Correction: Hill et al. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769
1. Explanation and Summary of Corrections
2. Error in Abstract
3. Error in Results
| FP-MD (n = 38) | Placebo (n = 37) | p-Value | p-Value ‡ | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
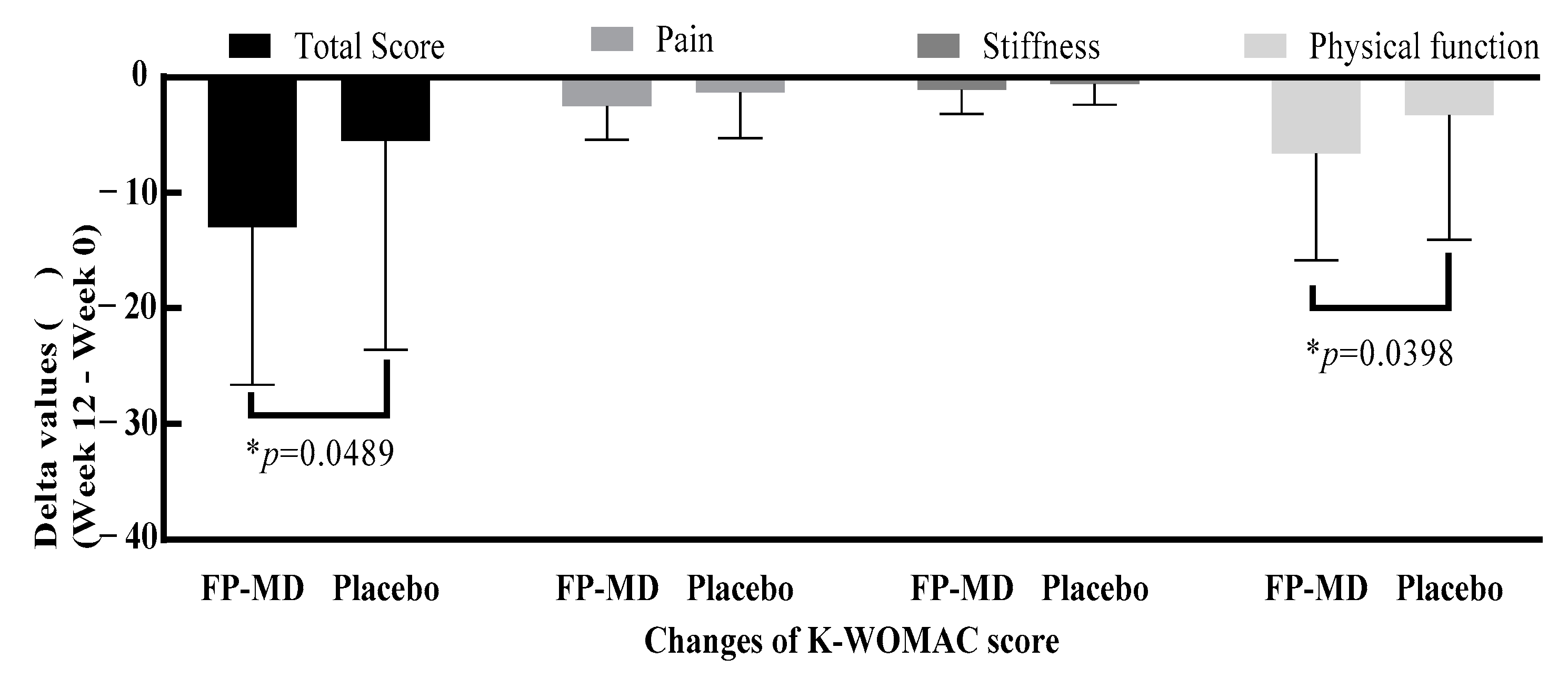
| 1. Total score | ||||
| Baseline | 30.7 ± 14.81 | 28.3 ± 13.55 | 0.4737 * | |
| Week 6 | 21.2 ± 13.10 | 23.5 ± 13.75 | 0.1304 * | 0.1658 |
| Change from baseline | −9.5 ± 12.57 | −4.8 ± 14.10 | ||
| p-value ** | <0.0001 | 0.0432 | ||
| Week 12 | 17.7 ± 15.06 | 22.8 ± 15.07 | 0.0489 * | 0.1063 |
| Change from baseline | −13.0 ± 13.62 | −5.5 ± 18.08 | ||
| p-value ** | <0.0001 | 0.0674 | ||
| 2. Pain score | ||||
| Baseline | 6.0 ± 3.22 | 5.7 ± 2.64 | 0.6582 † | |
| Week 6 | 4.0 ± 2.74 | 4.7 ± 2.98 | 0.1675 * | 0.1149 |
| Change from baseline | −2.0 ± 3.14 | −1.0 ± 6.07 | ||
| p-value ** | 0.0004 | 0.0518 | ||
| Week 12 | 3.5 ± 2.99 | 4.5 ± 3.45 | 0.2635 † | 0.1779 |
| Change from baseline | −2.5 ± 2.92 | −1.3 ± 3.94 | ||
| p-value | <0.0001 ** | 0.0173 # | ||
| 3. Stiffness score | ||||
| Baseline | 2.9 ± 1.61 | 2.6± 1.44 | 0.5240 † | |
| Week 6 | 2.0 ± 1.31 | 2.1 ± 1.35 | 0.4294 † | 0.5298 |
| Change from baseline | −0.9 ± 1.78 | −0.5 ± 1.45 | ||
| p-value | 0.0040 # | 0.0310 ** | ||
| Week 12 | 1.8 ± 1.57 | 2.0 ± 1.62 | 0.2819 † | 0.6330 |
| Change from baseline | −1.1 ± 2.08 | −0.6 ± 1.79 | ||
| p-value | 0.0039 ** | 0.0282 # | ||
