acids, Cardiometabolic Risk Factors and Outcomes in Older Men: The Concord Health and Ageing in Men Project. J. Gerontol. Ser. A 2020, 75, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.B.; et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes Risk and Amino Acid Profiles: Cross-Sectional and Prospective Analyses of Ethnicity, Amino Acids and Diabetes in a South Asian and European Cohort from the SABRE (Southall and Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Cardelo, M.M.P. Estrategias Dietéticas en el Manejo de la Diabetes Mellitus tipo 2 en Pacientes con Enfermedad Coronaria Establecida: Estudio Cordioprev. Ph.D. Thesis, University of Córdoba, Córdoba, Spain, 2023. [Google Scholar]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Fine, K.S.; Wilkins, J.T.; Sawicki, K.T. Circulating Branched Chain Amino Acids and Cardiometabolic Disease. J. Am. Heart Assoc. 2024, 13, e031617. [Google Scholar] [CrossRef]
- Chen, M.; Gao, C.; Yu, J.; Ren, S.; Wang, M.; Wynn, R.M.; Chuang, D.T.; Wang, Y.; Sun, H. Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure. J. Am. Heart Assoc. 2019, 8, e011625. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Jordan, J.; Dworak, M.; Merkel, T.; Burnstock, G. Myocardial Metabolism in Heart Failure: Purinergic Signalling and Other Metabolic Concepts. Pharmacol. Ther. 2019, 194, 132–144. [Google Scholar] [CrossRef]
- Ragni, M.; Greco, C.M.; Felicetta, A.; Ren, S.V.; Kunderfranco, P.; Ruocco, C.; Carullo, P.; Larcher, V.; Tedesco, L.; Severi, I.; et al. Dietary Essential Amino Acids for the Treatment of Heart Failure with Reduced Ejection Fraction. Cardiovasc. Res. 2023, 119, 982–997. [Google Scholar] [CrossRef]
- Li, R.; He, H.; Fang, S.; Hua, Y.; Yang, X.; Yuan, Y.; Liang, S.; Liu, P.; Tian, Y.; Xu, F.; et al. Time Series Characteristics of Serum Branched-Chain Amino Acids for Early Diagnosis of Chronic Heart Failure. J. Proteome Res. 2019, 18, 2121–2128. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Xia, Y.; Zhao, S.; Yan, W.; Wang, H.; Lee, Y.; Li, C.; Zhang, L.; Lian, K.; et al. Defective Branched Chain Amino Acid Catabolism Contributes to Cardiac Dysfunction and Remodeling Following Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1160–H1169. [Google Scholar] [CrossRef]
- Morio, A.; Tsutsumi, R.; Satomi, S.; Kondo, T.; Miyoshi, H.; Kato, T.; Kuroda, M.; Kitamura, T.; Hara, K.; Saeki, N.; et al. Leucine Imparts Cardioprotective Effects by Enhancing mTOR Activity and Mitochondrial Fusion in a Myocardial Ischemia/Reperfusion Injury Murine Model. Diabetol. Metab. Syndr. 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okumura, T.; Kazama, S.; Shibata, N.; Oishi, H.; Arao, Y.; Kuwayama, T.; Kato, H.; Yamaguchi, S.; Hiraiwa, H.; et al. Usefulness of Plasma Branched-Chain Amino Acid Analysis in Predicting Outcomes of Patients with Nonischemic Dilated Cardiomyopathy. Int. Heart J. 2020, 61, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, N.; Rau, C.D.; Lee, R.-P.; Yang, J.; Flach, R.J.R.; Petersen, L.; Zhu, C.; Pak, Y.-L.; Miller, R.A.; et al. Cell-Autonomous Effect of Cardiomyocyte Branched-Chain Amino Acid Catabolism in Heart Failure in Mice. Acta Pharmacol. Sin. 2023, 44, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.-Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, B.; Xie, M.; Li, T. Circulating Metabolic Signatures of Heart Failure in Precision Cardiology. Precis. Clin. Med. 2023, 6, pbad005. [Google Scholar] [CrossRef]
- Sansbury, B.E.; DeMartino, A.M.; Xie, Z.; Brooks, A.C.; Brainard, R.E.; Watson, L.J.; DeFilippis, A.P.; Cummins, T.D.; Harbeson, M.A.; Brittian, K.R.; et al. Metabolomic Analysis of Pressure-Overloaded and Infarcted Mouse Hearts. Circ. Heart Fail. 2014, 7, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, F.; Sorrentino, A.; Van Der Reest, J.; Yang, P.; Waldeck-Weiermair, M.; Steinhorn, B.; Eroglu, E.; Saeedi Saravi, S.S.; Yu, P.; Haigis, M.; et al. Metabolomic and Transcriptomic Signatures of Chemogenetic Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H451–H465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, X.; Cao, F.; Wang, Y. Mitochondrial Metabolism in Myocardial Remodeling and Mechanical Unloading: Implications for Ischemic Heart Disease. Front. Cardiovasc. Med. 2021, 8, 789267. [Google Scholar] [CrossRef]
- Lerman, J.B.; Giamberardino, S.N.; Hernandez, A.F.; Felker, G.M.; Shah, S.H.; McGarrah, R.W. Plasma Metabolites Associated with Functional and Clinical Outcomes in Heart Failure with Reduced Ejection Fraction with and without Type 2 Diabetes. Sci. Rep. 2022, 12, 9183. [Google Scholar] [CrossRef] [PubMed]
- Tso, S.-C.; Gui, W.-J.; Wu, C.-Y.; Chuang, J.L.; Qi, X.; Skvorak, K.J.; Dorko, K.; Wallace, A.L.; Morlock, L.K.; Lee, B.H.; et al. Benzothiophene Carboxylate Derivatives as Novel Allosteric Inhibitors of Branched-Chain α-Ketoacid Dehydrogenase Kinase. J. Biol. Chem. 2014, 289, 20583–20593. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.-C.; Yang, W.-H.; Haldeman, J.M.; Grenier-Larouche, T.; An, J.; Lapworth, A.L.; Astapova, I.; et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018, 27, 1281–1293.e7. [Google Scholar] [CrossRef]
- Hatahet, J.; Cook, T.M.; Bonomo, R.R.; Elshareif, N.; Gavini, C.K.; White, C.R.; Jesse, J.; Mansuy-Aubert, V.; Aubert, G. Fecal Microbiome Transplantation and Tributyrin Improves Early Cardiac Dysfunction and Modifies the BCAA Metabolic Pathway in a Diet Induced Pre-HFpEF Mouse Model. Front. Cardiovasc. Med. 2023, 10, 1105581. [Google Scholar] [CrossRef] [PubMed]
- Caragnano, A.; Aleksova, A.; Bulfoni, M.; Cervellin, C.; Rolle, I.G.; Veneziano, C.; Barchiesi, A.; Mimmi, M.C.; Vascotto, C.; Finato, N.; et al. Autophagy and Inflammasome Activation in Dilated Cardiomyopathy. J. Clin. Med. 2019, 8, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamashita, T.; Takahashi, T.; Tabata, T.; Watanabe, H.; Gotoh, Y.; Shinohara, M.; Kami, K.; Tanaka, H.; Matsumoto, K.; et al. Uncovering the Role of Gut Microbiota in Amino Acid Metabolic Disturbances in Heart Failure through Metagenomic Analysis. Front. Cardiovasc. Med. 2021, 8, 789325. [Google Scholar] [CrossRef]
- Biswas, D.; Duffley, L.; Pulinilkunnil, T. Role of Branched-chain Amino Acid–Catabolizing Enzymes in Intertissue Signaling, Metabolic Remodeling, and Energy Homeostasis. FASEB J. 2019, 33, 8711–8731. [Google Scholar] [CrossRef]
- Narita, K.; Amiya, E. Is Branched-Chain Amino Acid Nutritional Supplementation Beneficial or Detrimental in Heart Failure? World J. Cardiol. 2021, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Voronova, V.; Sokolov, V.; Morias, Y.; Boezelman, M.J.; Wågberg, M.; Henricsson, M.; Hansson, K.; Goltsov, A.; Peskov, K.; Sundqvist, M. Evaluation of Therapeutic Strategies Targeting BCAA Catabolism Using a Systems Pharmacology Model. Front. Pharmacol. 2022, 13, 993422. [Google Scholar] [CrossRef] [PubMed]