| 4. Physical function score | ||||
| Baseline | 21.8 ± 11.03 | 20.0 ± 10.25 | 0.4639 * | |
| Week 6 | 15.2 ± 9.84 | 16.7 ± 10.14 | 0.1528 * | 0.2148 |
| Change from baseline | −6.6 ± 9.25 | −3.3 ± 10.80 | ||
| p-value ** | 0.0001 | 0.0705 | ||
| Week 12 | 12.4 ± 10.83 | 16.3 ± 10.86 | 0.0398 * | 0.0890 |
| Change from baseline | −9.4 ± 9.99 | −3.7 ± 13.38 | ||
| p-value ** | <0.0001 | 0.1005 |
4. Error in Discussion
Reference
- Hill, W.S.; Dohnalek, M.H.; Ha, Y.; Kim, S.-J.; Jung, J.-C.; Kang, S.-B. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, W.S.; Dohnalek, M.H.; Ha, Y.; Kim, S.-J.; Jung, J.-C.; Kang, S.-B. Correction: Hill et al. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. Nutrients 2024, 16, 1961. https://doi.org/10.3390/nu16121961
Hill WS, Dohnalek MH, Ha Y, Kim S-J, Jung J-C, Kang S-B. Correction: Hill et al. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. Nutrients. 2024; 16(12):1961. https://doi.org/10.3390/nu16121961
Chicago/Turabian StyleHill, W. Stephen, Margaret H. Dohnalek, Yejin Ha, Seok-Jung Kim, Jae-Chul Jung, and Seung-Baik Kang. 2024. "Correction: Hill et al. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769" Nutrients 16, no. 12: 1961. https://doi.org/10.3390/nu16121961
APA StyleHill, W. S., Dohnalek, M. H., Ha, Y., Kim, S.-J., Jung, J.-C., & Kang, S.-B. (2024). Correction: Hill et al. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. Nutrients, 16(12), 1961. https://doi.org/10.3390/nu16121961