- Tanada, Y.; Shioi, T.; Kato, T.; Kawamoto, A.; Okuda, J.; Kimura, T. Branched-Chain Amino Acids Ameliorate Heart Failure with Cardiac Cachexia in Rats. Life Sci. 2015, 137, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Lau, E.S.H.; Fung, E.; Lee, H.; Ma, R.C.W.; Tam, C.H.T.; Wong, W.K.K.; Ng, A.C.W.; Chow, E.; Luk, A.O.Y.; et al. Circulating Branched-chain Amino Acids and Incident Heart Failure in Type 2 Diabetes: The Hong Kong Diabetes Register. Diabetes Metab. Res. Rev. 2020, 36, e3253. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Watanabe, M.; Takata, M.; Amiya, E.; Tsushima, K.; Adachi, T.; Hiroi, Y.; Funazaki, T.; Komuro, I. Effect of Oral Branched-Chain Amino Acids on Serum Albumin Concentration in Heart Failure Patients with Hypoalbuminemia: Results of a Preliminary Study. Am. J. Cardiovasc. Drugs 2018, 18, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, D.E.; Gibbs, J.J.; Li, J.; She, R.; Petucci, C.; Culver, J.A.; Tang, W.H.W.; Pinto, Y.M.; Williams, L.K.; Sabbah, H.N.; et al. Targeted Metabolomic Profiling of Plasma and Survival in Heart Failure Patients. JACC Heart Fail. 2017, 5, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Zhang, L.; Wagg, C.S.; Wang, W.; Ghandi, M.; Thai, D.; Yan, H.; Ussher, J.R.; Oudit, G.Y.; Lopaschuk, G.D. Targeting the Glucagon Receptor Improves Cardiac Function and Enhances Insulin Sensitivity Following a Myocardial Infarction. Cardiovasc. Diabetol. 2019, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; You, H.; Li, Y.; Wang, Y.; Hui, P.; Qiao, B.; Lu, J.; Zhang, W.; Zhou, S.; Zheng, Y.; et al. Relationships between Circulating Branched Chain Amino Acid Concentrations and Risk of Adverse Cardiovascular Events in Patients with STEMI Treated with PCI. Sci. Rep. 2018, 8, 15809. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Wang, Y.; You, H.; Hui, P.; Zheng, Y.; Du, J. Increased Branched-Chain Amino Acid Levels Are Associated with Long-Term Adverse Cardiovascular Events in Patients with STEMI and Acute Heart Failure. Life Sci. 2018, 209, 167–172. [Google Scholar] [CrossRef]
- Uddin, G.M.; Karwi, Q.G.; Pherwani, S.; Gopal, K.; Wagg, C.S.; Biswas, D.; Atnasious, M.; Wu, Y.; Wu, G.; Zhang, L.; et al. Deletion of BCATm Increases Insulin-Stimulated Glucose Oxidation in the Heart. Metabolism 2021, 124, 154871. [Google Scholar] [CrossRef]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High Dietary Intake of Branched-Chain Amino Acids Is Associated with an Increased Risk of Insulin Resistance in Adults. J. Diabetes 2018, 10, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Latimer, M.N.; Sonkar, R.; Mia, S.; Frayne, I.R.; Carter, K.J.; Johnson, C.A.; Rana, S.; Xie, M.; Rowe, G.C.; Wende, A.R.; et al. Branched Chain Amino Acids Selectively Promote Cardiac Growth at the End of the Awake Period. J. Mol. Cell. Cardiol. 2021, 157, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hsieh, P.N.; Sweet, D.R.; Jain, M.K. Krüppel-like Factor 15: Regulator of BCAA Metabolism and Circadian Protein Rhythmicity. Pharmacol. Res. 2018, 130, 123–126. [Google Scholar] [CrossRef]
- Shao, D.; Villet, O.; Zhang, Z.; Choi, S.W.; Yan, J.; Ritterhoff, J.; Gu, H.; Djukovic, D.; Christodoulou, D.; Kolwicz, S.C.; et al. Glucose Promotes Cell Growth by Suppressing Branched-Chain Amino Acid Degradation. Nat. Commun. 2018, 9, 2935. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.; Osté, M.; Kieneker, L.; Gruppen, E.; Wolak-Dinsmore, J.; Otvos, J.; Connelly, M.; Bakker, S.; Dullaart, R. Plasma Branched-Chain Amino Acids and Risk of Incident Type 2 Diabetes: Results from the PREVEND Prospective Cohort Study. J. Clin. Med. 2018, 7, 513. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Gogg, S.; Lotta, L.A.; Stančáková, A.; Nerstedt, A.; Boren, J.; Blüher, M.; Ferrannini, E.; Langenberg, C.; Wareham, N.J.; et al. Elevated Plasma Levels of 3-Hydroxyisobutyric Acid Are Associated with Incident Type 2 Diabetes. EBioMedicine 2018, 27, 151–155. [Google Scholar] [CrossRef]
- Bragg, F.; Trichia, E.; Aguilar-Ramirez, D.; Bešević, J.; Lewington, S.; Emberson, J. Predictive Value of Circulating NMR Metabolic Biomarkers for Type 2 Diabetes Risk in the UK Biobank Study. BMC Med. 2022, 20, 159. [Google Scholar] [CrossRef]
- Bragg, F.; Kartsonaki, C.; Guo, Y.; Holmes, M.; Du, H.; Yu, C.; Pei, P.; Yang, L.; Jin, D.; Chen, Y.; et al. The Role of NMR-Based Circulating Metabolic Biomarkers in Development and Risk Prediction of New Onset Type 2 Diabetes. Sci. Rep. 2022, 12, 15071. [Google Scholar] [CrossRef]
- Sawicki, K.T.; Ning, H.; Allen, N.B.; Carnethon, M.R.; Wallia, A.; Otvos, J.D.; Ben-Sahra, I.; McNally, E.M.; Snell-Bergeon, J.K.; Wilkins, J.T. Longitudinal Trajectories of Branched Chain Amino Acids through Young Adulthood and Diabetes in Later Life. JCI Insight 2023, 8, e166956. [Google Scholar] [CrossRef]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Li, X.S.; Gurmu, Y.; Bonaca, M.P.; Morrow, D.A.; Cohen, M.; Bhatt, D.L.; Steg, P.G.; Storey, R.F.; Johanson, P.; et al. Gut Microbiota-Dependent Trimethylamine N-oxide and Cardiovascular Outcomes in Patients with Prior Myocardial Infarction: A Nested Case Control Study From the PEGASUS-TIMI 54 Trial. J. Am. Heart Assoc. 2020, 9, e015331. [Google Scholar] [CrossRef] [PubMed]
- Croyal, M.; Saulnier, P.-J.; Aguesse, A.; Gand, E.; Ragot, S.; Roussel, R.; Halimi, J.-M.; Ducrocq, G.; Cariou, B.; Montaigne, D.; et al. Plasma Trimethylamine N-Oxide and Risk of Cardiovascular Events in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.; O’Brien, A.; Bernier, M.C.; Somogyi, A.; Wysocki, V.H.; Smart, S.; He, X.; Ambrosio, G.; Hsueh, W.A.; Raman, S.V. Trimethylamine N-Oxide and Incident Atherosclerotic Events in High-Risk Individuals with Diabetes: An ACCORD Trial Post Hoc Analysis. BMJ Open Diab Res. Care 2019, 7, e000718. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Van Dijk, P.R.; Connelly, M.A.; Garcia, E.; Bilo, H.J.G.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Circulating Trimethylamine N-Oxide Is Associated with Increased Risk of Cardiovascular Mortality in Type-2 Diabetes: Results from a Dutch Diabetes Cohort (ZODIAC-59). J. Clin. Med. 2021, 10, 2269. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Liu, L.; Han, T.; Yang, X.; Sun, C. Genetic Predisposition to Impaired Metabolism of the Branched Chain Amino Acids, Dietary Intakes, and Risk of Type 2 Diabetes. Genes. Nutr. 2021, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Fu, J.; Huang, A.; Fu, Z.; Yu, W.; et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Pallister, T.; Spector, T.; Cassidy, A. Associations between Branched Chain Amino Acid Intake and Biomarkers of Adiposity and Cardiometabolic Health Independent of Genetic Factors: A Twin Study. Int. J. Cardiol. 2016, 223, 992–998. [Google Scholar] [CrossRef]
- Siomkajło, M.; Rybka, J.; Mierzchała-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific Plasma Amino Acid Disturbances Associated with Metabolic Syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Hase, R.; Takahashi, H.; Ishimaru, Y.; Watanabe, R.; Saito, H.; Shimokawa, J.; Yamamoto, H.; Kikuchi, S.; et al. Plasma Branched-Chain and Aromatic Amino Acids in Relation to Hypertension. Nutrients 2020, 12, 3791. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Deussen, A. Effects of Natural Peptides from Food Proteins on Angiotensin Converting Enzyme Activity and Hypertension. Crit. Rev. Food Sci. Nutr. 2019, 59, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, J.; Yuan, R.; Li, N.; Li, C.; Zhang, X. mTOR Inhibitor Improves Testosterone-Induced Myocardial Hypertrophy in Hypertensive Rats. J. Endocrinol. 2022, 252, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Zhang, Y.; Jiang, X.; Liang, Y.; Wang, H.; Li, Y.; Sun, G. Association between Excessive Dietary Branched-Chain Amino Acids Intake and Hypertension Risk in Chinese Population. Nutrients 2022, 14, 2582. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Teymoori, F.; Asghari, G.; Azizi, F. Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Arch. Iran. Med. 2019, 22, 182–188. [Google Scholar] [PubMed]
- Wang, F.; Wang, B.; Chen, X.; Liu, W.; Wang, G.; Li, X.; Liu, X.; Li, N.; Zhang, J.; Yin, T.; et al. Association Between Blood Pressure and Branched-Chain/Aromatic Amino Acid Excretion Rate in 24-Hour Urine Samples from Elderly Hypertension Patients. Diabetes Metab. Syndr. Obes. 2021, 14, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Groothof, D.; Connelly, M.A.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. Concentration of Branched-Chain Amino Acids Is a Strong Risk Marker for Incident Hypertension. Hypertension 2019, 74, 1428–1435. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Mahbub, M.; Takahashi, H.; Hase, R.; Ishimaru, Y.; Sunagawa, H.; Amano, H.; Kobayashi- Miura, M.; Kanda, H.; Fujita, Y.; et al. Plasma Free Amino Acid Profiles Evaluate Risk of Metabolic Syndrome, Diabetes, Dyslipidemia, and Hypertension in a Large Asian Population. Environ. Health Prev. Med. 2017, 22, 35. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Fontana, M.; Giusti, A.M.; Pinto, A.; Iannucci, G.; Lenzi, A.; Donini, L.M. Amino Acids and Hypertension in Adults. Nutrients 2019, 11, 1459. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Welch, A.; Chowienczyk, P.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Inversely Associated with Arterial Stiffness andCentral Blood Pressure in Women. J. Nutr. 2015, 145, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Teymoori, F.; Asghari, G.; Mirmiran, P.; Azizi, F. Dietary Amino Acids and Incidence of Hypertension: A Principle Component Analysis Approach. Sci. Rep. 2017, 7, 16838. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M. Metabolomics Applications in Coronary Artery Disease Personalized Medicine. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 102, pp. 233–270. ISBN 978-0-12-824614-6. [Google Scholar]
- Xu, H.; Wang, X.; Geng, G.; Xu, X.; Liu, L.; Zhang, Y.; Wang, Z.; Wang, L.; Li, Y. Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients 2023, 15, 1580. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Aviram, M. Specific Amino Acids Affect Cardiovascular Diseases and Atherogenesis via Protection against Macrophage Foam Cell Formation: Review Article. Rambam Maimonides Med. J. 2018, 9, e0022. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.L.; Radovanovic, J.N.; Gluvic, Z.; Stewart, A.J.; Essack, M.; Motwalli, O.; Gojobori, T.; Isenovic, E.R. Atherosclerosis Linked to Aberrant Amino Acid Metabolism and Immunosuppressive Amino Acid Catabolizing Enzymes. Front. Immunol. 2020, 11, 551758. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, R.; Mu, H.; Zhang, W.; Zeng, J.; Li, H.; Wang, S.; Zhao, X.; Chen, W.; Dong, J.; et al. Oral Administration of Branched-Chain Amino Acids Attenuates Atherosclerosis by Inhibiting the Inflammatory Response and Regulating the Gut Microbiota in ApoE-Deficient Mice. Nutrients 2022, 14, 5065. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, H.; Li, L.; Chen, F.; Liu, Y.; Zhou, M.; Wang, J.; Jiang, J.; Li, X.; Fan, X.; et al. Branched-Chain Amino Acid Catabolism Promotes Thrombosis Risk by Enhancing Tropomodulin-3 Propionylation in Platelets. Circulation 2020, 142, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Kolwicz, S.C.; Abell, L.; Roe, N.D.; Kim, M.; Zhou, B.; Cao, Y.; Ritterhoff, J.; Gu, H.; et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, L.; Wang, Q.; Cao, J.-H.; Chen, Y.; Wang, W.; Zhu, B.-D.; Wei, Z.-H.; Li, R.; Li, C.-Y.; et al. Elevated Branched-Chain Amino Acid Promotes Atherosclerosis Progression by Enhancing Mitochondrial-to-Nuclear H2O2-Disulfide HMGB1 in Macrophages. Redox Biol. 2023, 62, 102696. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Sun, S.-J.; Cao, W.-X.; Lan, X.-T.; Ni, M.; Fu, H.; Li, D.-J.; Wang, P.; Shen, F.-M. Excessive ROS Production and Enhanced Autophagy Contribute to Myocardial Injury Induced by Branched-Chain Amino Acids: Roles for the AMPK-ULK1 Signaling Pathway and α7nAChR. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 165980. [Google Scholar] [CrossRef]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain Amino Acids Promote Endothelial Dysfunction through Increased Reactive Oxygen Species Generation and Inflammation. J. Cell Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Hu, F.; Qiu, Z.; Li, J.; Huang, C.; Xu, Y.; Cheng, X. Pim-2 Kinase Inhibits Inflammation by Suppressing the mTORC1 Pathway in Atherosclerosis. Aging 2021, 13, 22412–22431. [Google Scholar] [CrossRef] [PubMed]
- Reho, J.J.; Guo, D.; Rahmouni, K. Mechanistic Target of Rapamycin Complex 1 Signaling Modulates Vascular Endothelial Function Through Reactive Oxygen Species. J. Am. Heart Assoc. 2019, 8, e010662. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Smith, E.; Melander, O.; Fernandez, C. Altered Asparagine and Glutamate Homeostasis Precede Coronary Artery Disease and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Ma, Z.; Zhu, Y.; Chen, Z. Altered Amino Acid Metabolism between Coronary Heart Disease Patients with and without Type 2 Diabetes by Quantitative 1H NMR Based Metabolomics. J. Pharm. Biomed. Anal. 2021, 206, 114381. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Sun, J.-L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline Metabolomic Profiles Predict Cardiovascular Events in Patients at Risk for Coronary Artery Disease. Am. Heart J. 2012, 163, 844–850.e1. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Chen, Y.; Zhao, Y.-J.; Liu, L.-W.; Li, J.; Wang, S.-L.; Alolga, R.N.; Yin, Y.; Wang, X.-M.; et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J. Am. Coll. Cardiol. 2016, 68, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Castagné, R.; Boulangé, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Ebbels, T.M.D.; Kaluarachchi, M.R.; Chadeau-Hyam, M.; Mosen, D.; et al. Serum Metabolic Signatures of Coronary and Carotid Atherosclerosis and Subsequent Cardiovascular Disease. Eur. Heart J. 2019, 40, 2883–2896. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Granger, C.B.; Craig, D.; Haynes, C.; Bain, J.; Stevens, R.D.; Hauser, E.R.; Newgard, C.B.; Kraus, W.E.; Newby, L.K.; et al. Validation of the Association between a Branched Chain Amino Acid Metabolite Profile and Extremes of Coronary Artery Disease in Patients Referred for Cardiac Catheterization. Atherosclerosis 2014, 232, 191–196. [Google Scholar] [CrossRef]
- Vaarhorst, A.A.M.; Verhoeven, A.; Weller, C.M.; Böhringer, S.; Göraler, S.; Meissner, A.; Deelder, A.M.; Henneman, P.; Gorgels, A.P.M.; Van Den Brandt, P.A.; et al. A Metabolomic Profile Is Associated with the Risk of Incident Coronary Heart Disease. Am. Heart J. 2014, 168, 45–52.e7. [Google Scholar] [CrossRef]
- Paynter, N.P.; Balasubramanian, R.; Giulianini, F.; Wang, D.D.; Tinker, L.F.; Gopal, S.; Deik, A.A.; Bullock, K.; Pierce, K.A.; Scott, J.; et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018, 137, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Buring, J.E.; Lee, I.-M.; Manson, J.E.; Tobias, D.K. Association of Modifiable Lifestyle Factors with Plasma Branched-Chain Amino Acid Metabolites in Women. J. Nutr. 2022, 152, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Chevli, P.A.; Freedman, B.I.; Hsu, F.-C.; Xu, J.; Rudock, M.E.; Ma, L.; Parks, J.S.; Palmer, N.D.; Shapiro, M.D. Plasma Metabolomic Profiling in Subclinical Atherosclerosis: The Diabetes Heart Study. Cardiovasc. Diabetol. 2021, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.E.; Tahir, U.A.; Hu, J.; Ngo, D.; Chen, Z.-Z.; Robbins, J.M.; Katz, D.; Balasubramanian, R.; Peterson, B.; Deng, S.; et al. Metabolomic Analysis of Coronary Heart Disease in an African American Cohort from the Jackson Heart Study. JAMA Cardiol. 2022, 7, 184–194. [Google Scholar] [CrossRef] [PubMed]
- De La, O.V.; Zazpe, I.; Ruiz-Canela, M. Effect of Branched-Chain Amino Acid Supplementation, Dietary Intake and Circulating Levels in Cardiometabolic Diseases: An Updated Review. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 35–50. [Google Scholar] [CrossRef]
- Doestzada, M.; Zhernakova, D.V.; Van Den Munckhof, I.C.L.; Wang, D.; Kurilshikov, A.; Chen, L.; Bloks, V.W.; Van Faassen, M.; Rutten, J.H.W.; Joosten, L.A.B.; et al. Systematic Analysis of Relationships between Plasma Branched-Chain Amino Acid Concentrations and Cardiometabolic Parameters: An Association and Mendelian Randomization Study. BMC Med. 2022, 20, 485. [Google Scholar] [CrossRef]

| Study Design | Results | Ref. |
|---|---|---|
| Case–cohort of 970 patients PREDIMED trial | 70% excess risk of CVD and stroke when associated with high levels of BCAA | |
| Cohort | Increased BCAA levels in diabetic cardiomyopathy secondary to TAK1/P38 MAPK axis KLF15 inhibition | [66] |
| Cohort | BCAAs associated with cardiovascular mortality | [67] |
|
Randomized double- blinded placebo- controlled crossover study | Leucine does not influence insulin resistance | [68] |
| Prospective cohort of U.S. Women’s Health Study including 27,041 women | BCAA levels associated with incident CVD in women | [69] |
| Case–control study from Malmö Diet and Cancer Cardiovascular Cohort (MDC-CC) | BCAA levels predict CVD | [70] |
| Case–cohort study from FINRISK | BCAA levels as a CVD risk factor | [71] |
| Cohort from ADVANCED study | BCAA levels associated with major macrovascular complications in T2D | [72] |
| Cohort from Malmö Diet and Cancer Cardiovascular Cohort (MDC-CC) | Valine and isoleucine associated with an increased CVD risk | [73] |
| Cohort from Concord Health and Ageing in Men Project (CHAMP) | Lower BCAA levels associated with higher mortality and major cardiovascular endpoints (MACEs) | [74] |
| 685 participants without diabetes of the Insulin Resistance Atherosclerosis Study (IRAS) | BCAA levels associated with insulin resistance and T2D | [75] |
| Genome-wide study of 16,596 patients | BCAA levels associated with a higher risk of T2D | [76] |
| Case–control study of 2422 patients in the Framingham cohort | BCAA levels associated with a higher risk of T2D | [77] |
| Cohort of 1279 European and 1007 South Asian patients | BCAA levels associated with a 40% higher risk of T2D | [78] |
| Study Design | Results | Ref. |
|---|---|---|
| Experimental study (pigs) | Empagliflozin ameliorates left ventricular systolic function via BCAA myocardial consumption | [80] |
| Experimental study (Dahl salt-sensitive rats fed high-salt diet) | BCAA prolonged survival in HF | [104] |
| Experimental study (PP2Cm-knockout mice) | BCAAs impaired myocardial contractions | [90] |
| Experimental study (murine model) | BCAAs associated with post-MI HF | [86] |
| Prospective observational study of 29,103 patients | BCAAs levels associated with HF in T2D | [105] |
| Crossover controlled trial | BCAA supplementation improved serum albumin levels in HF | [106] |
| Randomized controlled study including 1032 patients | Leucine and valine associated with higher mortality in HF | [107] |
| Prospective study | BCAA associated with diastolic left ventricular function | [58] |
| Study Design | Results | Ref. |
|---|---|---|
| Prospective cohort of 4288 participants | BCAA intake (valine) associated with a higher incidence of hypertension | [137] |
| Cross-sectional study | Amino acid intake increases peripheral blood pressure | [142] |
| Cross-sectional study | BCAA intake associated with a higher incidence of hypertension | [143] |
| Study Design | Results | Ref. |
|---|---|---|
| Experimental study | BCAA levels associated with AS pathogenesis | [151] |
| Cohort | BCAA levels associated with coronary and carotid atherosclerosis | [160] |
| Experimental study (902 patients) | Increased BCAA levels associated with cardiovascular events in patients with STEMI and acute HF | [110] |
| Case–control study population of 1983 patients undergoing coronary angiography | BCAA independently associated with CAD diagnosis | [161] |
| Case–cohort, prospective, population based | BCAA levels associated with CAD | [162] |
| Case–control | BCAA levels associated with CAD | [163] |
| Experimental study (adult mice) | BCAA enhances I/R injury via CN2/ATF6/PPAR-α pathway | [53] |
| Experimental study (Wild-type C57BL/6 mice) | Overexpression of PP2Cm alleviates MI/R injury by reducing BCAA catabolic impairment | [28] |
| Experimental study (Male C57BL/6 mice and Wistar rats) | PI3K/Akt/GSK3β pathway attenuates myocardial I/R injury | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Valasciuc, E.; Costea, C.F.; Scripcariu, D.V.; Ouatu, A.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, D.E.; Ciocoiu, M.; Baroi, L.G.; et al. Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters. Nutrients 2024, 16, 1972. https://doi.org/10.3390/nu16121972
Tanase DM, Valasciuc E, Costea CF, Scripcariu DV, Ouatu A, Hurjui LL, Tarniceriu CC, Floria DE, Ciocoiu M, Baroi LG, et al. Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters. Nutrients. 2024; 16(12):1972. https://doi.org/10.3390/nu16121972
Chicago/Turabian StyleTanase, Daniela Maria, Emilia Valasciuc, Claudia Florida Costea, Dragos Viorel Scripcariu, Anca Ouatu, Loredana Liliana Hurjui, Claudia Cristina Tarniceriu, Diana Elena Floria, Manuela Ciocoiu, Livia Genoveva Baroi, and et al. 2024. "Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters" Nutrients 16, no. 12: 1972. https://doi.org/10.3390/nu16121972
APA StyleTanase, D. M., Valasciuc, E., Costea, C. F., Scripcariu, D. V., Ouatu, A., Hurjui, L. L., Tarniceriu, C. C., Floria, D. E., Ciocoiu, M., Baroi, L. G., & Floria, M. (2024). Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters. Nutrients, 16(12), 1972. https://doi.org/10.3390/nu16121972













